Nisoldipine (nisoldipine 8.5 mg) Dailymed
Generic: nisoldipine is used for the treatment of Angina Pectoris, Variant Hypertension Hypotension
IMPRINT: SCI 503
SHAPE: oval
COLOR: orange
All Imprints
nisoldipine 17 mg 24 hr extended release tablet - sci 501 round yellow
nisoldipine 34 mg - sci 503 oval orange
Go PRO for all pill images
Extended Release Tablets
For Oral Use
Description
Nisoldipine is an extended release tablet dosage form of the dihydropyridine calcium channel blocker nisoldipine. Nisoldipine is 3,5-pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-, methyl 2-methylpropyl ester, C20H24N2O6, and has the structural formula:
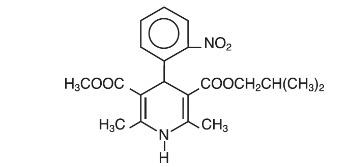
Nisoldipine is a yellow crystalline substance, practically insoluble in water but soluble in ethanol. It has a molecular weight of 388.4. Nisoldipine tablets comprise three layers: a top barrier layer, a middle layer containing nisoldipine, and a bottom barrier layer. The erodible barrier layers and the hydrogel middle layer provide for the controlled release of the drug. Nisoldipine tablets contain either 8.5, 17, or 34 mg of nisoldipine for once-a-day oral administration.
Inactive ingredients in the formulation include: Hypromellose, hypromellose phthalate, lactose, glyceryl behenate, povidone, magnesium stearate, silicon dioxide, methacrylic acid copolymer, and sodium lauryl sulfate. Inactive ingredients in the film coating include: polydextrose, titanium dioxide, hypromellose, polyethylene glycol, iron oxide, and carnauba wax. Additionally, the 17 mg formulation contains FD&C Yellow #5.
Clinical Pharmacology
Mechanism of Action
Nisoldipine is a member of the dihydropyridine class of calcium channel antagonists (calcium ion antagonists or slow channel blockers) that inhibit the transmembrane influx of calcium into vascular smooth muscle and cardiac muscle. It reversibly competes with other dihydropyridines for binding to the calcium channel. Because the contractile process of vascular smooth muscle is dependent upon the movement of extracellular calcium into the muscle through specific ion channels, inhibition of the calcium channel results in dilation of the arterioles. In vitro studies show that the effects of nisoldipine on contractile processes are selective, with greater potency on vascular smooth muscle than on cardiac muscle. Although, like other dihydropyridine calcium channel blockers, nisoldipine has negative inotropic effects in vitro, studies conducted in intact anesthetized animals have shown that the vasodilating effect occurs at doses lower than those that affect cardiac contractility. The effect of nisoldipine on blood pressure is principally a consequence of a dose-related decrease of peripheral vascular resistance. While nisoldipine, like other dihydropyridines, exhibits a mild diuretic effect, most of the antihypertensive activity is attributed to its effect on peripheral vascular resistance.
Pharmacokinetics and Metabolism
Nisoldipine pharmacokinetics are independent of the dose across the clinical dosage range of 17 to 51 mg, with plasma concentrations proportional to dose. Nisoldipine accumulation, during multiple dosing, is predictable from a single dose. Nisoldipine is relatively well absorbed into the systemic circulation with 87% of the radiolabeled drug recovered in urine and feces. The absolute bioavailability of nisoldipine is about 5%. Nisoldipine's low bioavailability is due, in part, to pre-systemic metabolism in the gut wall, and this metabolism decreases from the proximal to the distal parts of the intestine. A pronounced food-effect is observed when Nisoldipine is administered with a high-fat meal resulting in an increased peak concentration (Cmax) of up to 245%. Total exposure (AUC) is decreased by 25%. As a result, Nisoldipine should be taken on an empty stomach (1 hour before or 2 hours after a meal).
Maximal plasma concentrations of nisoldipine are reached at 9.2 ¬Ī 5.1 hours. The terminal elimination half-life (reflecting post absorption clearance of nisoldipine) ranges from 13.7 ¬Ī 4.3 hours. After oral administration, the concentration of (+)-nisoldipine, the active enantiomer, is about 6 times higher than the inactive (-) -nisoldipine enantiomer. The plasma protein binding of nisoldipine is very high, with less than 1% unbound over the plasma concentration range of 100 ng/mL to 10 mcg/mL.
