Diphenoxylate Hydrochloride and Atropine Sulfate (diphenoxylate hydrochloride 2.5 mg atropine sulfate 0.025 mg) Dailymed
Generic: diphenoxylate hydrochloride and atropine sulfate is used for the treatment of Tissue Adhesions Asthma Bradycardia Colitis, Ulcerative Heart Arrest Heart Block Intestinal Obstruction Iris Diseases Kidney Diseases Lens Diseases Liver Diseases Megacolon, Toxic Myasthenia Gravis Poisoning Sinoatrial Block Stomach Ulcer Tachycardia Thyrotoxicosis Ureteral Obstruction Uveitis Xerostomia Glaucoma, Angle-Closure Mydriasis Drug-Related Side Effects and Adverse Reactions Child, Preschool Dehydration Diarrhea Enterocolitis, Pseudomembranous Jaundice
IMPRINT: M 15
SHAPE: round
COLOR: white
All Imprints
diphenoxylate hydrochloride 2.5 mgatropine sulfate 0.025 mg - m 15 round white
atropine sulfate 0.025 mg / diphenoxylate hydrochloride 2.5 mg oral tablet - m 15 round white
Go PRO for all pill images
Description
Each tablet for oral administration contains:
diphenoxylatehydrochloride, USP 2.5 mg(Warning – May be habit forming)
atropinesulfate, USP 0.025 mg
Diphenoxylate hydrochloride, an antidiarrheal, is ethyl 1-(3-cyano-3, 3-diphenylpropyl)-4-phenyl-isonipecotate monohydrochloride and has the following structure:
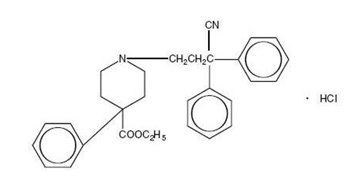
Atropine sulfate, an anticholinergic, is endo-(±)-alpha-(hydroxymethyl) benzeneacetic acid 8-methyl-8-azabicylo[3.2.1] oct-3-yl ester sulfate (2:1)] (salt) monohydrate and has the following structure:
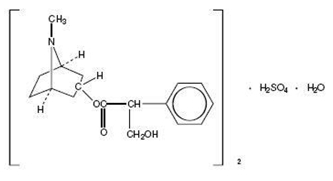
A subtherapeutic amount of atropine sulfate is present to discourage deliberate overdosage.
Each tablet for oral administration contains the following inactive ingredients: colloidal silicon dioxide, microcrystalline cellulose, pregelatinized starch and stearic acid.
Clinical Pharmacology
Diphenoxylate is rapidly and extensively metabolized in man by ester hydrolysis to diphenoxylic acid (difenoxine), which is biologically active and the major metabolite in the blood. After a 5 mg oral dose of carbon-14 labeled diphenoxylate hydrochloride in ethanolic solution was given to three healthy volunteers, an average of 14% of the drug plus its metabolites was excreted in the urine and 49% in the feces over a 4-day period. Urinary excretion of the unmetabolized drug constituted less than 1% of the dose, and diphenoxylic acid plus its glucuronide conjugate constituted about 6% of the dose. In a 16 subject cross-over bioavailability study, a linear relationship in the dose range of 2.5 mg to 10 mg was found between the dose of diphenoxylate hydrochloride (given as Diphenoxylate Hydrochloride and Atropine Sulfate Oral Solution) and the peak plasma concentration, the area under the plasma concentration-time curve, and the amount of diphenoxylic acid excreted in the urine. In the same study the bioavailability of the tablet compared with an equal dose of the liquid was approximately 90%. The average peak plasma concentration of diphenoxylic acid following ingestion of four 2.5 mg tablets was 163 ng/mL at about 2 hours, and the elimination half-life of diphenoxylic acid was approximately 12 to 14 hours.
In dogs, diphenoxylate hydrochloride has a direct effect on circular smooth muscle of the bowel, that conceivably results in segmentation and prolongation of gastrointestinal transit time. The clinical antidiarrheal action of diphenoxylate hydrochloride may thus be a consequence of enhanced segmentation that allows increased contact of the intraluminal contents with the intestinal mucosa.
Indications And Usage
Diphenoxylate hydrochloride and atropine sulfate tablets are effective as adjunctive therapy in the management of diarrhea.
Contraindications
Diphenoxylate hydrochloride and atropine sulfate tablets are contraindicated in patients with
- Known hypersensitivity to diphenoxylate or atropine,
- Obstructive jaundice,
- Diarrhea associated with pseudomembranous enterocolitis or enterotoxin-producing bacteria.
