Carbamazepine (carbamazepine 100 mg) Dailymed
Generic: carbamazepine is used for the treatment of Bipolar Disorder Bone Marrow Diseases Epilepsy, Tonic-Clonic Pregnancy Psychotic Disorders Restless Legs Syndrome Schizophrenia Stress Disorders, Post-Traumatic Substance Withdrawal Syndrome Trigeminal Neuralgia Epilepsy, Complex Partial
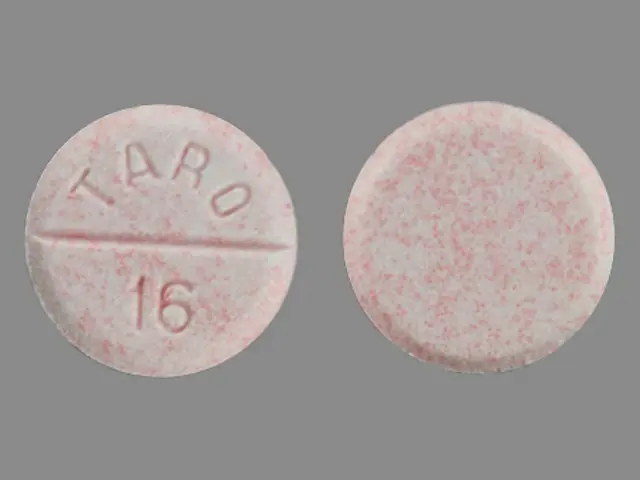
IMPRINT: TARO 16
SHAPE: round
COLOR: white SCORE: 2
All Imprints
carbamazepine 200 mg - taro 11 round white
carbamazepine 100 mg - taro 16 round white
Boxed Warning
Boxed Warning
warning
Go PRO for all pill images
Boxed Warning
WARNING
APLASTIC ANEMIA AND AGRANULOCYTOSIS HAVE BEEN REPORTED IN ASSOCIATION WITH THE USE OF CARBAMAZEPINE. DATA FROM A POPULATION-BASED CASE CONTROL STUDY DEMONSTRATE THAT THE RISK OF DEVELOPING THESE REACTIONS IS 5-8 TIMES GREATER THAN IN THE GENERAL POPULATION. HOWEVER, THE OVERALL RISK OF THESE REACTIONS IN THE UNTREATED GENERAL POPULATION IS LOW, APPROXIMATELY SIX PATIENTS PER ONE MILLION POPULATION PER YEAR FOR AGRANULOCYTOSIS AND TWO PATIENTS PER ONE MILLION POPULATION PER YEAR FOR APLASTIC ANEMIA.
ALTHOUGH REPORTS OF TRANSIENT OR PERSISTENT DECREASED PLATELET OR WHITE BLOOD CELL COUNTS ARE NOT UNCOMMON IN ASSOCIATION WITH THE USE OF CARBAMAZEPINE, DATA ARE NOT AVAILABLE TO ESTIMATE ACCURATELY THEIR INCIDENCE OR OUTCOME. HOWEVER, THE VAST MAJORITY OF THE CASES OF LEUKOPENIA HAVE NOT PROGRESSED TO THE MORE SERIOUS CONDITIONS OF APLASTIC ANEMIA OR AGRANULOCYTOSIS.
BECAUSE OF THE VERY LOW INCIDENCE OF AGRANULOCYTOSIS AND APLASTIC ANEMIA, THE VAST MAJORITY OF MINOR HEMATOLOGIC CHANGES OBSERVED IN MONITORING OF PATIENTS ON CARBAMAZEPINE ARE UNLIKELY TO SIGNAL THE OCCURRENCE OF EITHER ABNORMALITY. NONETHELESS, COMPLETE PRETREATMENT HEMATOLOGICAL TESTING SHOULD BE OBTAINED AS A BASELINE. IF A PATIENT IN THE COURSE OF TREATMENT EXHIBITS LOW OR DECREASED WHITE BLOOD CELL OR PLATELET COUNTS, THE PATIENT SHOULD BE MONITORED CLOSELY. DISCONTINUATION OF THE DRUG SHOULD BE CONSIDERED IF ANY EVIDENCE OF SIGNIFICANT BONE MARROW DEPRESSION DEVELOPS.
Before prescribing carbamazepine, the physician should be thoroughly familiar with the details of this prescribing information, particularly regarding use with other drugs, especially those which accentuate toxicity potential.
Description
Carbamazepine USP, is an anticonvulsant and specific analgesic for trigeminal neuralgia, available for oral administration as tablets of 200 mg. Its chemical name is 5H-dibenz[b,f]azepine-5-carboxamide, and its structural formula is:
Carbamazepine USP is a white to off-white powder, practically insoluble in water and soluble in alcohol and in acetone. Its molecular weight is 236.27.