Nisoldipine is highly metabolized; 5 major urinary metabolites have been identified. Although 60 - 80% of an oral dose undergoes urinary excretion, only traces of unchanged nisoldipine are found in urine. The major biotransformation pathway appears to be the hydroxylation of the isobutyl ester. A hydroxylated derivative of the side chain, present in plasma at concentrations approximately equal to the parent compound, appears to be the only active metabolite, and has about 10% of the activity of the parent compound. Cytochrome P450 enzymes are believed to play a major role in the metabolism of nisoldipine. The particular isoenzyme system responsible for its metabolism has not been identified, but other dihydropyridines are metabolized by cytochrome P450 IIIA4. Nisoldipine should not be administered with grapefruit juice, as this has been shown, in a study of 12 subjects, to interfere with nisoldipine metabolism, resulting in a mean increase in Cmax of about 3-fold (ranging up to about 7-fold) and AUC of almost 2-fold (ranging up to about 5-fold). A similar phenomenon has been seen with several other dihydropyridine calcium channel blockers.
Special Populations
Renal Dysfunction: Because renal elimination is not an important pathway, bioavailability and pharmacokinetics of Nisoldipine were not significantly different in patients with various degrees of renal impairment. Dosing adjustments in patients with mild to moderate renal impairment are not necessary.
Geriatric: Elderly patients have been found to have 2 to 3 fold higher plasma concentrations (Cmax and AUC) than young subjects. This should be reflected in more cautious dosing (see DOSAGE AND ADMINISTRATION).
Hepatic Insufficiency: In patients with liver cirrhosis given a dose bioequivalent to 8.5 mg Nisoldipine, plasma concentrations of the parent compound were 4 to 5 times higher than those in healthy young subjects. Lower starting and maintenance doses should be used in cirrhotic patients (see DOSAGE AND ADMINISTRATION).
Gender and Race: The effect of gender or race on the pharmacokinetics of nisoldipine has not been investigated.
Disease States: Hypertension does not significantly alter the pharmacokinetics of nisoldipine.
Pharmacodynamics
Administration of a single dose of nisoldipine leads to decreased systemic vascular resistance and blood pressure with a transient increase in heart rate. The change in heart rate is greater with immediate release nisoldipine preparations. The effect on blood pressure is directly related to the initial degree of elevation above normal. Chronic administration of nisoldipine results in a sustained decrease in vascular resistance and small increases in stroke index and left ventricular ejection fraction. A study of the immediate release formulation showed no effect of nisoldipine on the renin-angiotensin-aldosterone system or on plasma norepinephrine concentration in normals. Changes in blood pressure in hypertensive patients given Nisoldipine were dose related over the clinical dosage range.
Nisoldipine does not appear to have significant negative inotropic activity in intact animals or humans, and did not lead to worsening of clinical heart failure in three small studies of patients with asymptomatic and symptomatic left ventricular dysfunction. There is little information, however, in patients with severe congestive heart failure, and all calcium channel blockers should be used with caution in any patient with heart failure.
Nisoldipine has no clinically important chronotropic effects. Except for mild shortening of sinus cycle, SA conduction time and AH intervals, single oral doses up to 20 mg of immediate release nisoldipine did not significantly change other conduction parameters. Similar electrophysiologic effects were seen with single IV doses, which could be blunted in patients pre-treated with beta-blockers. Dose and plasma level related flattening or inversion of T-waves have been observed in a few small studies. Such reports were concentrated in patients receiving rapidly increased high doses in one study; the phenomenon has not been a cause of safety concern in large clinical trials.
