Cold and Flu (acetaminophen 325 mg dextromethorphan hydrobromide 10 mg doxylamine succinate 6.25 mg phenylephrine hydrochloride 5 mg) Dailymed
Generic: acetaminophen, dextromethorphan hbr, doxylamine succinate, guaifenesin, phenylephrine hcl is used for the treatment of Cough Fever Glucosephosphate Dehydrogenase Deficiency Liver Diseases Pain Headache Sleep Initiation and Maintenance Disorders Rhinitis, Allergic, Perennial Bronchitis Common Cold Laryngitis Pharyngitis Sinusitis Whooping Cough Fissure in Ano Glaucoma, Open-Angle Hemorrhoids Hypertension Hypotension Pruritus Ani Rhinitis, Vasomotor Shock, Septic Tachycardia, Supraventricular Mydriasis Tachycardia, Ventricular
IMPRINT: 44 677
SHAPE: oval
COLOR: green
All Imprints
acetaminophen 325 mg dextromethorphan hydrobromide 10 mg doxylamine succinate 6.25 mg phenylephrine hydrochloride 5 mg - 44 677 oval green
{16 (acetaminophen 325 mg / dextromethorphan hydrobromide 10 mg / doxylamine succinate 6.25 mg / phenylephrine hydrochloride 5 mg oral tablet) / 32 (acetaminophen 325 mg / dextromethorphan hydrobromide 10 mg / guaifenesin 200 mg / phenylephrine hydrochlor - 44 677 oval green
Go PRO for all pill images
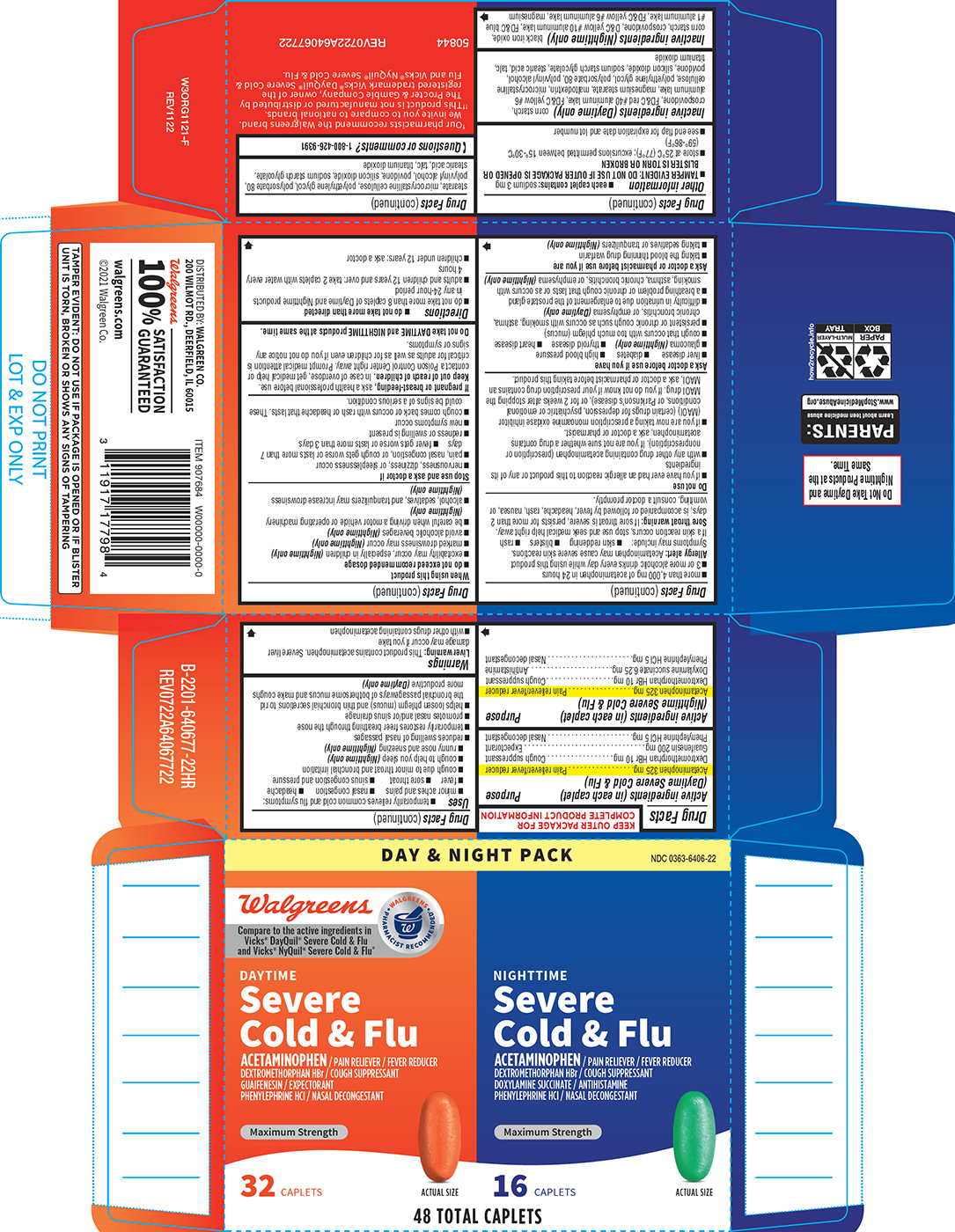
Active Ingredients (in Each Caplet) (daytime Severe Cold & Flu)
Acetaminophen 325 mg Dextromethorphan HBr 10 mg Guaifenesin 200 mg Phenylephrine HCl 5 mg
Purpose
Pain reliever/fever reducerCough suppressantExpectorantNasal decongestant
Active Ingredients (in Each Caplet) (nighttime Severe Cold & Flu)
Acetaminophen 325 mg Dextromethorphan HBr 10 mg Doxylamine succinate 6.25 mg Phenylephrine HCl 5 mg
Purpose
Pain reliever/fever reducerCough suppressantAntihistamineNasal decongestant
Uses
- temporarily relieves common cold and flu symptoms:
- minor aches and pains
- nasal congestion
- headache
- fever
- sore throat
- sinus congestion and pressure
- cough due to minor throat and bronchial irritation
- cough to help you sleep (Nighttime only)
- runny nose and sneezing (Nighttime only)
- reduces swelling of nasal passages
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive (Daytime only)
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- bulers
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- diabetes
- high blood pressure
- glaucoma (Nighttime only)
- thyroid disease
- heart disease
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema (Daytime only)
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis, or emphysema (Nighttime only)
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers (Nighttime only)
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children (Nighttime only)
- marked drowsiness may occur (Nighttime only)