The following inactive ingredients are contained in these tablets: lactose (monohydrate), microcrystalline cellulose, hypromellose, ethylcellulose, croscarmellose sodium, magnesium stearate.
Product meets USP Dissolution Test 3.
Clinical Pharmacology
In controlled clinical trials, carbamazepine has been shown to be effective in the treatment of psychomotor and grand mal seizures, as well as trigeminal neuralgia.
Mechanism Of Action: Carbamazepine has demonstrated anticonvulsant properties in rats and mice with electrically and chemically induced seizures. It appears to act by reducing polysynaptic responses and blocking the post-tetanic potentiation. Carbamazepine greatly reduces or abolishes pain induced by stimulation of the infraorbital nerve in cats and rats. It depresses thalamic potential and bulbar and polysynaptic reflexes, including the linguomandibular reflex in cats. Carbamazepine is chemically unrelated to other anticonvulsants or other drugs used to control the pain of trigeminal neuralgia. The mechanism of action remains unknown.
The principal metabolite of carbamazepine, carbamazepine-10, 11-epoxide, has anticonvulsant activity as demonstrated in several in vivo animal models of seizures. Though clinical activity for the epoxide has been postulated, the significance of its activity with respect to the safety and efficacy of carbamazepine has not been established.
Pharmacokinetics: In clinical studies, carbamazepine suspension, conventional tablets, and extended-release tablets delivered equivalent amounts of drug to the systemic circulation. However, the suspension was absorbed somewhat faster, and the extended-release tablet slightly slower, than the conventional tablet. The bioavailability of the extended-release tablet was 89% compared to suspension. Following a b.i.d. dosage regimen, the suspension provides higher peak levels and lower trough levels than those obtained from the conventional tablet for the same dosage regimen. On the other hand, following a t.i.d. dosage regimen, carbamazepine suspension affords steady-state plasma levels comparable to carbamazepine tablets given b.i.d. when administered at the same total mg daily dose. Following a b.i.d. dosage regimen, carbamazepine extended-release tablets afford steady-state plasma levels comparable to conventional carbamazepine tablets given q.i.d., when administered at the same total mg daily dose. Carbamazepine in blood is 76% bound to plasma proteins. Plasma levels of carbamazepine are variable and may range from 0.5-25 mcg/mL, with no apparent relationship to the daily intake of the drug. Usual adult therapeutic levels are between 4 and 12 mcg/mL. In polytherapy, the concentration of carbamazepine and concomitant drugs may be increased or decreased during therapy, and drug effects may be altered (see PRECAUTIONS, Drug Interactions). Following chronic oral administration of suspension, plasma levels peak at approximately 1.5 hours compared to 4-5 hours after administration of conventional carbamazepine tablets, and 3-12 hours after administration of carbamazepine extended-release tablets. The CSF/serum ratio is 0.22, similar to the 24% unbound carbamazepine in serum. Because carbamazepine induces its own metabolism, the half-life is also variable. Autoinduction is completed after 3-5 weeks of a fixed dosing regimen. Initial half-life values range from 25-65 hours, decreasing to 12-17 hours on repeated doses. Carbamazepine is metabolized in the liver. Cytochrome P450 3A4 was identified as the major isoform responsible for the formation of carbamazepine-10,11-epoxide from carbamazepine. After oral administration of 14C-carbamazepine, 72% of the administered radioactivity was found in the urine and 28% in the feces. This urinary radioactivity was composed largely of hydroxylated and conjugated metabolites, with only 3% of unchanged carbamazepine.
The pharmacokinetic parameters of carbamazepine disposition are similar in children and in adults. However, there is a poor correlation between plasma concentrations of carbamazepine and carbamazepine dose in children. Carbamazepine is more rapidly metabolized to carbamazepine-10,11-epoxide (a metabolite shown to be equipotent to carbamazepine as an anticonvulsant in animal screens) in the younger age groups than in adults. In children below the age of 15, there is an inverse relationship between CBZ-E/CBZ ratio and increasing age (in one report from 0.44 in children below the age of 1 year to 0.18 in children between 10-15 years of age).
The effects of race and gender on carbamazepine pharmacokinetics have not been systematically evaluated.
Indications And Usage
Epilepsy: Carbamazepine tablets are indicated for use as an anticonvulsant drug. Evidence supporting efficacy of carbamazepine as an anticonvulsant was derived from active drug-controlled studies that enrolled patients with the following seizure types:
1. Partial seizures with complex symptomatology (psychomotor, temporal lobe). Patients with these seizures appear to show greater improvement than those with other types.