Clinical Studies in Hypertension
The antihypertensive efficacy of Nisoldipine was studied in 5 double-blind, placebo-controlled, randomized studies, in which over 600 patients were treated with Nisoldipine as monotherapy and about 300 with placebo; 4 of the five studies compared 2 or 3 fixed doses while the fifth allowed titration from doses bioequivalent to 8.5 - 34 mg. Once daily administration of Nisoldipine produced sustained reductions in systolic and diastolic blood pressures over the 24 hour dosing interval in both supine and standing positions. The mean placebo-subtracted reductions in supine systolic and diastolic blood pressure at trough, 24 hours post-dose, in these studies, are shown below. Changes in standing blood pressure were similar:
 MEAN SUPINE TROUGH SYSTOLIC AND DIASTOLIC BLOOD PRESSURE CHANGES (mm Hg)  Nisoldipine  Doses bioequivalent to:(mg/day)  8.5 mg  17 mg  25.5 mg  34 mg  8.5-34 mgtitrated Systolic  8  11  11  14  15 Diastolic  3  5  7  7  8
In patients receiving atenolol, supine blood pressure reductions with Nisoldipine at doses bioequivalent to 17 and 34 mg once daily were 12/6 and 19/8 mm Hg, respectively. The sustained antihypertensive effect of Nisoldipine was demonstrated by 24 hour blood pressure monitoring and examination of peak and trough effects. The trough/peak ratios ranged from 70 to 100% for diastolic and systolic blood pressure. The mean change in heart rate in these studies was less than one beat per minute. In 4 of the 5 studies, patients received initial doses bioequivalent to 17-25.5 mg Nisoldipine without incident (excessive effects on blood pressure or heart rate). The fifth study started patients on lower doses of Nisoldipine.
Patient race and gender did not influence the blood pressure lowering effect of Nisoldipine. Despite the higher plasma concentration of nisoldipine in the elderly, there was no consistent difference in their blood pressure response except that the lowest clinical dose was somewhat more effective than in non-elderly patients. No postural effect on blood pressure was apparent and there was no evidence of tolerance to the antihypertensive effect of Nisoldipine in patients treated for up to one year.
Indications And Usage
Nisoldipine is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
Contraindications
Nisoldipine is contraindicated in patients with known hypersensitivity to dihydropyridine calcium channel blockers.
Warnings
Increased angina and/or myocardial infarction in patients with coronary artery disease: Rarely, patients, particularly those with severe obstructive coronary artery disease, have developed increased frequency, duration and/or severity of angina, or acute myocardial infarction on starting calcium channel blocker therapy or at the time of dosage increase. The mechanism of this effect has not been established. In controlled studies of Nisoldipine in patients with angina this was seen about 1.5% of the time in patients given nisoldipine, compared with 0.9% in patients given placebo.
Precautions
General
Hypotension : Because nisoldipine, like other vasodilators, decreases peripheral vascular resistance, careful monitoring of blood pressure during the initial administration and titration of Nisoldipine is recommended. Close observation is especially important for patients already taking medications that are known to lower blood pressure. Although in most patients the hypotensive effect of Nisoldipine is modest and well tolerated, occasional patients have had excessive and poorly tolerated hypotension. These responses have usually occurred during initial titration or at the time of subsequent upward dosage adjustment.
Congestive Heart Failure : Although acute hemodynamic studies of nisoldipine in patients with NYHA Class II-IV heart failure have not demonstrated negative inotropic effects, safety of Nisoldipine in patients with heart failure has not been established. Caution therefore should be exercised when using Nisoldipine in patients with heart failure or compromised ventricular function, particularly in combination with a beta-blocker.
Patients with Hepatic Impairment : Because nisoldipine is extensively metabolized by the liver and, in patients with cirrhosis, it reaches blood concentrations about 5 times those in normals, Nisoldipine should be administered cautiously in patients with severe hepatic dysfunction (see DOSAGE AND ADMINISTRATION). 
Information for Patients
Nisoldipine is an extended release tablet and should be swallowed whole. Tablets should not be chewed, divided or crushed. Nisoldipine should be taken on an empty stomach (1 hour before or 2 hours after a meal). Grapefruit juice, which has been shown to increase significantly the bioavailability of nisoldipine and other dihydropyridine type calcium channel blockers, should not be taken with Nisoldipine.
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Laboratory Tests
Nisoldipine is not known to interfere with the interpretation of laboratory tests.
Drug Interactions
A 30 to 45% increase in AUC and Cmax of nisoldipine was observed with concomitant administration of cimetidine 400 mg twice daily. Ranitidine 150 mg twice daily did not interact significantly with nisoldipine (AUC was decreased by 15 - 20%). No pharmacodynamic effects of either histamine H2 receptor antagonist were observed.
