Albendazole (albendazole 200 mg) Dailymed
Generic: albendazole is used for the treatment of Ancylostomiasis Clonorchiasis Echinococcosis Giardiasis Hymenolepiasis Necatoriasis Opisthorchiasis Toxocariasis Trichuriasis Enterobiasis Neurocysticercosis
Go PRO for all pill images
1 Indications And Usage
Albendazole tablets are an anthelmintic drug indicated for:
- Treatment of parenchymal neurocysticercosis due to active lesions caused by larval forms of the pork tapeworm, Taenia solium. (
1.1 )- Treatment of cystic hydatid disease of the liver, lung, and peritoneum, caused by the larval form of the dog tapeworm, Echinococcus granulosus. (
1.2 )1.1 Neurocysticercosis
Albendazole tablets are indicated for the treatment of parenchymal neurocysticercosis due to active lesions caused by larval forms of the pork tapeworm, Taenia solium.
1.2 Hydatid Disease
Albendazole tablets are indicated for the treatment of cystic hydatid disease of the liver, lung, and peritoneum, caused by the larval form of the dog tapeworm, Echinococcus granulosus.
2 Dosage And Administration
Patients weighing 60 kg or greater, 400 mg twice daily; less than 60 kg, 15 mg/kg/day in divided doses twice daily (maximum total daily dose 800 mg). Albendazole tablets should be taken with food. (2 )
- Hydatid disease: 28-day cycle followed by 14-day albendazole-free interval for a total of 3 cycles. (
2 )- Neurocysticercosis: 8 to 30 days. (
2 )
See additional important information in the Full Prescribing Information. (2 )
2.1 Dosage
Dosing of albendazole will vary depending upon the indication. Albendazole tablets may be crushed or chewed and swallowed with a drink of water. Albendazole tablets should be taken with food [see Clinical Pharmacology (12.3)].
Table 1: Albendazole Dosage
Indication
Patient Weight
Dose
Duration
Hydatid Disease
60 kg or greater
400 mg twice daily, with meals
28-day cycle followed by a 14-day albendazole-free interval, for a total of 3 cycles
Less than 60 kg
15 mg/kg/day given in divided doses twice daily with meals (maximum total daily dose 800 mg)
Neurocysticercosis
60 kg or greater
400 mg twice daily, with meals
8 to 30 days
Less than 60 kg
15 mg/kg/day given in divided doses twice daily with meals (maximum total daily dose 800 mg)
2.2 Concomitant Medication to Avoid Adverse Reactions
Patients being treated for neurocysticercosis should receive appropriate steroid and anticonvulsant therapy as required. Oral or intravenous corticosteroids should be considered to prevent cerebral hypertensive episodes during the first week of treatment [see Warnings and Precautions (5.3)].
2.3 Monitoring for Safety Before and During Treatment
- Monitor blood counts at the beginning of each 28-day cycle of therapy, and every 2 weeks while on therapy with albendazole in all patients [see Warnings and Precautions (5.1)].
- Monitor liver enzymes (transaminases) at the beginning of each 28-day cycle of therapy, and at least every 2 weeks during treatment with albendazole in all patients [see Warnings and Precautions (5.5)].
- Obtain a pregnancy test in females of reproductive potential prior to therapy [see Warnings and Precautions (5.2)].
3 Dosage Forms And Strengths
- Tablet: 200 mg
- Tablet: 200 mg (
3 )
4 Contraindications
Albendazole is contraindicated in patients with known hypersensitivity to the benzimidazole class of compounds or any components of albendazole.
Patients with known hypersensitivity to the benzimidazole class of compounds or any components of albendazole. (4 )
5 Warnings And Precautions
- Bone Marrow Suppression: Fatalities have been reported due to bone marrow suppression; monitor blood counts in all patients at the beginning of each 28-day cycle of therapy, and every 2 weeks while on therapy. Discontinue albendazole if clinically significant changes in blood counts occur. (
5.1 ,5.4 )- Embryo-Fetal Toxicity: May cause fetal harm. Pregnancy testing is recommended for females of reproductive potential prior to therapy. Advise females of reproductive potential of the potential risk to a fetus and to use an effective method of contraception. (
2.3 ,5.2 ,8.1 ,8.3 )- Risk of Neurologic Symptoms: Neurocysticercosis patients may experience cerebral hypertensive episodes, seizures or focal neurologic deficits after initiation of therapy; begin appropriate steroid and anticonvulsant therapy. (
5.3 )- Risk of Retinal Damage in Retinal Cysticercosis: Cases of retinal involvement have been reported; examine the patient for the presence of retinal lesions before initiating therapy for neurocysticercosis. (
5.4 )- Hepatic Effects. Elevations of liver enzymes may occur. Monitor liver enzymes before the start of each treatment cycle and at least every 2 weeks while on albendazole therapy and discontinue if clinically significant elevations occur. (
5.5 )5.1 Bone Marrow Suppression
Fatalities associated with the use of albendazole have been reported due to granulocytopenia or pancytopenia. Albendazole may cause bone marrow suppression, aplastic anemia, and agranulocytosis. Monitor blood counts at the beginning of each 28-day cycle of therapy, and every 2 weeks while on therapy with albendazole in all patients. Patients with liver disease and patients with hepatic echinococcosis are at increased risk for bone marrow suppression and warrant more frequent monitoring of blood counts. Discontinue albendazole if clinically significant decreases in blood cell counts occur.
