Cymbalta (duloxetine hydrochloride 30 mg) Dailymed
Generic: duloxetine hydrochloride
Boxed Warning
Warning: Suicidality And Antidepressant Drugs
Go PRO for all pill images
Recent Major Changes Section
Indications and Usage:      Chronic Musculoskeletal Pain (1.5 )        11/2010
Dosage and Administration:      Chronic Musculoskeletal Pain (2.1 ,2.2 )        11/2010      Dosing in Special Populations, Pregnancy (2.3 )        11/2010
Warnings and Precautions:      Hepatotoxicity (5.2 )        04/2011      Activation of Mania/Hypomania (5.7 )        04/2011      Seizures (5.8 )        04/2011      Effect on Blood Pressure (5.9 )        04/2011
Warning: Suicidality And Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of Cymbalta or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Cymbalta is not approved for use in pediatric patients[see Warnings and Precautions (5.1), Use in Specific Populations (8.4), and Information for Patients (17.2)].
WARNING: Suicidality and Antidepressant Drugs
See full prescribing information for complete boxed warning.
Increased risk of suicidal thinking and behavior in children, adolescents, and young adults taking antidepressants for major depressive disorder (MDD) and other psychiatric disorders. Cymbalta is not approved for use in pediatric patients (5.1 ).
1 Indications And Usage
Cymbalta¬ģ is a serotonin and norepinephrine reuptake inhibitor¬†(SNRI) indicated for:
- Major Depressive Disorder (MDD). (
1.1 )Efficacy was established in four short-term and one maintenance trial in adults. (14.1 )- Generalized Anxiety Disorder (GAD). (
1.2 )Efficacy was established in three short-term and one maintenance trial in adults. (14.2 )- Diabetic Peripheral Neuropathic Pain (DPNP). (
1.3 )- Fibromyalgia (FM). (
1.4 )- Chronic Musculoskeletal Pain. (
1.5 )1.1 Major Depressive Disorder
Cymbalta is indicated for the treatment of major depressive disorder (MDD). The efficacy of Cymbalta was established in four short-term and one maintenance trial in adults [see Clinical Studies (14.1)].
A major depressive episode (DSM-IV) implies a prominent and relatively persistent (nearly every day for at least 2 weeks) depressed or dysphoric mood that usually interferes with daily functioning, and includes at least 5 of the following 9 symptoms: depressed mood, loss of interest in usual activities, significant change in weight and/or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, or a suicide attempt or suicidal ideation.
1.2 Generalized Anxiety Disorder
Cymbalta is indicated for the treatment of generalized anxiety disorder (GAD). The efficacy of Cymbalta was established in three short-term trials and one maintenance trial in adults [see Clinical Studies (14.2)].
Generalized anxiety disorder is defined by the DSM-IV as excessive anxiety and worry, present more days than not, for at least 6 months. The excessive anxiety and worry must be difficult to control and must cause significant distress or impairment in normal functioning. It must be associated with at least 3 of the following 6 symptoms: restlessness or feeling keyed up or on edge, being easily fatigued, difficulty concentrating or mind going blank, irritability, muscle tension, and/or sleep disturbance.
1.3 Diabetic Peripheral Neuropathic Pain
Cymbalta is indicated for the management of neuropathic pain (DPNP) associated with diabetic peripheral neuropathy [see Clinical Studies (14.3)].
1.4 Fibromyalgia
Cymbalta is indicated for the management of fibromyalgia (FM) [see Clinical Studies (14.4)].
1.5 Chronic Musculoskeletal Pain
Cymbalta is indicated for the management of chronic musculoskeletal pain. This has been established in studies in patients with chronic low back pain (CLBP) and chronic pain due to osteoarthritis [see Clinical Studies (14.5)].
2 Dosage And Administration
Cymbalta should be swallowed whole and should not be chewed or crushed, nor should the capsule be opened and its contents sprinkled on food or mixed with liquids. All of these might affect the enteric coating. Cymbalta can be given without regard to meals.