2. Generalized tonic-clonic seizures (grand mal).
3. Mixed seizure patterns which include the above, or other partial or generalized seizures. Absence seizures (petit mal) do not appear to be controlled by carbamazepine (see PRECAUTIONS, General).
Trigeminal Neuralgia: Carbamazepine tablets are indicated in the treatment of the pain associated with true trigeminal neuralgia.
Beneficial results have also been reported in glossopharyngeal neuralgia.
This drug is not a simple analgesic and should not be used for the relief of trivial aches or pains.
Contraindications
Carbamazepine should not be used in patients with a history of previous bone marrow depression, hypersensitivity to the drug, or known sensitivity to any of the tricyclic compounds, such as amitriptyline, desipramine, imipramine, protriptyline, nortriptyline, etc. Likewise, on theoretical grounds its use with monoamine oxidase inhibitors is not recommended. Before administration of carbamazepine, MAO inhibitors should be discontinued for a minimum of 14 days, or longer if the clinical situation permits.
Co-administration of carbamazepine and nefazodone may result in insufficient plasma concentrations of nefazodone and its active metabolite to achieve a therapeutic effect. Co-administration of carbamazepine with nefazodone is contraindicated.
Warnings
Patients with a history of adverse hematologic reaction to any drug may be particularly at risk.
Severe dermatologic reactions, including toxic epidermal necrolysis (Lyell’s syndrome) and Stevens-Johnson syndrome, have been reported with carbamazepine. These reactions have been extremely rare. However, a few fatalities have been reported.
Carbamazepine has shown mild anticholinergic activity; therefore, patients with increased intraocular pressure should be closely observed during therapy.
Because of the relationship of the drug to other tricyclic compounds, the possibility of activation of a latent psychosis and, in elderly patients, of confusion or agitation should be borne in mind.
The used of carbamazepine should be avoided in patients with a history of hepatic porphyria (e.g., acute intermittent porphyria, variegate porphyria, porphyria cutanea tarda). Acute attacks have been reported in such patients receiving carbamazepine therapy. Carbamazepine administration has also been demonstrated to increase porphyrin precursors in rodents, a presumed mechanism for the induction of acute attacks of porphyria.
As with all antiepileptic drugs, carbamazepine should be withdrawn gradually to minimize the potential of increased seizure frequency.
Usage In Pregnancy: Carbamazepine can cause fetal harm when administered to a pregnant woman.
Epidemiological data suggest that there may be an association between the use of carbamazepine during pregnancy and congenital malformations, including spina bifida. There have also been reports that associate carbamazepine with developmental disorders and congenital anomalies (e.g., craniofacial defects, cardiovascular malformations and anomalies involving various body systems). Developmental delays based on neurobehavioral assessments have been reported. In treating or counseling women of childbearing potential, the prescribing physician will wish to weigh the benefits of therapy against the risks. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Retrospective case reviews suggest that, compared with monotherapy, there may be a higher prevalence of teratogenic effects associated with the use of anticonvulsants in combination therapy. Therefore, if therapy is to be continued, monotherapy may be preferable for pregnant women.
In humans, transplacental passage of carbamazepine is rapid (30-60 minutes), and the drug is accumulated in the fetal tissues, with higher levels found in liver and kidney than in brain and lung.
Carbamazepine has been shown to have adverse effects in reproduction studies in rats when given orally in dosages 10-25 times the maximum human daily dosage (MHDD) of 1200 mg on a mg/kg basis or 1.5-4 times the MHDD on a mg/m2 basis. In rat teratology studies, 2 of 135 offspring showed kinked ribs at 250 mg/kg and 4 of 119 offspring at 650 mg/kg showed other anomalies (cleft palate, 1; talipes, 1; anophthalmos, 2). In reproduction studies in rats, nursing offspring demonstrated a lack of weight gain and an unkempt appearance at a maternal dosage level of 200 mg/kg.
Antiepileptic drugs should not be discontinued abruptly in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorder are such that removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy, although it cannot be said with any confidence that even minor seizures do not pose some hazard to the developing embryo or fetus.
Tests to detect defects using currently accepted procedures should be considered a part of routine prenatal care in childbearing women receiving carbamazepine.
There have been a few cases of neonatal seizures and/or respiratory depression associated with maternal carbamazepine and other concomitant anticonvulsant drug use. A few cases of neonatal vomiting, diarrhea, and/or decreased feeding have also been reported in association with maternal carbamazepine use. These symptoms may represent a neonatal withdrawal syndrome.
Precautions
General
Before initiating therapy, a detailed history and physical examination should be made.
Carbamazepine should be used with caution in patients with a mixed seizure disorder that includes atypical absence seizures, since in these patients carbamazepine has been associated with increased frequency of generalized convulsions (see INDICATIONS AND USAGE).