CYP3A4 inhibitors and inducers : Nisoldipine is substrate of CYP3A4 and coadministration of Nisoldipine with any known inducer or inhibitor of CYP3A4 should be avoided in general.
Coadministration of phenytoin with a dose bioequivalent to 34 mg Nisoldipine tablets in epileptic patients lowered the nisoldipine plasma concentrations to undetectable levels. Coadministration of Nisoldipine with phenytoin should be avoided and alternative antihypertensive therapy should be considered. Pharmacokinetic interactions between nisoldipine and beta-blockers (atenolol, propranolol) were variable and not significant. Propranolol attenuated the heart rate increase following administration of immediate release nisoldipine. The blood pressure effect of Nisoldipine tended to be greater in patients on atenolol than in patients on no other antihypertensive therapy. Quinidine at 648 mg bid decreased the bioavailability (AUC) of nisoldipine by 26%, but not the peak concentration. Immediate release nisoldipine increased plasma quinidine concentrations by about 20%. This interaction was not accompanied by ECG changes and its clinical significance is not known. No significant interactions were found between nisoldipine and warfarin or digoxin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Dietary administration of nisoldipine to male and female rats for up to 24 months (mean doses up to 82 and 111 mg/kg/day, 16 and 19 times the maximum recommended human dose [MRHD] on a mg/m2 basis, respectively) and female mice for up to 21 months (mean doses of up to 217 mg/kg/day, 20 times the MRHD on a mg/m2 basis) revealed no evidence of tumorigenic effect of nisoldipine. In male mice receiving a mean dose of 163 mg nisoldipine/kg/day (16 times the MRHD of 60 mg/day on a mg/m2 basis), an increased frequency of stomach papilloma, but still within the historical range, was observed. No evidence of stomach neoplasia was observed at lower doses (up to 58 mg/kg/day). Nisoldipine was negative when tested in a battery of genotoxicity assays including the Ames test and the CHO/HGRPT assay for mutagenicity and the in vivo mouse micronucleus test and in vitro CHO cell test for clastogenicity.
When administered to male and female rats at doses of up to 30 mg/kg/day (about 5 times the MRHD on a mg/m2 basis) nisoldipine had no effect on fertility.
Pregnancy
Nisoldipine was neither teratogenic nor fetotoxic at doses that were not maternally toxic. Nisoldipine was fetotoxic but not teratogenic in rats and rabbits at doses resulting in maternal toxicity (reduced maternal body weight gain). In pregnant rats, increased fetal resorption (postimplantation loss) was observed at 100 mg/kg/day and decreased fetal weight was observed at both 30 and 100 mg/kg/day. These doses are, respectively, about 5 and 16 times the MRHD when compared on a mg/m2 basis. In pregnant rabbits, decreased fetal and placental weights were observed at a dose of 30 mg/kg/day, about 10 times the MRHD when compared on a mg/m2 basis. In a study in which pregnant monkeys (both treated and control) had high rates of abortion and mortality, the only surviving fetus from a group exposed to a maternal dose of 100 mg nisoldipine/kg/day (about 30 times the MRHD when compared on a mg/m2 basis) presented with forelimb and vertebral abnormalities not previously seen in control monkeys of the same strain. There are no adequate and well controlled studies in pregnant women. Nisoldipine should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether nisoldipine is excreted in human milk. Because many drugs are excreted in human milk, a decision should be made to discontinue nursing, or to discontinue Nisoldipine, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of nisoldipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Patients over 65 are expected to develop higher plasma concentrations of nisoldipine. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Adverse Experiences
More than 6000 patients world-wide have received nisoldipine in clinical trials for the treatment of hypertension, either as the immediate release or the Nisoldipine extended release formulation. Of about 1,500 patients who received Nisoldipine in hypertension studies, about 55% were exposed for at least 2 months and about one third were exposed for over 6 months, the great majority at doses equivalent to 17 mg and above.