- Cymbalta should generally be administered once daily without regard to meals. Cymbalta should be swallowed whole and should not be chewed or crushed, nor should the capsule be opened and its contents be sprinkled on food or mixed with liquids. (
2.1 )
Indication
Starting Dose
Target Dose
Maximum Dose
MDD (2.1 ,2.2 )
40 mg/day to 60 mg/day
Acute Treatment: 40 mg/day (20 mg twice daily) to 60 mg/day (once daily or as 30 mg twice daily); Maintenance Treatment: 60 mg/day
120 mg/day
GAD (2.1 )
60 mg/day
60 mg/day (once daily)
120 mg/day
DPNP (2.1 )
60 mg/day
60 mg/day (once daily)
60 mg/day
FM (2.1 )
30 mg/day
60 mg/day (once daily)
60 mg/day
Chronic Musculoskeletal Pain (2.1 )
30 mg/day
60 mg/day (once daily)
60 mg/day
- Some patients may benefit from starting at 30 mg once daily.
- There is no evidence that doses greater than 60 mg/day confers additional benefit, while some adverse reactions were observed to be dose-dependent.
- Discontinuing Cymbalta: A gradual dose reduction is recommended to avoid discontinuation symptoms. (
5.6 )2.1 Initial Treatment
Major Depressive Disorder ‚ÄĒ Cymbalta should be administered at a total dose of 40¬†mg/day (given as 20¬†mg¬†twice daily) to 60¬†mg/day (given either once daily or as 30¬†mg¬†twice daily). For some patients, it may be desirable to start at 30¬†mg once¬†daily for 1¬†week, to allow patients to adjust to the medication before increasing to 60¬†mg once¬†daily. While a 120 mg/day dose was shown to be effective, there is no evidence that doses greater than 60¬†mg/day confer any additional benefits. The safety of doses above 120¬†mg/day has not been adequately evaluated [see Clinical Studies (14.1)].
Generalized Anxiety Disorder ‚ÄĒ For most patients, the recommended starting dose for Cymbalta is 60¬†mg administered once¬†daily. For some patients, it may be desirable to start at 30¬†mg once¬†daily for 1¬†week, to allow patients to adjust to the medication before increasing to 60¬†mg once¬†daily. While a 120 mg once daily dose was shown to be effective, there is no evidence that doses greater than 60¬†mg/day confer additional benefit. Nevertheless, if a decision is made to increase the dose beyond 60¬†mg once daily, dose increases should be in increments of 30 mg once daily. The safety of doses above 120 mg once daily has not been adequately evaluated [see Clinical Studies (14.2)].
Diabetic Peripheral Neuropathic Pain ‚ÄĒ The recommended dose for Cymbalta is 60 mg administered once daily. There is no evidence that doses higher than 60¬†mg confer additional significant benefit and the higher dose is clearly less well tolerated [see Clinical Studies (14.3)]. For patients for whom tolerability is a concern, a lower starting dose may be considered.
Since diabetes is frequently complicated by renal disease, a lower starting dose and gradual increase in dose should be considered for patients with renal impairment [see Clinical Pharmacology (12.3) and Dosage and Administration (2.3)].
Fibromyalgia ‚ÄĒ The recommended dose for Cymbalta is 60 mg administered once daily. Treatment should begin at 30 mg once daily for 1 week, to allow patients to adjust to the medication before increasing to 60 mg once daily. Some patients may respond to the starting dose. There is no evidence that doses greater than 60 mg/day confer additional benefit, even in patients who do not respond to a 60 mg dose, and higher doses are associated with a higher rate of adverse reactions [see Clinical Studies (14.4)].
Chronic Musculoskeletal Pain ‚ÄĒ The recommended dose for Cymbalta is 60 mg once daily. Dosing may be started at 30 mg for one week, to allow patients to adjust to the medication before increasing to 60¬†mg once¬†daily. There is no evidence that higher doses confer additional benefit, even in patients who do not respond to a 60 mg dose, and higher doses are associated with a higher rate of adverse reactions [see Clinical Studies (14.5)].
2.2 Maintenance/Continuation/Extended Treatment
Major Depressive Disorder ‚ÄĒ It is generally agreed that acute episodes of major depression require several¬†months or longer of sustained pharmacologic therapy. Maintenance of efficacy in MDD was demonstrated with Cymbalta as monotherapy. Cymbalta should be administered at a total dose of 60¬†mg once¬†daily. Patients should be periodically reassessed to determine the need for maintenance treatment and the appropriate dose for such treatment [see Clinical Studies (14.1)].
Generalized Anxiety Disorder ‚ÄĒ It is generally agreed that episodes of generalized anxiety disorder require several months or longer of sustained pharmacological therapy. Maintenance of efficacy in GAD was demonstrated with Cymbalta as monotherapy. Cymbalta should be administered in a dose range of 60-120 mg once daily. Patients should be periodically reassessed to determine the continued need for maintenance treatment and the appropriate dose for such treatment [see Clinical Studies (14.2)].