Therapy should be prescribed only after critical benefit-to-risk appraisal in patients with a history of cardiac conduction disturbance, including second and third degree AV heart block; cardiac, hepatic, or renal damage; adverse hematologic or hypersensitivity reaction to other drugs, including reactions to other anticonvulsants; or interrupted courses of therapy with carbamazepine.
AV heart block, including second and third degree block, have been reported following, carbamazepine treatment. This occurred generally, but not solely, in patients with underling EKG abnormalities or risk factors for conduction disturbances.
Hepatic effects, ranging from slight elevations in liver enzymes to rare cases of hepatic failure have been reported (see ADVERSE REACTIONSand PRECAUTIONS, Laboratory Tests). In some cases, hepatic effects may progress despite discontinuation of the drug.
Multi-organ hypersensitivity reactions occurring days to weeks or months after initiating treatment have been reported in rare cases (see ADVERSE REACTIONS, Otherand PRECAUTIONS, Information for Patients).
Discontinuation of carbamazepine should be considered if any evidence of hypersensitivity develops.
Hypersensitivity reactions to carbamazepine have been reported in patients who previously experienced this reaction to anticonvulsants including phenytoin and phenobarbital. A history of hypersensitivity reactions should be obtained for a patient and the immediate family members. If positive, caution should be used in prescribing carbamazepine.
Information for Patients
Patients should be made aware of the early toxic signs and symptoms of a potential hematologic problem, as well as dermatologic, hypersensitivity or hepatic reactions. These symptoms may include, but are not limited to, fever, sore throat, rash, ulcers in the mouth, easy bruising, lymphadenopathy and petechial or purpuric hemorrhage, and in the case of liver reactions, anorexia, nausea/vomiting, or jaundice. The patient should be advised that, because these signs and symptoms may signal a serious reaction, that they must report any occurrence immediately to a physician. In addition, the patient should be advised that these signs and symptoms should be reported even if mild or when occurring after extended use.
Carbamzepine may interact with some drugs. Therefore, patients should be advised to report to their doctors the use of any other prescription or non-prescription medications or herbal products.
Caution should be exercised if alcohol is taken in combination with carbamazepine therapy, due to a possible additive sedative effect.
Since dizziness and drowsiness may occur, patients should be cautioned about the hazards of operating machinery or automobiles or engaging in other potentially dangerous tasks.
Laboratory Tests
Complete pretreatment blood counts, including platelets and possibly reticulocytes and serum iron, should be obtained as a baseline. If a patient in the course of treatment exhibits low or decreased white blood cell or platelet counts, the patient should be monitored closely. Discontinuation of the drug should be considered if any evidence of significant bone marrow depression develops.
Baseline and periodic evaluations of liver function, particularly in patients with a history of liver disease, must be performed during treatment with this drug since liver damage may occur (see PRECAUTIONS, Generaland ADVERSE REACTIONS). Carbamazepine should be discontinued, based on clinical judgement, if indicated by newly occurring or worsening clinical or laboratory evidence of liver dysfunction or hepatic damage, or in the case of active liver disease.
Baseline and periodic eye examinations, including slit-lamp, funduscopy, and tonometry, are recommended since many phenothiazines and related drugs have been shown to cause eye changes.
Baseline and periodic complete urinalysis and BUN determinations are recommended for patients treated with this agent because of observed renal dysfunction.
Monitoring of blood levels (see CLINICAL PHARMACOLOGY) has increased the efficacy and safety of anticonvulsants. This monitoring may be particularly useful in cases of dramatic increase in seizure frequency and for verification of compliance. In addition, measurement of drug serum levels may aid in determining the cause of toxicity when more than one medication is being used.
Thyroid function tests have been reported to show decreased values with carbamazepine administered alone.
Hyponatremia has been reported in association with carbamazepine use, either alone or in combination with other drugs.
Interference with some pregnancy tests has been reported.
Drug Interactions
Clinically meaningful drug interactions have occurred with concomitant medications and include, but are not limited to, the following:
Agents That May Affect Carbamazepine Plasma Levels
CYP 3A4 inhibitors inhibit carbamazepine metabolism and can thus increase plasma carbamazepine levels. Drugs that have been shown, or would be expected to increase plasma carbamazepine levels include:
cimetidine, danazol, diltiazem, macrolides, erythromycin, troleandomycin, clarithromycin, fluoxetine, fluvoxamine, nefazodone, loratadine, terfenadine, isoniazid, niacinamide, nicotinamide, propoxyphene, azoles (e.g., ketaconazole, itraconazole, fluconazole), acetazolamide, verapamil, grapefruit juice, protease inhibitors, valproate.*
CYP 3A4 inducers can increase the rate of carbamazepine metabolism. Drugs that have been shown, or that would be expected, to decrease plasma carbamazepine levels include:
cisplatin, doxorubicin HCl, felbamate,†rifampin, phenobarbital, phenytoin, primidone, methsuximide, theophylline.