5.2 Embryo-Fetal Toxicity
Based on findings from animal reproduction studies, albendazole may cause fetal harm when administered to a pregnant woman. Embryotoxicity and skeletal malformations were reported in rats and rabbits when treated during the period of organogenesis (at oral doses approximately 0.1 to 0.6 times the recommended human dose normalized for total body surface area). Advise pregnant women of the potential risk to a fetus. Pregnancy testing is recommended for females of reproductive potential prior to initiating albendazole [see Dosage and Administration (2.3)]. Advise females of reproductive potential to use an effective method of contraception during treatment with albendazole and for 3 days after the final dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.3)].
5.3 Risk of Neurologic Symptoms in Neurocysticercosis
Patients being treated for neurocysticercosis should receive steroid and anticonvulsant therapy to prevent neurological symptoms (e.g. seizures, increased intracranial pressure and focal signs) as a result of an inflammatory reaction caused by death of the parasite within the brain.
5.4 Risk of Retinal Damage in Patients with Retinal Neurocysticercosis
Cysticercosis may involve the retina. Before initiating therapy for neurocysticercosis, examine the patient for the presence of retinal lesions. If such lesions are visualized, weigh the need for anticysticeral therapy against the possibility of retinal damage resulting from inflammatory damage caused by albendazole-induced death of the parasite.
5.5 Hepatic Effects
In clinical trials, treatment with albendazole has been associated with mild to moderate elevations of hepatic enzymes in approximately 16% of patients. These elevations have generally returned to normal upon discontinuation of therapy. There have also been case reports of acute liver failure of uncertain causality and hepatitis [see Adverse Reactions (6)].
Monitor liver enzymes (transaminases) before the start of each treatment cycle and at least every 2 weeks during treatment. If hepatic enzymes exceed twice the upper limit of normal, consideration should be given to discontinuing albendazole therapy based on individual patient circumstances. Restarting albendazole treatment in patients whose hepatic enzymes have normalized off treatment is an individual decision that should take into account the risk/benefit of further albendazole usage. Perform laboratory tests frequently if albendazole treatment is restarted.
Patients with elevated liver enzyme test results are at increased risk for hepatotoxicity and bone marrow suppression [see Warnings and Precautions (5.1)]. Discontinue therapy if liver enzymes are significantly increased or if clinically significant decreases in blood cell counts occur.
5.6 Unmasking of Neurocysticercosis in Hydatid Patients
Undiagnosed neurocysticercosis may be uncovered in patients treated with albendazole for other conditions. Patients with epidemiologic factors who are at risk for neurocysticercosis should be evaluated prior to initiation of therapy.
6 Adverse Reactions
- Adverse reactions 1% or greater in hydatid disease: abnormal liver function tests, abdominal pain, nausea/vomiting, reversible alopecia, headache, dizziness/vertigo, fever. (
6.1 )- Adverse reactions 1% or greater in neurocysticercosis: headache, nausea/vomiting, raised intracranial pressure, meningeal signs. (
6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872Â or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse reaction profile of albendazole differs between hydatid disease and neurocysticercosis. Adverse reactions occurring with a frequency of 1% or greater in either disease are described in Table 2 below.
These symptoms were usually mild and resolved without treatment. Treatment discontinuations were predominantly due to leukopenia (0.7%) or hepatic abnormalities (3.8% in hydatid disease). The following incidence reflects adverse reactions that were reported to be at least possibly or probably related to albendazole.