Diabetic Peripheral Neuropathic Pain ‚ÄĒ As the progression of diabetic peripheral neuropathy is highly variable and management of pain is empirical, the effectiveness of Cymbalta must be assessed individually. Efficacy beyond 12 weeks has not been systematically studied in placebo-controlled trials.
Fibromyalgia ‚ÄĒ Fibromyalgia is recognized as a chronic condition. The efficacy of Cymbalta in the management of fibromyalgia has been demonstrated in placebo-controlled studies up to 3 months. The efficacy of Cymbalta was not demonstrated in longer studies; however, continued treatment should be based on individual patient response.
Chronic Musculoskeletal Pain ‚ÄĒ The efficacy of Cymbalta has not been established in placebo-controlled studies beyond 13 weeks.
2.3 Dosing in Special Populations
Hepatic Insufficiency ‚ÄĒ It is recommended that Cymbalta should ordinarily not be administered to patients with any hepatic insufficiency [see Warnings and Precautions (5.12) and Use in Specific Populations (8.9)].
Severe Renal Impairment ‚ÄĒ Cymbalta is not recommended for patients with end-stage renal disease or severe renal impairment (estimated creatinine clearance <30 mL/min) [see Warnings and Precautions (5.12) and Use in Specific Populations (8.10)].
Elderly Patients ‚ÄĒ No dose adjustment is recommended for elderly patients on the basis of age. As with any drug, caution should be exercised in treating the elderly. When individualizing the dosage in elderly patients, extra care should be taken when increasing the dose [see Use in Specific Populations (8.5)].
Pregnant Women ‚ÄĒ There are no adequate and well-controlled studies in pregnant women; therefore, Cymbalta should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus [see Use in Specific Populations (8.1)].
Lilly maintains a pregnancy registry to monitor the pregnancy outcomes of women exposed to Cymbalta while pregnant. Healthcare providers are encouraged to register any patient who is exposed to Cymbalta during pregnancy by calling the Cymbalta Pregnancy Registry at 1-866-814-6975 or by visiting www.cymbaltapregnancyregistry.com
Nursing Mothers ‚ÄĒ Because the safety of duloxetine in infants is not known, nursing while on Cymbalta is not recommended [see Use in Specific Populations (8.3)].
2.4 Discontinuing Cymbalta
Symptoms associated with discontinuation of Cymbalta and other SSRIs and SNRIs have been reported. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible [see Warnings and Precautions (5.6)].
2.5 Switching Patients to or from a Monoamine Oxidase Inhibitor
At least 14 days should elapse between discontinuation of an MAOI and initiation of therapy with Cymbalta. In addition, at least 5 days should be allowed after stopping Cymbalta before starting an MAOI [see Contraindications (4.1) and Warnings and Precautions (5.4)].
3 Dosage Forms And Strengths
Cymbalta is available as delayed release capsules:
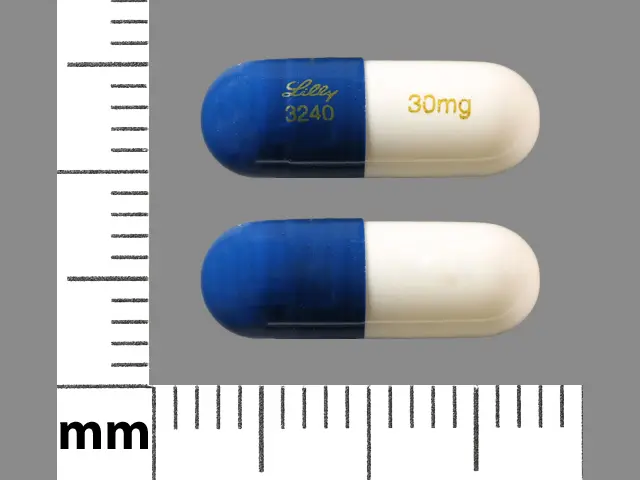
20 mg opaque green capsules imprinted with ‚ÄúLilly 3235 20mg‚ÄĚ 30 mg opaque white and blue capsules imprinted with ‚ÄúLilly 3240 30mg‚ÄĚ 60 mg opaque green and blue capsules imprinted with ‚ÄúLilly 3237 60mg‚ÄĚ 60 mg opaque green and blue capsules imprinted with ‚ÄúLilly 3270 60mg‚ÄĚ
20 mg, 30 mg, and 60 mg capsules (3 )
4 Contraindications
- Use of a monoamine oxidase inhibitor concomitantly or in close temporal proximity. (
4.1 )- Use in patients with uncontrolled narrow-angle glaucoma. (
4.2 )4.1 Monoamine Oxidase Inhibitors
Concomitant use in patients taking monoamine oxidase inhibitors (MAOIs) is contraindicated due to the risk of serious, sometimes fatal, drug interactions with serotonergic drugs. These interactions may include hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma. These reactions have also been reported in patients who have recently discontinued serotonin reuptake inhibitors and are then started on an MAOI. Some cases presented with features resembling neuroleptic malignant syndrome [see Dosage and Administration (2.5) and Warnings and Precautions (5.4)].