When carbamazepine is given with drugs that can increase or decrease carbamazepine levels, close monitoring of carbamazepine levels is indicated and dosage adjustment may be required.
______________________________________
*increased levels of the active 10,11-epoxide
†decreased levels of carbamazepine and increased levels of the 10,11-epoxide
Effect Of Carbamazepine On Plasma Levels Of Concomitant Agents
Increased levels: clomipramine HCl, phenytoin, primidone
Carbamazepine induces hepatic CYP activity. Carbamazepine causes, or would be expected to cause, decreased levels of the following:
acetaminophen, alprazolam, dihydropyridine calcium channel blockers (e.g., felodipine), cyclosporine, corticosteroids (e.g., prednisolone, dexamethasone), clonazepam, clozapine, dicumarol, doxycycline, ethosuximide, haloperidol, itraconazole, lamotrigine, levothyroxine, methadone, methsuximide, midazolam, olanzapine, oral and other hormonal contraceptives, oxcarbazepine, phensuximide, phenytoin, praziquantel, protease inhibitors, risperidone, theophylline, tiagabine, topiramate, tramadol, tricyclic antidepressants (e.g., imipramine, amitriptyline, nortriptyline), valproate, warfarin,ziprasidone, zonisamide.
In concomitant use with carbamazepine, dosage adjustment of the above agents may be necessary.
Co-administration of carbamazepine with nefazodone results in insufficient plasma concentrations of nefazodone and its active metabolite to achieve a therapeutic effect. Co-administration of carbamazepine with nefazodone is contraindicated. (See CONTRAINDICATIONS).
Concomitant administration of carbamazepine and lithium may increase the risk of neurotoxic side effects.
Alterations of thyroid function have been reported in combination therapy with other anticonvulsant medications.
Concomitant use of carbamazepine with hormonal contraceptive products (e.g. oral, and levonorgestrel subdermal implant contraceptives) may render the contraceptives less effective because the plasma concentrations of the hormones may be decreased. Breakthrough bleeding and unintended pregnancies have been reported. Alternative or back-up methods of contraception should be considered.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carbamazepine, when administered to Sprague-Dawley rats for two years in the diet at doses of 25, 75, and 250 mg/kg/day, resulted in a dose-related increase in the incidence of hepatocellular tumors in females and of benign interstitial cell adenomas in the testes of males.
Carbamazepine must, therefore, be considered to be carcinogenic in Sprague-Dawley rats. Bacterial and mammalian mutagenicity studies using carbamazepine produced negative results. The significance of these findings relative to the use of carbamazepine in humans is, at present, unknown.
Pregnancy
Usage in Pregnancy: Pregnancy Category D(see WARNINGS).
Labor and Delivery
The effect of carbamazepine on human labor and delivery is unknown.
Nursing Mothers
Carbamazepine and its epoxide metabolite are transferred to breast milk. The ratio of the concentration in breast milk to that in maternal plasma is about 0.4 for carbamazepine and about 0.5 for the epoxide. The estimated doses given to the newborn during breast feeding are in the range of 2-5 mg daily for carbamazepine and 1-2 mg daily for the epoxide.
Because of the potential for serious adverse reactions in nursing infants from carbamazepine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Substantial evidence of carbamazepine’s effectiveness for use in the management of children with epilepsy (see INDICATIONS AND USAGEfor specific seizure types) is derived from clinical investigations performed in adults and from studies in several in vitro systems which support the conclusion that (1) the pathogenetic mechanisms underlying seizure propagation are essentially identical in adults and children, and (2) the mechanism of action of carbamazepine in treating seizures is essentially identical in adults and children.
Taken as a whole, this information supports a conclusion that the generally accepted therapeutic range of total carbamazepine in plasma (i.e., 4-12 mcg/mL) is the same in children and adults.
The evidence assembled was primarily obtained from short-term use of carbamazepine. The safety of carbamazepine in children has been systematically studied up to 6 months. No longer-term data from clinical trials is available.
Geriatric Use
No systematic studies in geriatric patients have been conducted.
Adverse Reactions
If adverse reactions are of such severity that the drug must be discontinued, the physician must be aware that abrupt discontinuation of any anticonvulsant drug in a responsive epileptic patient may lead to seizures or even status epilepticus with its life-threatening hazards.
The most severe adverse reactions have been observed in the hemopoietic system (see boxed WARNING), the skin, liver, and the cardiovascular system.