Nisoldipine is generally well-tolerated. In the U.S. clinical trials of Nisoldipine in hypertension, 10.9% of the 921 Nisoldipine patients discontinued treatment due to adverse events compared with 2.9% of 280 placebo patients. The frequency of discontinuations due to adverse experiences was related to dose, with a 5.4% and 10.9% discontinuation rate at the lowest and highest daily dose, respectively.
The most frequently occurring adverse experiences with Nisoldipine are those related to its vasodilator properties; these are generally mild and only occasionally lead to patient withdrawal from treatment. The table below, from U.S. placebo-controlled parallel dose response trials of Nisoldipine using doses across the clinical dosage range in patients with hypertension, uls all of the adverse events, regardless of the causal relationship to Nisoldipine, for which the overall incidence on Nisoldipine was both >1% and greater with Nisoldipine than with placebo.
Adverse Event Nisoldipine (%)(n=663) Placebo (%)(n=280) Peripheral Edema 22 10 Headache 22 15 Dizziness 5  4 Pharyngitis 5  4 Vasodilation 4  2 Sinusitis 3  2 Palpitation 3 1 Chest Pain  2 1 Nausea 2 1 Rash 2 1 Only peripheral edema and possibly dizziness appear to be dose related.
Nisoldipine, dose bioequivalent to: Adverse Event  Placebo  8.5 mg  17 mg 25.5 mg  34 mg (Rates in %)  N=280 N=30  N=170  N=105  N=139  Peripheral Edema  10 7  15  20  27  Dizziness  4 7  3  3  4 
The common adverse events occurred at about the same rate in men as in women, and at a similar rate in patients over age 65 as in those under that age, except that headache was much less common in older patients. Except for peripheral edema and vasodilation, which were more common in whites, adverse event rates were similar in blacks and whites.
The following adverse events occurred in ‚ȧ1% of all patients treated for hypertension in U.S. and foreign clinical trials, or with unspecified incidence in other studies. Although a causal relationship of Nisoldipine to these events cannot be established, they are uled to alert the physician to a possible relationship with Nisoldipine treatment.
Body As A Whole : cellulitis, chills, facial edema, fever, flu syndrome, malaise
Cardiovascular : atrial fibrillation, cerebrovascular accident, congestive heart failure, first degree AV block, hypertension, hypotension, jugular venous distension, migraine, myocardial infarction, postural hypotension, ventricular extrasystoles, supraventricular tachycardia, syncope, systolic ejection murmur, T wave abnormalities on ECG (flattening, inversion, nonspecific changes), venous insufficiency
Digestive : abnormal liver function tests, anorexia, colitis, diarrhea, dry mouth, dyspepsia, dysphagia, flatulence, gastritis, gastrointestinal hemorrhage, gingival hyperplasia, glossitis, hepatomegaly, increased appetite, melena, mouth ulceration
Endocrine : diabetes mellitus, thyroiditis
Hemic and Lymphatic : anemia, ecchymoses, leukopenia, petechiae
Metabolic and Nutritional : gout, hypokalemia, increased serum creatine kinase, increased nonprotein nitrogen, weight gain, weight loss
Musculoskeletal : arthralgia, arthritis, leg cramps, myalgia, myasthenia, myositis, tenosynovitis
Nervous : abnormal dreams, abnormal thinking and confusion, amnesia, anxiety, ataxia, cerebral ischemia, decreased libido, depression, hypesthesia, hypertonia, insomnia, nervousness, paresthesia, somnolence, tremor, vertigo
Respiratory : asthma, dyspnea, end inspiratory wheeze and fine rales, epistaxis, increased cough, laryngitis, pharyngitis, pleural effusion, rhinitis, sinusitis
Skin and Appendages : acne, alopecia, dry skin, exfoliative dermatitis, fungal dermatitis, herpes simplex, herpes zoster, maculopapular rash, pruritus, pustular rash, skin discoloration, skin ulcer, sweating, urticaria
Special Senses: abnormal vision, amblyopia, blepharitis, conjunctivitis, ear pain, glaucoma, itchy eyes, keratoconjunctivitis, otitis media, retinal detachment, tinnitus, watery eyes, taste disturbance, temporary unilateral loss of vision, vitreous floater
Urogenital : dysuria, hematuria, impotence, nocturia, urinary frequency, increased BUN and serum creatinine, vaginal hemorrhage, vaginitis
The following postmarketing event has been reported very rarely in patients receiving Nisoldipine: systemic hypersensitivity reaction which may include one or more of the following; angioedema, shortness of breath, tachycardia, chest tightness, hypotension, and rash. A definite causal relationship with Nisoldipine has not been established. An unusual event observed with immediate release nisoldipine but not observed with Nisoldipine was one case of photosensitivity. Gynecomastia has been associated with the use of calcium channel blockers.