4.2 Uncontrolled Narrow-Angle Glaucoma
In clinical trials, Cymbalta use was associated with an increased risk of mydriasis; therefore, its use should be avoided in patients with uncontrolled narrow-angle glaucoma [see Warnings and Precautions (5.12)].
5 Warnings And Precautions
- Suicidality: Monitor for clinical worsening and suicide risk. (
5.1 )- Hepatotoxicity: Hepatic failure, sometimes fatal, has been reported in patients treated with Cymbalta. Cymbalta should be discontinued in patients who develop jaundice or other evidence of clinically significant liver dysfunction and should not be resumed unless another cause can be established. Cymbalta should not be prescribed to patients with substantial alcohol use or evidence of chronic liver disease. (
5.2 )- Orthostatic Hypotension and Syncope: Cases have been reported with duloxetine therapy. (
5.3 )- Serotonin Syndrome, or Neuroleptic Malignant Syndrome (NMS)-like reactions: Serotonin syndrome or NMS-like reactions have been reported with SSRIs and SNRIs. Discontinue Cymbalta and initiate supportive treatment. (
5.4 ,7.14 )- Abnormal Bleeding: Cymbalta may increase the risk of bleeding events. Patients should be cautioned about the risk of bleeding associated with the concomitant use of duloxetine and NSAIDs, aspirin, or other drugs that affect coagulation. (
5.5 ,7.4 )- Discontinuation: May result in symptoms, including dizziness, nausea, headache, paresthesia, fatigue, vomiting, irritability, insomnia, diarrhea, anxiety, and hyperhidrosis. (
5.6 )- Activation of mania or hypomania has occurred. (
5.7 )- Seizures: Prescribe with care in patients with a history of seizure disorder. (
5.8 )- Blood Pressure: Monitor blood pressure prior to initiating treatment and periodically throughout treatment. (
5.9 )- Inhibitors of CYP1A2 or Thioridazine: Should not administer with Cymbalta. (
5.10 )- Hyponatremia: Cases of hyponatremia have been reported. (
5.11 )- Hepatic Insufficiency and Severe Renal Impairment: Should ordinarily not be administered to these patients. (
5.12 )- Controlled Narrow-Angle Glaucoma: Use cautiously in these patients. (
5.12 )- Glucose Control in Diabetes: In diabetic peripheral neuropathic pain patients, small increases in fasting blood glucose, HbA1c, and total cholesterol have been observed. (
5.12 )- Conditions that Slow Gastric Emptying: Use cautiously in these patients. (
5.12 )- Urinary Hesitation and Retention. (
5.13 )5.1 Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment.
Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk of differences (drug vs placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
Table 1 Age Range Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated Increases Compared to Placebo <18 14 additional cases 18-24 5 additional cases Decreases Compared to Placebo 25-64 1 fewer case ‚Č•65 6 fewer cases
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
If the decision has been made to discontinue treatment, medication should be tapered, as rapidly as is feasible, but with recognition that discontinuation can be associated with certain symptoms [see Dosage and Administration (2.4) and Warnings and Precautions (5.6) for descriptions of the risks of discontinuation of Cymbalta].
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for Cymbalta should be written for the smallest quantity of capsules consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder ‚ÄĒ A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that Cymbalta (duloxetine) is not approved for use in treating bipolar depression.
5.2 Hepatotoxicity
There have been reports of hepatic failure, sometimes fatal, in patients treated with Cymbalta. These cases have presented as hepatitis with abdominal pain, hepatomegaly, and elevation of transaminase levels to more than twenty times the upper limit of normal with or without jaundice, reflecting a mixed or hepatocellular pattern of liver injury. Cymbalta should be discontinued in patients who develop jaundice or other evidence of clinically significant liver dysfunction and should not be resumed unless another cause can be established.