Table 2: Adverse Reaction Incidence 1% or Greater in Hydatid Disease and Neurocysticercosis
Adverse Reaction
Hydatid Disease
Neurocysticercosis
Gastrointestinal
Abdominal Pain
6
0
Nausea
4
6
Vomiting
4
6
General disorders and administration site conditions
Fever
1
0
Investigations
Elevated Hepatic Enzymes
16
less than 1
Nervous system disorders
Dizziness
1
less than 1
Headache
1
11
Meningeal Signs
0
1
Raised Intracranial Pressure
0
2
Vertigo
1
less than 1
Skin and subcutaneous tissue disorders
Reversible Alopecia
2
less than 1
The following adverse events were observed at an incidence of less than 1%:
Blood and Lymphatic System Disorders: There have been reports of leukopenia, granulocytopenia, pancytopenia, agranulocytosis, or thrombocytopenia [see Warnings and Precautions (5.1)].
Immune System Disorders: Hypersensitivity reactions, including rash and urticaria.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of albendazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Aplastic anemia, bone marrow suppression, neutropenia.
Eye Disorders: Vision blurred.
Gastrointestinal Disorders: Diarrhea.
General System Disorders: Asthenia.
Hepatobiliary Disorders: Elevations of hepatic enzymes, hepatitis, acute liver failure.
Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis.
Nervous System Disorders: Somnolence, convulsion.
Renal and Urinary Disorders: Acute renal failure.
Skin and Subcutaneous Tissue Disorders: Erythema multiforme, Stevens-Johnson syndrome.
7 Drug Interactions
- Dexamethasone: Steady-state trough concentrations of albendazole sulfoxide were about 56% higher when dexamethasone was coadministered with each dose of albendazole. (
7.1 )- Praziquantel: In the fed state increased mean maximum plasma concentration and area under the curve of albendazole sulfoxide by about 50% in healthy subjects. (
7.2 )- Cimetidine: Increased albendazole sulfoxide concentrations in bile and cystic fluid by about 2-fold in hydatid cyst patients. (
7.3 )- Theophylline: Albendazole induces cytochrome P450 1A in human hepatoma cells; therefore, it is recommended that plasma concentrations of theophylline be monitored during and after treatment. (
5.5 ,7.4 )7.1 Dexamethasone
Steady-state trough concentrations of albendazole sulfoxide were about 56% higher when 8 mg dexamethasone was co-administered with each dose of albendazole (15 mg/kg/day) in 8 neurocysticercosis patients.
7.2 Praziquantel
In the fed state, praziquantel (40 mg/kg) increased mean maximum plasma concentration and area under the curve of albendazole sulfoxide by about 50% in healthy subjects (n = 10) compared with a separate group of subjects (n = 6) given albendazole alone. Mean Tmax and mean plasma elimination half-life of albendazole sulfoxide were unchanged. The pharmacokinetics of praziquantel were unchanged following co-administration with albendazole (400 mg).
7.3 Cimetidine
Albendazole sulfoxide concentrations in bile and cystic fluid were increased (about 2-fold) in hydatid cyst patients treated with cimetidine (10 mg/kg/day) (n = 7) compared with albendazole (20 mg/kg/day) alone (n = 12). Albendazole sulfoxide plasma concentrations were unchanged 4 hours after dosing.
7.4 Theophylline
Following a single dose of albendazole (400 mg), the pharmacokinetics of theophylline (aminophylline 5.8 mg/kg infused over 20 minutes) were unchanged. Albendazole induces cytochrome P450 1A in human hepatoma cells; therefore, it is recommended that plasma concentrations of theophylline be monitored during and after treatment.
8 Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on findings from animal reproduction studies, albendazole may cause fetal harm when administered to a pregnant woman. However, available human data from a small number of published case series and reports on the use of multiple-dose albendazole in the 1st trimester of pregnancy, and several published studies on single-dose albendazole use later in pregnancy, have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproductive studies, oral administration of albendazole during the period of organogenesis caused embryotoxicity and skeletal malformations in pregnant rats (at doses of 0.10 times and 0.32 times the maximum recommended human dose based on body surface area in mg/m2) and pregnant rabbits (at doses of 0.60 times the maximum recommended human dose based on body surface area in mg/m2). Albendazole was also associated with maternal toxicity in rabbits (at doses of 0.60 times the recommended human dose based on body surface area in mg/m2) (see Data). Advise a pregnant woman of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Albendazole has been shown to be teratogenic (to cause embryotoxicity and skeletal malformations) in pregnant rats and rabbits. The teratogenic response in the rat was shown at oral doses of 10 and 30 mg/kg/day (0.10 times and 0.32 times the maximum recommended human dose based on body surface area in mg/m2, respectively) during organogenesis (gestation days 6 to 15) and in pregnant rabbits at oral doses of 30 mg/kg/day (0.60 times the maximum recommended human dose based on body surface area in mg/m2) administered during organogenesis (gestation days 7 to 19). In the rabbit study, maternal toxicity (33% mortality) was noted at 30 mg/kg/day. In mice, no teratogenic effects were observed at oral doses up to 30 mg/kg/day (0.16 times the recommended human dose based on body surface area in mg/m2), administered during gestation days 6 to 15.
