Butalbital, Acetaminophen and Caffeine (butalbital 50 mg acetaminophen 325 mg caffeine 40 mg) Dailymed
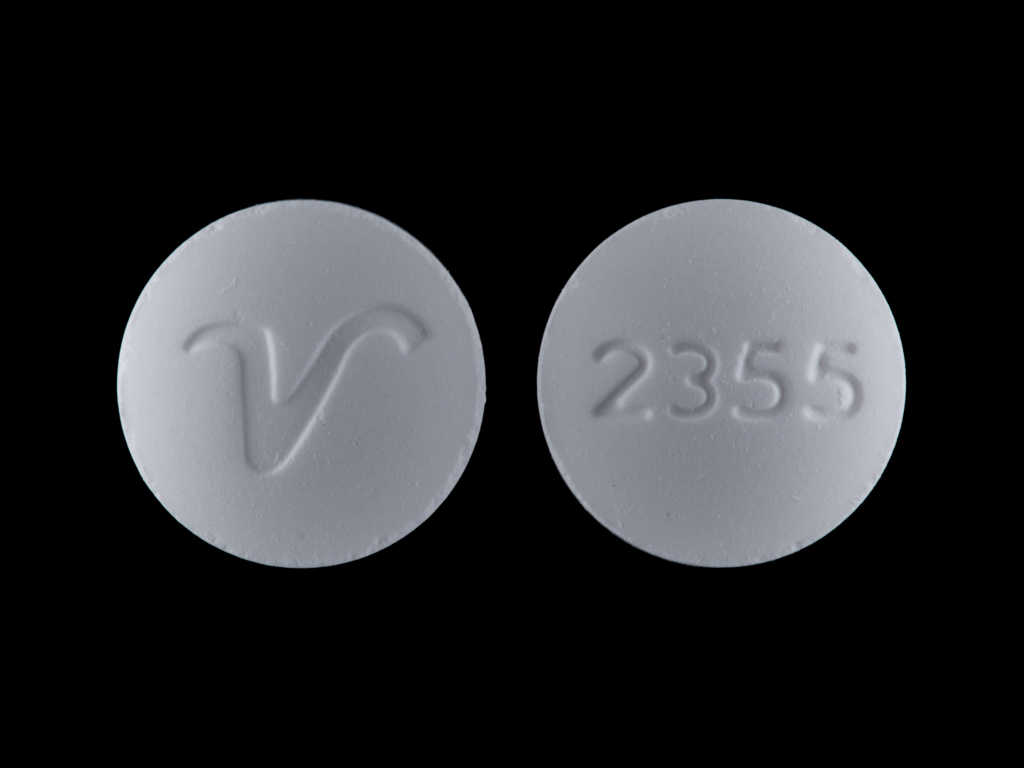
IMPRINT: 2355 V
SHAPE: round
COLOR: white
All Imprints
butalbital, acetaminophen and caffeine - acetaminophen 325 mg / butalbital 50 mg / caffeine 40 mg oral tablet - 2355 v round white
acetaminophen 325 mg / butalbital 50 mg / caffeine 40 mg oral tablet - 2355 v round white
apap 325 mg / butalbital 50 mg / caffeine 40 mg oral tablet - 2355 v round white
butalbital 50 mgacetaminophen 325 mgcaffeine 40 mg - 2355 v round white
Go PRO for all pill images
Rx only
Description
Butalbital, Acetaminophen and Caffeine Tablets, USP are supplied in tablet form for oral administration.
Each tablet contains the following active ingredients:
 butalbital, USP ...................................................................................... 50 mg acetaminophen, USP ........................................................................... 325 mg caffeine, USP ........................................................................................ 40 mg
Inactive Ingredients:
colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and stearic acid.
Butalbital (5-allyl-5-isobutylbarbituric acid), is a short to intermediate-acting barbiturate. It has the following structural formula:
![]()
Acetaminophen (4'-hydroxyacetanilide), is a non-opiate, non-salicylate analgesic and antipyretic. It has the following structural formula.
![]()
Caffeine (1,3,7-trimethylxanthine), is a central nervous system stimulant. It has the following structural formula:
![]()
Clinical Pharmacology
This combination drug product is intended as a treatment for tension headache.
It consists of a fixed combination of butalbital, acetaminophen and caffeine. The role each component plays in the relief of the complex of symptoms known as tension headache is incompletely understood.