Cases of cholestatic jaundice with minimal elevation of transaminase levels have also been reported. Other postmarketing reports indicate that elevated transaminases, bilirubin, and alkaline phosphatase have occurred in patients with chronic liver disease or cirrhosis.
Cymbalta increased the risk of elevation of serum transaminase levels in development program clinical trials. Liver transaminase elevations resulted in the discontinuation of 0.3% (91/31,268) of Cymbalta-treated patients. In most patients, the median time to detection of the transaminase elevation was about two months. In placebo-controlled trials in any indication, for patients with normal and abnormal baseline ALT values, elevation of ALT >3 times the upper limit of normal occurred in 1.32% (135/10,244) of Cymbalta-treated patients compared to 0.49% (37/7556) of placebo-treated patients. In placebo-controlled studies using a fixed dose design, there was evidence of a dose response relationship for ALT and AST elevation of >3 times the upper limit of normal and >5 times the upper limit of normal, respectively.
Because it is possible that duloxetine and alcohol may interact to cause liver injury or that duloxetine may aggravate pre-existing liver disease, Cymbalta should not be prescribed to patients with substantial alcohol use or evidence of chronic liver disease.
5.3 Orthostatic Hypotension and Syncope
Orthostatic hypotension and syncope have been reported with therapeutic doses of duloxetine. Syncope and orthostatic hypotension tend to occur within the first week of therapy but can occur at any time during duloxetine treatment, particularly after dose increases. The risk of blood pressure decreases may be greater in patients taking concomitant medications that induce orthostatic hypotension (such as antihypertensives) or are potent CYP1A2 inhibitors [see Warnings and Precautions (5.10) and Drug Interactions (7.1)] and in patients taking duloxetine at doses above 60 mg daily. Consideration should be given to discontinuing duloxetine in patients who experience symptomatic orthostatic hypotension and/or syncope during duloxetine therapy.
5.4 Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
The development of a potentially life-threatening serotonin syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions have been reported with SNRIs and SSRIs alone, including Cymbalta treatment, but particularly with concomitant use of serotonergic drugs (including triptans) with drugs which impair metabolism of serotonin (including MAOIs), or with antipsychotics or other dopamine antagonists. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Serotonin syndrome, in its most severe form can resemble neuroleptic malignant syndrome, which includes hyperthermia, muscle rigidity, autonomic instability with possible rapid fluctuation of vital signs, and mental status changes. Patients should be monitored for the emergence of serotonin syndrome or NMS-like signs and symptoms.
The concomitant use of Cymbalta with MAOIs intended to treat depression is contraindicated [see Contraindications (4.1)].
If concomitant treatment of Cymbalta with a 5-hydroxytryptamine receptor agonist (triptan) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases [see Drug Interactions (7.15)].
The concomitant use of Cymbalta with serotonin precursors (such as tryptophan) is not recommended [see Drug Interactions (7.14)].
Treatment with duloxetine and any concomitant serotonergic or antidopaminergic agents, including antipsychotics, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
5.5 Abnormal Bleeding
SSRIs and SNRIs, including duloxetine, may increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs, warfarin, and other anti-coagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to SSRIs and SNRIs use have ranged from ecchymoses, hematomas, epistaxis, and petechiae to life-threatening hemorrhages.
Patients should be cautioned about the risk of bleeding associated with the concomitant use of duloxetine and NSAIDs, aspirin, or other drugs that affect coagulation.
5.6 Discontinuation of Treatment with Cymbalta
Discontinuation symptoms have been systematically evaluated in patients taking duloxetine. Following abrupt or tapered discontinuation in placebo-controlled clinical trials, the following symptoms occurred at 1% or greater and at a significantly higher rate in duloxetine-treated patients compared to those discontinuing from placebo: dizziness, nausea, headache, paresthesia, fatigue, vomiting, irritability, insomnia, diarrhea, anxiety, and hyperhidrosis.
During marketing of other SSRIs and SNRIs (serotonin and norepinephrine reuptake inhibitors), there have been spontaneous reports of adverse events occurring upon discontinuation of these drugs, particularly when abrupt, including the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesias such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. Although these events are generally self-limiting, some have been reported to be severe.