8.2 Lactation
Risk Summary
Concentrations of albendazole and the active metabolite, albendazole sulfoxide, have been reported to be low in human breast milk. There are no reports of adverse effects on the breastfed infant and no information on the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for albaendazole and any potential adverse effects on the breastfed infant from albendazole or from the underlying maternal condition.
8.3 Females and Males ofReproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential prior to initiating albendazole.
Contraception
Females
Albendazole may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with albendazole and for 3 days after the final dose.
8.4 Pediatric Use
Hydatid disease is uncommon in infants and young children. In neurocysticercosis, the efficacy of albendazole in children appears to be similar to that in adults.
8.5 Geriatric Use
In patients aged 65 and older with either hydatid disease or neurocysticercosis, there was insufficient data to determine whether the safety and effectiveness of albendazole is different from that of younger patients.
8.6 Patients with Impaired Renal Function
The pharmacokinetics of albendazole in patients with impaired renal function has not been studied.
8.7 Patients with Extra-Hepatic Obstruction
In patients with evidence of extrahepatic obstruction (n = 5), the systemic availability of albendazole sulfoxide was increased, as indicated by a 2-fold increase in maximum serum concentration and a 7-fold increase in area under the curve. The rate of absorption/conversion and elimination of albendazole sulfoxide appeared to be prolonged with mean Tmax and serum elimination half-life values of 10 hours and 31.7 hours, respectively. Plasma concentrations of parent albendazole were measurable in only 1 of 5 patients.
10 Overdosage
In case of overdosage, symptomatic therapy and general supportive measures are recommended.
11 Description
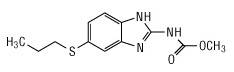
Albendazole, USP is an orally administered anthelmintic drug. Chemically, it is methyl 5-(propylthio)-2-benzimidazolecarbamate. Its molecular formula is C12H15N3O2S. Its molecular weight is 265.34. It has the following chemical structure:
Albendazole, USP is a white to faintly yellowish powder. It is freely soluble in anhydrous formic acid; very slightly soluble in ether and in methylene chloride; practically insoluble in alcohol and in water. Each white to off-white, film-coated, unscored tablet contains 200 mg of albendazole, USP.
Inactive ingredients consist of: corn starch, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400 and 8000, povidone, sodium lauryl sulfate, sodium starch glycolate type A, and sucralose.
12 Clinical Pharmacology
12.1 Mechanism of Action
Albendazole is a synthetic, anthelmintic drug of the class benzimidazole [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Absorption
Albendazole is poorly absorbed from the gastrointestinal tract due to its low aqueous solubility. Albendazole concentrations are negligible or undetectable in plasma as it is rapidly converted to the sulfoxide metabolite prior to reaching the systemic circulation. The systemic anthelmintic activity has been attributed to the primary metabolite, albendazole sulfoxide. Oral bioavailability appears to be enhanced when albendazole is coadministered with a fatty meal (estimated fat content 40 grams) as evidenced by higher (up to 5-fold on average) plasma concentrations of albendazole sulfoxide as compared to the fasted state.
Maximal plasma concentrations of albendazole sulfoxide were achieved 2 hours to 5 hours after dosing and were on average 1310 ng/mL (range 460 ng/mL to 1580 ng/mL) following oral doses of albendazole (400 mg) in 6 hydatid disease patients, when administered with a fatty meal. Plasma concentrations of albendazole sulfoxide increased in a dose-proportional manner over the therapeutic dose range following ingestion of a high-fat meal (fat content 43.1 grams). The mean apparent terminal elimination half-life of albendazole sulfoxide ranged from 8 hours to 12 hours in 25 healthy subjects, as well as in 14 hydatid and 8 neurocysticercosis patients.
Following 4 weeks of treatment with albendazole (200 mg three times daily), 12 patients’ plasma concentrations of albendazole sulfoxide were approximately 20% lower than those observed during the first half of the treatment period, suggesting that albendazole may induce its own metabolism.