The most frequently observed adverse reactions, particularly during the initial phases of therapy, are dizziness, drowsiness, unsteadiness, nausea, and vomiting. To minimize the possibility of such reactions, therapy should be initiated at the low dosage recommended.
The following additional adverse reactions have been reported:
Hemopoietic System: Aplastic anemia, agranulocytosis, pancytopenia, bone marrow depression, thrombocytopenia, leukopenia, leukocytosis, eosinophilia, acute intermittent porphyria.
Skin:Pruritic and erythematous rashes, urticaria, toxic epidermal necrolysis (Lyell’s syndrome) (see WARNINGS), Stevens-Johnson syndrome (see WARNINGS), photosensitivity reactions, alterations in skin pigmentation, exfoliative dermatitis, erythema multiforme and nodosum, purpura, aggravation of disseminated lupus erythematosus, alopecia, and diaphoresis. In certain cases, discontinuation of therapy may be necessary. Isolated cases of hirsutism have been reported, but a causal relationship is not clear.
Cardiovascular System:Congestive heart failure, edema, aggravation of hypertension, hypotension, syncope and collapse, aggravation of coronary artery disease, arrhythmias and AV block, thrombophlebitis, thromboembolism, and adenopathy or lymphadenopathy.
Some of these cardiovascular complications have resulted in fatalities. Myocardial infarction has been associated with other tricyclic compounds.
Liver:Abnormalities in liver function tests, cholestatic and hepatocellular jaundice, hepatitis, very rare cases of hepatic failure.
Pancreatic:Pancreatitis.
Respiratory System: Pulmonary hypersensitivity characterized by fever, dyspnea, pneumonitis, or pneumonia.
Genitourinary System:Urinary frequency, acute urinary retention, oliguria with elevated blood pressure, azotemia, renal failure, and impotence. Albuminuria, glycosuria, elevated BUN, and microscopic deposits in the urine have also been reported.
Testicular atrophy occurred in rats receiving carbamazepine orally from 4-52 weeks at dosage levels of 50-400 mg/kg/day. Additionally, rats receiving carbamazepine in the diet for 2 years at dosage levels of 25, 75, and 250 mg/kg/day had a dose-related incidence of testicular atrophy and aspermatogenesis. In dogs, it produced a brownish discoloration, presumably a metabolite, in the urinary bladder at dosage levels of 50 mg/kg and higher. Relevance of these findings to humans is unknown.
Nervous System:Dizziness, drowsiness, disturbances of coordination, confusion, headache, fatigue, blurred vision, visual hallucinations, transient diplopia, oculomotor disturbances, nystagmus, speech disturbances, abnormal involuntary movements, peripheral neuritis and paresthesias, depression with agitation, talkativeness, tinnitus, and hyperacusis.
There have been reports of associated paralysis and other symptoms of cerebral arterial insufficiency, but the exact relationship of these reactions to the drug has not been established. Isolated cases of neuroleptic malignant syndrome have been reported with concomitant use of psychotropic drugs.
Digestive System: Nausea, vomiting, gastric distress and abdominal pain, diarrhea, constipation, anorexia, and dryness of the mouth and pharynx, including glossitis and stomatitis.
Eyes:Scattered punctate cortical lens opacities, as well as conjunctivitis, have been reported. Although a direct causal relationship has not been established, many phenothiazines and related drugs have been shown to cause eye changes.
Musculoskeletal System:Aching joints and muscles, and leg cramps.
Metabolism:Fever and chills. Inappropriate antidiuretic hormone (ADH) secretion syndrome has been reported. Cases of frank water intoxication, with decreased serum sodium (hyponatremia) and confusion, have been reported in association with carbamazepine use (see PRECAUTIONS: Laboratory Tests). Decreased levels of plasma calcium have been reported.
Other:Multi-organ hypersensitivity reactions occurring days to weeks or months after initiating treatment have been reported in rare cases. Signs or symptoms may include, but are not limited to fever, skin rashes, vasculitis, lymphadenopathy, disorders mimicking lymphoma, arthralgia, leukopenia, eosinophilia, hepato-splenomegaly and abnormal liver function tests. These signs and symptoms may occur in various combinations and not necessarily concurrently. Signs and symptoms may initially be mild. Various organs, including but not limited to, liver, skin, immune system, lungs, kidneys, pancreas, myocardium, and colon may be affected (see PRECAUTIONS, Generaland PRECAUTIONS, Information for Patients).
Isolated cases of a lupus erythematosus-like syndrome have been reported. There have been occasional reports of elevated levels of cholesterol, HDL cholesterol, and triglycerides in patients taking anticonvulsants.