Patients should be monitored for these symptoms when discontinuing treatment with Cymbalta. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate [see Dosage and Administration (2.4)].
5.7 Activation of Mania/Hypomania
In placebo-controlled trials in patients with major depressive disorder, activation of mania or hypomania was reported in 0.1% (3/3007) of duloxetine-treated patients and 0.1% (1/1883) of placebo-treated patients. No activation of mania or hypomania was reported in GAD, fibromyalgia, or chronic musculoskeletal pain placebo-controlled trials. Activation of mania or hypomania has been reported in a small proportion of patients with mood disorders who were treated with other marketed drugs effective in the treatment of major depressive disorder. As with these other agents, Cymbalta should be used cautiously in patients with a history of mania.
5.8 Seizures
Duloxetine has not been systematically evaluated in patients with a seizure disorder, and such patients were excluded from clinical studies. In placebo-controlled clinical trials, seizures/convulsions occurred in 0.03% (3/11,305) of patients treated with duloxetine and 0.01% (1/8224) of patients treated with placebo. Cymbalta should be prescribed with care in patients with a history of a seizure disorder.
5.9 Effect on Blood Pressure
In placebo-controlled clinical trials across indications from baseline to endpoint, duloxetine treatment was associated with mean increases of 0.5 mm Hg in systolic blood pressure and 0.8 mm Hg in diastolic blood pressure compared to mean decreases of 0.6 mm Hg systolic and 0.3 mm Hg diastolic in placebo-treated patients. There was no significant difference in the frequency of sustained (3 consecutive visits) elevated blood pressure. In a clinical pharmacology study designed to evaluate the effects of duloxetine on various parameters, including blood pressure at supratherapeutic doses with an accelerated dose titration, there was evidence of increases in supine blood pressure at doses up to 200 mg twice daily. At the highest 200 mg twice daily dose, the increase in mean pulse rate was 5.0 to 6.8 beats and increases in mean blood pressure were 4.7 to 6.8 mm Hg (systolic) and 4.5 to 7 mm Hg (diastolic) up to 12 hours after dosing.
Blood pressure should be measured prior to initiating treatment and periodically measured throughout treatment [see Adverse Reactions (6.7)].
5.10 Clinically Important Drug Interactions
Both CYP1A2 and CYP2D6 are responsible for duloxetine metabolism.
Potential for Other Drugs to Affect Cymbalta
CYP1A2 Inhibitors ‚ÄĒ Co-administration of Cymbalta with potent CYP1A2 inhibitors should be avoided [see Drug Interactions (7.1)].
CYP2D6 Inhibitors ‚ÄĒ Because CYP2D6 is involved in duloxetine metabolism, concomitant use of duloxetine with potent inhibitors of CYP2D6 would be expected to, and does, result in higher concentrations (on average of 60%) of duloxetine [see Drug Interactions (7.2)].
Potential for Cymbalta to Affect Other Drugs
Drugs Metabolized by CYP2D6 ‚ÄĒ Co-administration of Cymbalta with drugs that are extensively metabolized by CYP2D6 and that have a narrow therapeutic index, including certain antidepressants (tricyclic antidepressants [TCAs], such as nortriptyline, amitriptyline, and imipramine), phenothiazines and Type 1C antiarrhythmics (e.g., propafenone, flecainide), should be approached with caution. Plasma TCA concentrations may need to be monitored and the dose of the TCA may need to be reduced if a TCA is co-administered with Cymbalta. Because of the risk of serious ventricular arrhythmias and sudden death potentially associated with elevated plasma levels of thioridazine, Cymbalta and thioridazine should not be co-administered [see Drug Interactions (7.9)].
Other Clinically Important Drug Interactions
Alcohol ‚ÄĒ Use of Cymbalta concomitantly with heavy alcohol intake may be associated with severe liver injury. For this reason, Cymbalta should not be prescribed for patients with substantial alcohol use [see Warnings and Precautions (5.2) and Drug Interactions (7.16)].
CNS Acting Drugs ‚ÄĒ Given the primary CNS effects of Cymbalta, it should be used with caution when it is taken in combination with or substituted for other centrally acting drugs, including those with a similar mechanism of action [see Warnings and Precautions (5.10) and Drug Interactions (7.17)].