Overdosage
There is no experience with nisoldipine overdosage. Generally, overdosage with other dihydropyridines leading to pronounced hypotension calls for active cardiovascular support including monitoring of cardiovascular and respiratory function, elevation of extremities, judicious use of calcium infusion, pressor agents and fluids. Clearance of nisoldipine would be expected to be slowed in patients with impaired liver function. Since nisoldipine is highly protein bound, dialysis is not likely to be of any benefit; however, plasmapheresis may be beneficial.
Dosage And Administration
The dosage of Nisoldipine must be adjusted to each patient’s needs. Therapy usually should be initiated with 17 mg orally once daily, then increased by 8.5 mg per week or longer intervals, to attain adequate control of blood pressure. Usual maintenance dosage is 17 to 34 mg once daily. Blood pressure response increases over the 8.5 - 34 mg daily dose range but adverse event rates also increase. Doses beyond 34 mg once daily are not recommended. Nisoldipine has been used safely with diuretics, ACE inhibitors, and beta-blocking agents. Patients over age 65, or patients with impaired liver function, are expected to develop higher plasma concentrations of nisoldipine. Their blood pressure should be monitored closely during any dosage adjustment. A starting dose not exceeding 8.5 mg daily is recommended in these patient groups. Nisoldipine tablets should be administered orally once daily. Nisoldipine should be taken on an empty