Pharmacokinetics
The behavior of the individual components is described below.
Butalbital is well absorbed from the gastrointestinal tract and is expected to distribute to most tissues in the body. Barbiturates in general may appear in breast milk and readily cross the placental barrier. They are bound to plasma and tissue proteins to a varying degree and binding increases directly as a function of lipid solubility.
Elimination of butalbital is primarily via the kidney (59% to 88% of the dose) as unchanged drug or metabolites. The plasma half-life is about 35 hours. Urinary excretion products include parent drug (about 3.6% of the dose), 5-isobutyl-5-(2, 3-dihydroxypropyl) barbituric acid (about 24% of the dose), 5-allyl-5(3-hydroxy-2-methyl-1-propyl) barbituric acid (about 4.8% of the dose), products with the barbituric acid ring hydrolyzed with excretion of urea (about 14% of the dose), as well as unidentified materials. Of the material excreted in the urine, 32% is conjugated.
The in vitro plasma protein binding of butalbital is 45% over the concentration range of 0.5–20 mcg/mL. This falls within the range of plasma protein binding (20%–45%) reported with other barbiturates such as phenobarbital, pentobarbital, and secobarbital sodium. The plasma-to-blood concentration ratio was almost unity, indicating that there is no preferential distribution of butalbital into either plasma or blood cells.
See OVERDOSAGEÂ for toxicity information.
Acetaminophen is rapidly absorbed from the gastrointestinal tract and is distributed throughout most body tissues. The plasma half-life is 1.25 to 3 hours, but may be increased by liver damage and following overdosage. Elimination of acetaminophen is principally by liver metabolism (conjugation) and subsequent renal excretion of metabolites. Approximately 85% of an oral dose appears in the urine within 24 hours of administration, most as the glucuronide conjugate, with small amounts of other conjugates and unchanged drug.
See OVERDOSAGE Â for toxicity information.
Like most xanthines, caffeine is rapidly absorbed and distributed in all body tissues and fluids, including the CNS, fetal tissues, and breast milk.
Caffeine is cleared through metabolism and excretion in the urine. The plasma half-life is about 3 hours. Hepatic biotransformation prior to excretion results in about equal amounts of 1-methylxanthine and 1-methyluric acid. Of the 70% of the dose that is recovered in the urine, only 3% is unchanged drug.
See OVERDOSAGE Â for toxicity information.
Indications And Usage
Butalbital, acetaminophen and caffeine tablets are indicated for the relief of the symptom complex of tension (or muscle contraction) headache.
Evidence supporting the efficacy and safety of this combination product in the treatment of multiple recurrent headaches is unavailable. Caution in this regard is required because butalbital is habit-forming and potentially abusable.
Contraindications
This product is contraindicated under the following conditions:
• –Hypersensitivity or intolerance to any component of this product• –Patients with porphyria.
Warnings
Butalbital is habit-forming and potentially abusable. Consequently, the extended use of this product is not recommended.
Precautions
General
Butalbital, acetaminophen and caffeine tablets should be prescribed with caution in certain special-risk patients, such as the elderly or debilitated, and those with severe impairment of renal or hepatic function, or acute abdominal conditions.
Information for Patients
This product may impair mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. Such tasks should be avoided while taking this product.
Alcohol and other CNS depressants may produce an additive CNS depression, when taken with this combination product, and should be avoided.
Butalbital may be habit-forming. Patients should take the drug only for as long as it is prescribed, in the amounts prescribed, and no more frequently than prescribed.
For information on use in geriatric patients, see PRECAUTIONS/Geriatric Use .
Laboratory Tests
In patients with severe hepatic or renal disease, effects of therapy should be monitored with serial liver and/or renal function tests.
Drug Interactions
The CNS effects of butalbital may be enhanced by monoamine oxidase (MAO) inhibitors.
Butalbital, acetaminophen and caffeine may enhance the effects of: other narcotic analgesics, alcohol, general anesthetics, tranquilizers such as chlordiazepoxide, sedative-hypnotics, or other CNS depressants, causing increased CNS depression.
Drug/Laboratory Test Interactions
Acetaminophen may produce false-positive test results for urinary 5-hydroxyindoleacetic acid.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate studies have been conducted in animals to determine whether acetaminophen or butalbital have a potential for carcinogenesis, mutagenesis or impairment of fertility.