Warnings
THIS IS NOT AN INNOCUOUS DRUG AND DOSAGE RECOMMENDATIONS SHOULD BE STRICTLY ADHERED TO, ESPECIALLY IN CHILDREN. DIPHENOXYLATE HYDROCHLORIDE AND ATROPINE SULFATE IS NOT RECOMMENDED FOR CHILDREN UNDER 2 YEARS OF AGE. OVERDOSAGE MAY RESULT IN SEVERE RESPIRATORY DEPRESSION AND COMA, POSSIBLY LEADING TO PERMANENT BRAIN DAMAGE OR DEATH (See OVERDOSAGE). THEREFORE, KEEP THIS MEDICATION OUT OF THE REACH OF CHILDREN.
THE USE OF DIPHENOXYLATE HYDROCHLORIDE AND ATROPINE SULFATE SHOULD BE ACCOMPANIED BY APPROPRIATE FLUID AND ELECTROLYTE THERAPY, WHEN INDICATED. IF SEVERE DEHYDRATION OR ELECTROLYTE IMBALANCE IS PRESENT, THIS PRODUCT SHOULD BE WITHHELD UNTIL APPROPRIATE CORRECTIVE THERAPY HAS BEEN INITIATED. DRUG-INDUCED INHIBITION OF PERISTALSIS MAY RESULT IN FLUID RETENTION IN THE INTESTINE, WHICH MAY FURTHER AGGRAVATE DEHYDRATION AND ELECTROLYTE IMBALANCE.
DIPHENOXYLATE HYDROCHLORIDE AND ATROPINE SULFATE SHOULD BE USED WITH SPECIAL CAUTION IN YOUNG CHILDREN BECAUSE THIS AGE GROUP MAY BE PREDISPOSED TO DELAYED DIPHENOXYLATE TOXICITY AND BECAUSE OF THE GREATER VARIABILITY OF RESPONSE IN THIS AGE GROUP.
Antiperistaltic agents may prolong and/or worsen diarrhea associated with organisms that penetrate the intestinal mucosa (toxigenic E. coli, Salmonella, Shigella), and pseudomembranous enterocolitis associated with broad-spectrum antibiotics. Antiperistaltic agents should not be used in these conditions.
In some patients with acute ulcerative colitis, agents that inhibit intestinal motility or prolong intestinal transit time have been reported to induce toxic megacolon. Consequently, patients with acute ulcerative colitis should be carefully observed and therapy should be discontinued promptly if abdominal distention occurs or if other untoward symptoms develop.
Since the chemical structure of diphenoxylate hydrochloride is similar to that of meperidine hydrochloride, the concurrent use of this product with monoamine oxidase (MAO) inhibitors may, in theory, precipitate hypertensive crisis.
This product should be used with extreme caution in patients with advanced hepatorenal disease and in all patients with abnormal liver function since hepatic coma may be precipitated.
Diphenoxylate hydrochloride may potentiate the action of barbiturates, tranquilizers, and alcohol. Therefore, the patient should be closely observed when any of these are used concomitantly.
Adverse Reactions
At therapeutic doses, the following have been reported: they are uled in decreasing order of severity, but not of frequency:
Nervous system: Numbness of extremities, euphoria, depression, malaise/lethargy, confusion, sedation/drowsiness, dizziness, restlessness, headache.
Allergic: anaphylaxis, angioneurotic edema, urticaria, swelling of the gums, pruritus.
Gastrointestinal system: toxic megacolon, paralytic ileus, pancreatitis, vomiting, nausea, anorexia, abdominal discomfort.
The following atropine sulfate effects are uled in decreasing order of severity, but not of frequency: hyperthermia, tachycardia, urinary retention, flushing, dryness of the skin and mucous membranes. These effects may occur especially in children.
THIS MEDICATION SHOULD BE KEPT IN A CHILD-RESISTANT CONTAINER AND OUT OF THE REACH OF CHILDREN SINCE AN OVERDOSAGE MAY RESULT IN SEVERE RESPIRATORY DEPRESSION AND COMA, POSSIBLY LEADING TO PERMANENT BRAIN DAMAGE OR DEATH.
Overdosage
RECOMMENDED DOSAGE SCHEDULES SHOULD BE STRICTLY FOLLOWED. THIS MEDICATION SHOULD BE KEPT IN A CHILD-RESISTANT CONTAINER AND OUT OF THE REACH OF CHILDREN, SINCE AN OVERDOSAGE MAY RESULT IN SEVERE, EVEN FATAL, RESPIRATORY DEPRESSION.