stomach (1 hour before or 2 hours after a meal). Grapefruit products should be avoided before and after dosing. Nisoldipine is an extended release dosage form and tablets should be swallowed whole, not bitten, divided or crushed. 
How Supplied
Nisoldipine extended release tablets are supplied as 8.5 mg and 17 mg round film coated tablets and 34 mg elliptic film coated tablets. The different strengths can be identified as follows:
Strength Color Markings 8.5 mg Oyster SCI 500 17 mg Yellow Cream SCI 501 34 mg Burnt Orange SCI 503
Nisoldipine Tablets are supplied in bottles of 100:
NDC Code Strength  66993-472-02  8.5 mg 66993-473-02  17 mg 66993-475-02  34 mg
Protect from light and moisture. Store at 20¬į-25¬įC (68¬į-77¬įF); excursions permitted to 15¬į-30¬įC (59¬į-86¬įF) [see USP Controlled Room Temperature]. Dispense in tight, light-resistant containers.
Rx only
Manufactured for:  Prasco Laboratories Mason, OH 45040 USA
Rev 10/2021 362168
Principal Display Panel - 8.5 Mg Tablet Bottle Carton
NDC 66993-472-02
Nisoldipine Extended Release Tablets 8.5 mg
100 Tablets
Rx only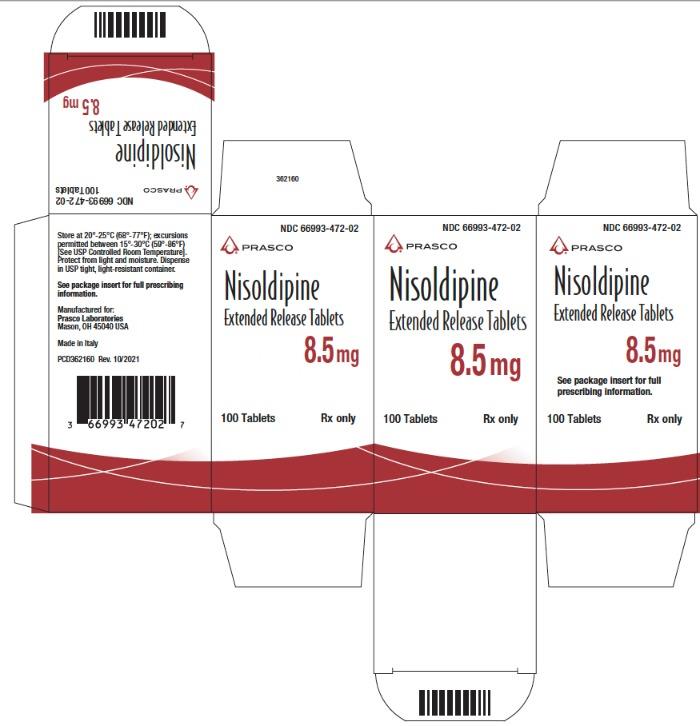
Principal Display Panel - 17 Mg Tablet Bottle Carton
NDC 66993-473-02
Nisoldipine Extended Release Tablets 17 mg
100 Tablets
Rx Only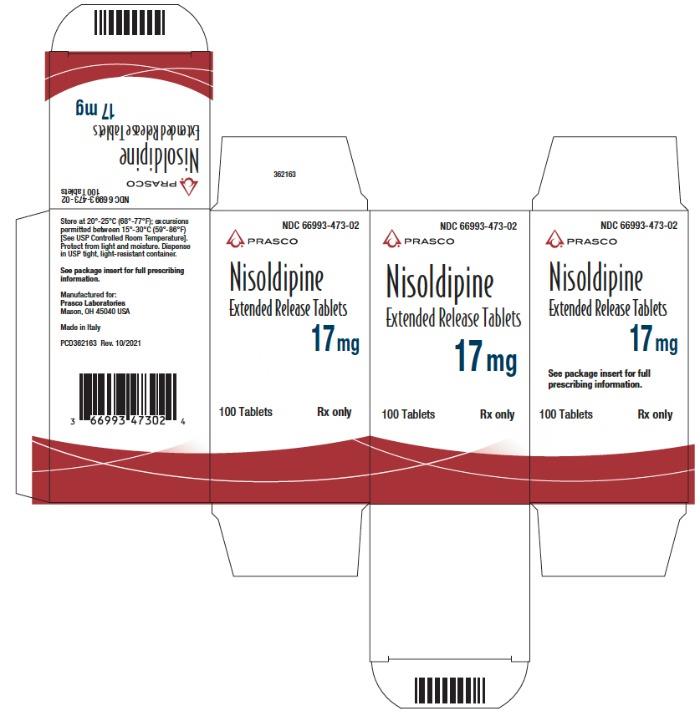
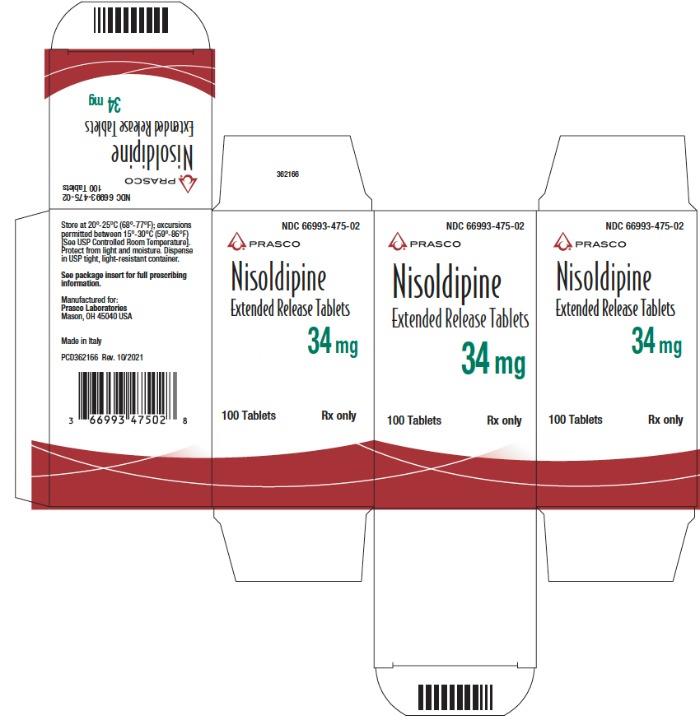
Principal Display Panel - 34 Mg Tablet Bottle Carton
NDC 66993-475-02
Nisoldipine Extended Release Tablets 34 mg
100 Tablets
Rx Only
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