A case of aseptic meningitis, accompanied by myoclonus and peripheral eosinophilia, has been reported in a patient taking carbamazepine in combination with other medications. The patient was successfully dechallenged, and the meningitis reappeared upon rechallenge with carbamazepine.
Drug Abuse And Dependence
No evidence of abuse potential has been associated with carbamazepine, nor is there evidence of psychological or physical dependence in humans.
Overdosage
Acute Toxicity: Lowest known lethal dose: adults, 3.2 g (a 24-year-old woman died of a cardiac arrest and a 24-year-old man died of pneumonia and hypoxic encephalopathy); children, 4 g (a 14-year-old girl died of a cardiac arrest), 1.6 g (a 3-year-old girl died of aspiration pneumonia).
Oral LD50 in animals (mg/kg): mice, 1100-3750; rats, 3850-4025; rabbits, 1500-2680; guinea pigs, 920.
Signs And Symptoms: The first signs and symptoms appear after 1-3 hours. Neuromuscular disturbances are the most prominent. Cardiovascular disorders are generally milder, and severe cardiac complications occur only when very high doses (>60 g) have been ingested.
Respiration: Irregular breathing, respiratory depression.
Cardiovascular System:Tachycardia, hypotension or hypertension, shock, conduction disorders.
Nervous System And Muscles:Impairment of consciousness ranging in severity to deep coma. Convulsions, especially in small children. Motor restlessness, muscular twitching, tremor, athetoid movements, opisthotonos, ataxia, drowsiness, dizziness, mydriasis, nystagmus, adiadochokinesia, ballism, psychomotor disturbances, dysmetria. Initial hyperreflexia, followed by hyporeflexia.
Gastrointestinal Tract:Nausea, vomiting.
Kidneys and Bladder:Anuria or oliguria, urinary retention.
Laboratory Findings:Isolated instances of overdosage have included leukocytosis, reduced leukocyte count, glycosuria, and acetonuria. EEG may show dysrhythmias.
Combined Poisoning:When alcohol, tricyclic antidepressants, barbiturates, or hydantoins are taken at the same time, the signs and symptoms of acute poisoning with carbamazepine may be aggravated or modified.
Treatment: The prognosis in cases of severe poisoning is critically dependent upon prompt elimination of the drug, which may be achieved by inducing vomiting, irrigating the stomach, and by taking appropriate steps to diminish absorption. If these measures cannot be implemented without risk on the spot, the patient should be transferred at once to a hospital, while ensuring that vital functions are safeguarded. There is no specific antidote.
Elimination Of The Drug:Induction of vomiting.
Gastric lavage. Even when more than 4 hours have elapsed following ingestion of the drug, the stomach should be repeatedly irrigated, especially if the patient has also consumed alcohol.
Measures To Reduce Absorption:Activated charcoal, laxatives.
Measures To Accelerate Elimination:Forced diuresis.
Dialysis is indicated only in severe poisoning associated with renal failure. Replacement transfusion is indicated in severe poisoning in small children.
Respiratory Depression:Keep the airways free; resort, if necessary, to endotracheal intubation, artificial respiration, and administration of oxygen.
Hypotension, Shock:Keep the patient’s legs raised and administer a plasma expander. If blood pressure fails to rise despite measures taken to increase plasma volume, use of vasoactive substances should be considered.
Convulsions:Diazepam or barbiturates.
Warning:Diazepam or barbiturates may aggravate respiratory depression (especially in children), hypotension, and coma. However, barbiturates should not be used if drugs that inhibit monoamine oxidase have also been taken by the patient either in overdosage or in recent therapy (within 1 week).
Surveillance:Respiration, cardiac function (ECG monitoring), blood pressure, body temperature, pupillary reflexes, and kidney and bladder function should be monitored for several days.
Treatment Of Blood Count Abnormalities:If evidence of significant bone marrow depression develops, the following recommendations are suggested: (1) stop the drug, (2) perform daily CBC, platelet, and reticulocyte counts, (3) do a bone marrow aspiration and trephine biopsy immediately and repeat with sufficient frequency to monitor recovery.
Special periodic studies might be helpful as follows: (1) white cell and platelet antibodies, (2) 59Fe-ferrokinetic studies, (3) peripheral blood cell typing, (4) cytogenetic studies on marrow and peripheral blood, (5) bone marrow culture studies for colony-forming units, (6) hemoglobin electrophoresis for A2 and F hemoglobin, and (7) serum folic acid and B12 levels.
A fully developed aplastic anemia will require appropriate, intensive monitoring and therapy, for which specialized consultation should be sought.