Distribution
Albendazole sulfoxide is 70% bound to plasma protein and is widely distributed throughout the body; it has been detected in urine, bile, liver, cyst wall, cyst fluid, and cerebrospinal fluid (CSF). Concentrations in plasma were 3-fold to 10-fold and 2-fold to 4-fold higher than those simultaneously determined in cyst fluid and CSF, respectively.
Metabolism and Excretion
Albendazole is rapidly converted in the liver to the primary metabolite, albendazole sulfoxide, which is further metabolized to albendazole sulfone and other primary oxidative metabolites that have been identified in human urine. Following oral administration, albendazole has not been detected in human urine. Urinary excretion of albendazole sulfoxide is a minor elimination pathway with less than 1% of the dose recovered in the urine. Biliary elimination presumably accounts for a portion of the elimination as evidenced by biliary concentrations of albendazole sulfoxide similar to those achieved in plasma.
Specific Populations
Pediatrics
Following single-dose administration of 200 mg to 300 mg (approximately 10 mg/kg) albendazole to 3 fasted and 2 fed pediatric patients with hydatid cyst disease (age range 6 to 13 years), albendazole sulfoxide pharmacokinetics were similar to those observed in fed adults.
Geriatrics
Although no studies have investigated the effect of age on albendazole sulfoxide pharmacokinetics, data in 26 hydatid cyst patients (up to 79 years) suggest pharmacokinetics similar to those in young healthy subjects.
12.4 Microbiology
Mechanism of Action
Albendazole binds to the colchicine-sensitive site of β-tubulin inhibiting their polymerization into microtubules. The decrease in microtubules in the intestinal cells of the parasites decreases their absorptive function, especially the uptake of glucose by the adult and larval forms of the parasites, and also depletes glycogen storage. Insufficient glucose results in insufficient energy for the production of adenosine trisphosphate (ATP) and the parasite eventually dies.
Mechanism of Resistance
Parasitic resistance to albendazole is caused by changes in amino acids that result in changes in the β-tubulin protein. This causes reduced binding of the drug to β-tubulin.
In the specified treatment indications albendazole appears to be active against the larval forms of the following organisms:
Echinococcus granulosus
Taenia solium
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies were conducted in mice and rats.
No evidence of increased incidence of tumors was found in the mice or rats at up to 400 mg/kg/day or 20 mg/kg/day respectively (2 times and 0.2 times the recommended human dose on a body surface area basis).
In genotoxicity tests, albendazole was found negative in an Ames Salmonella/Microsome Plate mutation assay, Chinese Hamster Ovary chromosomal aberration test, and in vivo mouse micronucleus test. In the in vitro BALB/3T3 cells transformation assay, albendazole produced weak activity in the presence of metabolic activation while no activity was found in the absence of metabolic activation.
Albendazole did not adversely affect male or female fertility in the rat at an oral dose of 30 mg/kg/day (0.32 times the recommended human dose based on body surface area in mg/m2).
16 How Supplied/storage And Handling
16.1 How Supplied
Albendazole Tablets, USP are available as follows:
200 mg — Each white to off white, round, film-coated, unscored tablet debossed with ‘A210’ on one side and plain on the other contains 200 mg of albendazole, USP. Tablets are supplied in bottles of 2 (NDC 0591-2712-02) with a child-resistant closure.
Â
16.2 Storage and Handling
Store at 20° to 25°C (68° to 77°F)[See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
17 Patient Counseling Information
Patients should be advised that:
- Some people, particularly children, may experience difficulties swallowing the albendazole tablets whole.
- Take albendazole tablets with food.
- Advise pregnant women and females of reproductive potential of the potential risk to the fetus. Advise females to inform their prescriber of a known or suspected pregnancy [see Warnings and Precautions (5.2), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with albendazole and for 3 days after the final dose [see Use in Specific Populations (8.3)].
- During albendazole tablets therapy, monitor blood counts and liver enzymes every 2 weeks because of the possibility of harm to the liver or bone marrow [see Warnings and Precautions (5.5)]
Manufactured In India By:Watson Pharma Private LimitedVerna, Salcette Goa 403 722 INDIA
Distributed By:Teva Pharmaceuticals USA, Inc.North Wales, PA 19454
Rev. A 7/2019
Principal Display Panel
NDC 0591-2712-02Albendazole Tablets USPÂ 200 mgRx only2 Tablets

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