Diagnosis
Initial signs of overdosage may include dryness of the skin and mucous membranes, mydriasis, restlessness, flushing, hyperthermia, and tachycardia followed by lethargy or coma, hypotonic reflexes, nystagmus, pinpoint pupils, and respiratory depression. Respiratory depression may be evidenced as late as 30 hours after ingestion and may recur in spite of an initial response to narcotic antagonists. TREAT ALL POSSIBLE OVERDOSAGES AS SERIOUS AND MAINTAIN MEDICAL OBSERVATION FOR AT LEAST 48 HOURS, PREFERABLY UNDER CONTINUOUS HOSPITAL CARE.
Treatment
In the event of overdose, induction of vomiting, gastric lavage, establishment of a patent airway, and possibly mechanically assisted respiration are advised. In vitro and animal studies indicate that activated charcoal may significantly decrease the bioavailability of diphenoxylate. In non-comatose patients, a slurry of 100 g of activated charcoal can be administered immediately after the induction of vomiting or gastric lavage.
A pure narcotic antagonist (e.g., naloxone) should be used in the treatment of respiratory depression caused by diphenoxylate hydrochloride and atropine sulfate. When a narcotic antagonist is administered intravenously, the onset of action is generally apparent within 2 minutes. It may also be administered subcutaneously or intramuscularly, providing a slightly less rapid onset of action but a more prolonged effect.
To counteract respiratory depression caused by diphenoxylate/atropine overdosage, the following dosage schedule for the narcotic antagonist naloxone hydrochloride should be followed:
Adult Dosage: The usual initial adult dose of naloxone hydrochloride is 0.4 mg administered intravenously. If respiratory function does not adequately improve after the initial-dose, the same IV dose may be repeated at 2 to 3 minute intervals.
Children: The usual initial dose of naloxone hydrochloride for children is 0.01 mg/kg of body weight administered intravenously and repeated at 2 to 3 minute intervals if necessary.
Following initial improvement of respiratory function, repeated doses of naloxone hydrochloride may be required to counteract recurrent respiratory depression. Supplemental intramuscular doses of naloxone hydrochloride may be utilized to produce a longer-lasting effect.
Since the duration of action of diphenoxylate hydrochloride is longer than that of naloxone hydrochloride, improvement of respiration following administration may be followed by recurrent respiratory depression. Consequently, continued observation is necessary until the effect of diphenoxylate hydrochloride on respiration has passed. This effect may persist for many hours. The period of observation should extend over at least 48 hours, preferably under continuous hospital care. Although signs of overdosage and respiratory depression may not be evident soon after ingestion of diphenoxylate hydrochloride, respiratory depression may occur from 12 to 30 hours later.
Dosage And Administration
DO NOT EXCEED RECOMMENDED DOSAGE.
Adults
The recommended initial dosage is two tablets four times daily (20 mg per day). Most patients will require this dosage until initial control has been achieved, after which the dosage may be reduced to meet individual requirements. Control may often be maintained with as little as 5 mg (two tablets) daily.
Clinical improvement of acute diarrhea is usually observed within 48 hours. If clinical improvement of chronic diarrhea after treatment with a maximum daily dose of 20 mg of diphenoxylate hydrochloride is not observed within 10 days, symptoms are unlikely to be controlled by further administration.
Children
Diphenoxylate hydrochloride and atropine sulfate is not recommended in children under 2 years of age and should be used with special caution in young children (see WARNINGS and PRECAUTIONS). The nutritional status and degree of dehydration must be considered. In children under 13 years of age, use oral solution. Do not use tablets for this age group.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
How Supplied
Diphenoxylate Hydrochloride and Atropine Sulfate Tablets, USP are available containing 2.5 mg of diphenoxylate hydrochloride, USP (Warning: May be habit forming) and 0.025 mg of atropine sulfate, USP. The tablets are white round, unscored tablets debossed with M over 15 on one side of the tablet and blank on the other side. They are available as follows:
NDC 66336-0437-15bottles of 15 tablets
NDC 66336-0437-20bottles of 20 tablets
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Pharmacist: Dispense with a child-resistant closure only.
Manufacture:
Mylan Pharmaceuticals Inc.Morgantown, WV 26505
REVISED NOVEMBER 2009DPXAS:R12
Principal Display Panel

NDC 66336-0437-XX
NDC 66336-0437-15NDC 66336-0437-20
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