Dosage And Administration
(See table below)
Monitoring of blood levels has increased the efficacy and safety of anticonvulsants (see PRECAUTIONS: Laboratory Tests). Dosage should be adjusted to the needs of the individual patient. A low initial daily dosage with a gradual increase is advised. As soon as adequate control is achieved, the dosage may be reduced very gradually to the minimum effective level. Tablets should be taken with meals.
Conversion of patients from oral carbamazepine tablets to carbamazepine suspension: Patients should be converted by administering the same number of mg per day in smaller, more frequent doses (i.e., b.i.d. tablets to t.i.d. suspension).
Epilepsy (see INDICATIONS AND USAGE):
Adults And Children Over 12 Years Of Age — Initial: 200 mg b.i.d. Increase at weekly intervals by adding up to 200 mg/day using a t.i.d. or q.i.d. regimen until the optimal response is obtained. Dosage generally should not exceed 1000 mg daily in children 12 to 15 years of age, and 1200 mg daily in patients above 15 years of age. Doses up to 1600 mg daily have been used in adults in rare instances. Maintenance: Adjust dosage to the minimum effective level, usually 800 to 1200 mg daily.
Children 6 to 12 years of age — Initial: 100 mg b.i.d. Increase at weekly intervals by adding up to 100 mg/day using a t.i.d. or q.i.d. regimen until the optimal response is obtained. Dosage generally should not exceed 1000 mg daily. Maintenance: Adjust dosage to the minimum effective level, usually 400 to 800 mg daily.
Children Under 6 Years Of Age — Initial: 10 to 20 mg/kg/day b.i.d. or t.i.d. Increase weekly to achieve optimal clinical response administered t.i.d. or q.i.d. Maintenance: Ordinarily, optimal clinical response is achieved at daily doses below 35 mg/kg. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the therapeutic range. No recommendation regarding the safety of carbamazepine for use at doses above 35 mg/kg/24 hours can be made.
Combination Therapy:Carbamazepine may be used alone or with other anticonvulsants. When added to existing anticonvulsant therapy, the drug should be added gradually while the other anticonvulsants are maintained or gradually decreased, except phenytoin, which may have to be increased (see PRECAUTIONS: Drug Interactions,and Pregnancy Category D).
Trigeminal Neuralgia (see INDICATIONS AND USAGE):
Initial:On the first day, 100 mg b.i.d., for a total daily dose of 200 mg. This daily dose may be increased by up to 200 mg/day using increments of 100 mg every 12 hours for tablets, only as needed to achieve freedom from pain. Do not exceed 1200 mg daily. Maintenance:Control of pain can be maintained in most patients with 400 to 800 mg daily. However, some patients may be maintained on as little as 200 mg daily, while others may require as much as 1200 mg daily. At least once every 3 months throughout the treatment period, attempts should be made to reduce the dose to the minimum effective level or even to discontinue the drug.
Dosage Information Initial Dose Subsequent Dose Maximum Daily Dose Indication Epilepsy Under 6 yr 10 to 20 mg/kg/dayb.i.d. or t.i.d. Increase weekly to achieve optimal clinical response, t.i.d. or q.i.d. 35 mg/kg/24 hr (see DOSAGE AND ADMINISTRATION section above) 6 to 12 yr 100 mg b.i.d.(200 mg/day) Add up to 100 mg/dayat weekly intervals,t.i.d. or q.i.d. 1000 mg/24 hr Over 12 yr 200 mg b.i.d.(400 mg/day) Add up to200 mg/day at weekly intervals, t.i.d. or q.i.d. 1000 mg/24 hr (12 to 15 yr)1200 mg/24 hr (>15 yr)1600 mg/24 hr (adults, in rare instances) Trigeminal Neuralgia 100 mg b.i.d. (200 mg/day) Add up to 200 mg/day in increments of 100 mg every 12 hr 1200 mg/24 hr
How Supplied
Carbamazepine Tablets, USP are supplied by State of Florida DOH Central Pharmacy as follows:
NDC Strength Quantity/Form Color Source Prod. Code 53808-0913-1 100 mg 30 Tablets in a Buler Pack WHITE 51672-4041 53808-0916-1 200 mg 30 Tablets in a Buler Pack WHITE 51672-4005 53808-0915-1 200 mg 30 Tablets in a Buler Pack WHITE 60505-0183
Store at controlled room temperature 15°-30°C (59°-86°F) (see USP).
Store in a dry place. Protect from moisture.
Rx only
Manufactured by:
Taro HAWTHORNE, NY or Apotex Indianapolis, IN
This Product was Repackaged By:
State of Florida DOH Central Pharmacy 104-2 Hamilton Park Drive Tallahassee, FL 32304 United States
Label Image (taro) 100mg
Label Image (taro) 200mg
Label Image (apotex) 200mg
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site