- avoid alcoholic beverages (Nighttime only)
- be careful when driving a motor vehicle or operating machinery (Nighttime only)
- alcohol, sedatives, and tranquilizers may increase drowsiness (Nighttime only)
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Do not take DAYTIME and NIGHTTIME products at the same time.
Directions
- do not take more than directed
- do not take more than 8 caplets of Daytime and Nighttime products in any 24-hour period
- adults and children 12 years and over: take 2 caplets with water every 4 hours
- children under 12 years: ask a doctor
Other Information
- each caplet contains: sodium 3 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive Ingredients (daytime Only)
corn starch, crospovidone, FD&C red #40 aluminum lake, FD&C yellow #6 aluminum lake, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polysorbate 80, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, talc, titanium dioxide
Inactive Ingredients (nighttime Only)
black iron oxide, corn starch, crospovidone, D&C yellow #10 aluminum lake, FD&C blue #1 aluminum lake, FD&C yellow #6 aluminum lake, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, talc, titanium dioxide
Questions Or Comments?
1-800-426-9391
Principal Display Panel
DAY & NIGHT PACK
Walgreens
WALGREENS PHARMACIST RECOMMENDED â€
Compare to the active ingredients in Vicks® DayQuil® VapoCOOL® SEVERE COLD & FLU + CONGESTION and Vicks® NyQuil® VapoCOOL® SEVERE COLD & FLU + CONGESTION†â€
DAYTIME
Severe Cold & Flu
ACETAMINOPHENÂ / PAIN RELIEVER / FEVER REDUCER DEXTROMETHORPHAN HBr / COUGH SUPPRESSANT GUAIFENESIN / EXPECTORANT PHENYLEPHRINE HCl / NASAL DECONGESTANT
Maximum Strength
32Â CAPLETS
ACTUAL SIZE
NIGHTTIME
Severe Cold & Flu
ACETAMINOPHENÂ / PAIN RELIEVER / FEVER REDUCER DEXTROMETHORPHAN HBr / COUGH SUPPRESSANT DOXYLAMINE SUCCINATE / ANTIHISTAMINE PHENYLEPHRINE HCl / NASAL DECONGESTANT
Maximum Strength
16Â CAPLETS
ACTUAL SIZE
48 TOTAL CAPLETS
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Do Not Take Daytime and Nighttime Products at the Same Time.
PARENTS: Learn about teen medicine abuse www.StopMedicineAbuse.org
†Our pharmacists recommend the Walgreens brand. We invite you to compare to national brands. ††This product is not manufactured or distributed by The Procter & Gamble Company, owner of the registered trademark Vicks® DayQuil® VapoCOOL® SEVERE COLD & FLU + CONGESTION and Vicks® NyQuil® VapoCOOL® SEVERE COLD & FLU + CONGESTION.
50844Â Â Â Â Â Â Â Â Â Â Â Â REV0722B64067722
NDC 0363-6406-22
DISTRIBUTED BY: WALGREEN CO. DEERFIELD, IL 60015 Walgreens 100 % SATISFACTION GUARANTEED walgreens.com ©2024 Walgreen Co.
Walgreens 44-640677
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