Pregnancy
Animal reproduction studies have not been conducted with this combination product. It is also not known whether butalbital, acetaminophen and caffeine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. This product should be given to a pregnant woman only when clearly needed.
Withdrawal seizures were reported in a two-day-old male infant whose mother had taken a butalbital-containing drug during the last two months of pregnancy. Butalbital was found in the infant's serum. The infant was given phenobarbital 5 mg/kg, which was tapered without further seizure or other withdrawal symptoms.
Nursing Mothers
Caffeine, barbiturates and acetaminophen are excreted in breast milk in small amounts, but the significance of their effects on nursing infants is not known. Because of potential for serious adverse reactions in nursing infants from butalbital, acetaminophen and caffeine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 12 have not been established.
Geriatric Use
Clinical studies of butalbital, acetaminophen and caffeine tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Butalbital is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Adverse Reactions
Frequently Observed
The most frequently reported adverse reactions are drowsiness, lightheadedness, dizziness, sedation, shortness of breath, nausea, vomiting, abdominal pain, and intoxicated feeling.
Infrequently Observed
All adverse events tabulated below are classified as infrequent.
Central Nervous System: Â headache, shaky feeling, tingling, agitation, fainting, fatigue, heavy eyelids, high energy, hot spells, numbness, sluggishness, seizure. Mental confusion, exclient, or depression can also occur due to intolerance, particularly in elderly or debilitated patients, or due to overdosage of butalbital.
Autonomic Nervous System: Â dry mouth, hyperhidrosis.
Gastrointestinal: Â difficulty swallowing, heartburn, flatulence, constipation.
Cardiovascular: Â tachycardia.
Musculoskeletal: Â leg pain, muscle fatigue.
Genitourinary: Â diuresis.
Miscellaneous: Â pruritus, fever, earache, nasal congestion, tinnitus, euphoria, allergic reactions.
Several cases of dermatological reactions, including toxic epidermal necrolysis and erythema multiforme, have been reported.
The following adverse drug events may be borne in mind as potential effects of the components of this product. Potential effects of high dosage are uled in the OVERDOSAGEÂ section.
Acetaminophen:Â allergic reactions, rash, thrombocytopenia, agranulocytosis.
Caffeine: Â cardiac stimulation, irritability, tremor, dependence, nephrotoxicity, hyperglycemia.
Drug Abuse And Dependence
Abuse and Dependence
Barbiturates may be habit-forming:
Tolerance, psychological dependence, and physical dependence may occur especially following prolonged use of high doses of barbiturates. The average daily dose for the barbiturate addict is usually about 1500 mg. As tolerance to barbiturates develops, the amount needed to maintain the same level of intoxication increases; tolerance to a fatal dosage, however, does not increase more than two-fold. As this occurs, the margin between an intoxication dosage and fatal dosage becomes smaller. The lethal dose of a barbiturate is far less if alcohol is also ingested. Major withdrawal symptoms (convulsions and delirium) may occur within 16 hours and last up to 5 days after abrupt cessation of these drugs. Intensity of withdrawal symptoms gradually declines over a period of approximately 15 days. Treatment of barbiturate dependence consists of cautious and gradual withdrawal of the drug. Barbiturate-dependent patients can be withdrawn by using a number of different withdrawal regimens. One method involves initiating treatment at the patient's regular dosage level and gradually decreasing the daily dosage as tolerated by the patient.
Overdosage
Following an acute overdosage of butalbital, acetaminophen and caffeine, toxicity may result from the barbiturate or the acetaminophen. Toxicity due to caffeine is less likely, due to the relatively small amounts in this formulation.
Signs and Symptoms
Toxicity frombarbiturate  poisoning includes drowsiness, confusion, and coma; respiratory depression; hypotension; and hypovolemic shock.
Inacetaminophen  overdosage: dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necroses, hypoglycemic coma and thrombocytopenia may also occur. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion. In adults hepatic toxicity has rarely been reported with acute overdoses of less than 10 grams, or fatalities with less than 15 grams.
Acutecaffeine  poisoning may cause insomnia, restlessness, tremor, and delirium, tachycardia and extrasystoles.
Treatment
A single or multiple overdose with this combination product is a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended.
Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption. Vomiting should be induced mechanically, or with syrup of ipecac, if the patient is alert (adequate pharyngeal and laryngeal reflexes). Oral activated charcoal (1 g/kg) should follow gastric emptying. The first dose should be accompanied by an appropriate cathartic. If repeated doses are used, the cathartic might be included with alternate doses as required. Hypotension is usually hypovolemic and should respond to fluids. Pressors should be avoided. A cuffed endotracheal tube should be inserted before gastric lavage of the unconscious patient and, when necessary, to provide assisted respiration. If renal function is normal, forced diuresis may aid in the elimination of the barbiturate. Alkalinization of the urine increases renal excretion of some barbiturates, especially phenobarbital.
Meticulous attention should be given to maintaining adequate pulmonary ventilation. In severe cases of intoxication, peritoneal dialysis, or preferably hemodialysis may be considered. If hypoprothrombinemia occurs due to acetaminophen overdose, vitamin K should be administered intravenously.
If the dose of acetaminophen may have exceeded 140 mg/kg, acetylcysteine should be administered as early as possible. Serum acetaminophen levels should be obtained, since levels four or more hours following ingestion help predict acetaminophen toxicity. Do not await acetaminophen assay results before initiating treatment. Hepatic enzymes should be obtained initially, and repeated at 24-hour intervals.
Methemoglobinemia over 30% should be treated with methylene blue by slow intravenous administration.
Toxic Doses (for adults)
Butalbital:
toxic dose
1 g
(20 tablets)
Acetaminophen:
toxic dose
10 g
(30 tablets)
Caffeine:
toxic dose
1 g
(25 tablets)
In all cases of suspected overdosage, call your Regional Poison Control Center to obtain the most up-to-date information about the treatment of overdosage. Telephone numbers of certified Regional Poison Control Centers are uled in the Physicians' Desk Reference®*.
Dosage And Administration
One or 2 tablets every 4 hours as needed. Total daily dosage should not exceed 6 tablets.
Extended and repeated use of this product is not recommended because of the potential for physical dependence.
How Supplied
Butalbital, Acetaminophen and Caffeine Tablets, USP
Containing 50 mg butalbital, 325 mg acetaminophen and 40 mg caffeine. Available as white, round shaped tablets, debossed "2355" on one side, and debossed "V" on the reverse side. Bottles of 100, 500 and 1000.
Store and Dispense
Store at 20°-25°C (68°-77°F) with excursions permitted between 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]; dispense in a tight, light-resistant container as defined in the USP.
*Trademark of Medical Economics Company, Inc.
Manufactured for: QUALITEST PHARMACEUTICALS Huntsville, AL 35811
8181466R3/08-R2
Butalbital, Acetaminophen and Caffeine Tablets, USP, containing 50 mg of butalbital,
325 mg acetaminophen and 40 mg caffeine are available from Cardinal Health in unit
dose packages of 100.
Unit dose package of 100 tablets, NDC 55154-0832-4
Cardinal Health
Zanesville OH 43701
IU42964890410
Principal Display Panel - Carton
NDC: 55154-0832-4
Butalbital, Acetaminophen and Caffeine Tablets, USP
50 mg/ 325 mg/ 40 mg
100 Tablets
EACH TABLET CONTAINS:
Butalbital, USP 50 mg
Acetaminophen, USP 325 mg
Caffeine, USP 40 mg
See product insert for prescribing information, precautions and warnings.
USUAL ADULT DOSAGE: 1 or 2 tablets every four hours as needed. Total daily dose should not exceed 6 tablets.
STORAGE: Store at 20° - 25°C (68° - 77°F) with excursions permitted between 15° - 30°C (59° - 86°F) [see USP Controlled Room Temperature].
DISPENSE in a tight, light-resistant container as defined in the USP.
RX ONLY
WARNING: This package is intended for institutional use only. This package is not child resistant. Keep this and all drugs out of the reach of children.
See window for lot number and expiration date.
Manufactured for: QUALITEST PHARMACEUTICALS
HUNTERSVILLE, AL 35811
Repackaged by: Cardinal Health
Zanesville, OH 43701
LUC42964890610
![]()
Principal Display Panel - Pouch
Butalbital, Acetaminophen and Caffeine Tablet, USP
50 mg/ 325 mg/ 40 mg
![]()
Principal Display Panel
Butalbital, Acetaminophen and Caffeine Tablets, USP
50 mg/ 325 mg/ 40 mg
10 Tablets
![]()
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site