5.11 Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs and SNRIs, including Cymbalta. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Cases with serum sodium lower than 110 mmol/L have been reported and appeared to be reversible when Cymbalta was discontinued. Elderly patients may be at greater risk of developing hyponatremia with SSRIs and SNRIs. Also, patients taking diuretics or who are otherwise volume depleted may be at greater risk [see Use in Specific Populations (8.5)]. Discontinuation of Cymbalta should be considered in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. More severe and/or acute cases have been associated with hallucination, syncope, seizure, coma, respiratory arrest, and death.
5.12 Use in Patients with Concomitant Illness
Clinical experience with Cymbalta in patients with concomitant systemic illnesses is limited. There is no information on the effect that alterations in gastric motility may have on the stability of Cymbalta's enteric coating. In extremely acidic conditions, Cymbalta, unprotected by the enteric coating, may undergo hydrolysis to form naphthol. Caution is advised in using Cymbalta in patients with conditions that may slow gastric emptying (e.g., some diabetics).
Cymbalta has not been systematically evaluated in patients with a recent history of myocardial infarction or unstable coronary artery disease. Patients with these diagnoses were generally excluded from clinical studies during the product's premarketing testing.
Hepatic Insufficiency ‚ÄĒ Cymbalta should ordinarily not be used in patients with hepatic insufficiency [see Dosage and Administration (2.3), Warnings and Precautions (5.2), and Use in Specific Populations (8.9)].
Severe Renal Impairment ‚ÄĒ Cymbalta should ordinarily not be used in patients with end-stage renal disease or severe renal impairment (creatinine clearance <30¬†mL/min). Increased plasma concentration of duloxetine, and especially of its metabolites, occur in patients with end-stage renal disease (requiring dialysis) [see Dosage and Administration (2.3) and Use in Specific Populations (8.10)].
Controlled Narrow-Angle Glaucoma ‚ÄĒ In clinical trials, Cymbalta was associated with an increased risk of mydriasis; therefore, it should be used cautiously in patients with controlled narrow-angle glaucoma [see Contraindications (4.2)].
Glycemic Control in Patients with Diabetes ‚ÄĒ As observed in DPNP trials, Cymbalta treatment worsens glycemic control in some patients with diabetes. In three clinical trials of Cymbalta for the management of neuropathic pain associated with diabetic peripheral neuropathy, the mean duration of diabetes was approximately 12 years, the mean baseline fasting blood glucose was 176 mg/dL, and the mean baseline hemoglobin A1c (HbA1c) was 7.8%. In the 12-week acute treatment phase of these studies, Cymbalta was associated with a small increase in mean fasting blood glucose as compared to placebo. In the extension phase of these studies, which lasted up to 52 weeks, mean fasting blood glucose increased by 12 mg/dL in the Cymbalta group and decreased by 11.5 mg/dL in the routine care group. HbA1c increased by 0.5% in the Cymbalta and by 0.2% in the routine care groups.
5.13 Urinary Hesitation and Retention
Cymbalta is in a class of drugs known to affect urethral resistance. If symptoms of urinary hesitation develop during treatment with Cymbalta, consideration should be given to the possibility that they might be drug-related.
In post marketing experience, cases of urinary retention have been observed. In some instances of urinary retention associated with duloxetine use, hospitalization and/or catheterization has been needed.
5.14 Laboratory Tests
No specific laboratory tests are recommended.
6 Adverse Reactions
- Most common adverse reactions (‚Č•5% and at least twice the incidence of placebo patients): nausea, dry mouth, somnolence, fatigue, constipation, decreased appetite, and hyperhidrosis. (
6.3 )
To report SUSPECTED ADVERSE REACTIONS, contact Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
6.1 Clinical Trial Data Sources
The data described below reflect exposure to duloxetine in placebo-controlled trials for MDD (N=3007), GAD (N=910), OA (N=239), CLBP (N=600), DPNP (N=906), and FM (N=1139). The population studied was 17 to 91 years of age; 65.0%, 62.5%, 61.5%, 42.9%, and 94.4% female; and 82.7%, 81.2%, 86.2 %, 74.0%, and 85.7% Caucasian for MDD, GAD, OA and CLBP, DPNP, and FM, respectively. Most patients received doses of a total of 60 to 120 mg per day [see Clinical Studies (14)].
The stated frequencies of adverse reactions represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse reaction of the type uled. A reaction was considered treatment-emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation. Reactions reported during the studies were not necessarily caused by the therapy, and the frequencies do not reflect investigator impression (assessment) of causality.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Adverse Reactions Reported as Reasons for Discontinuation of Treatment in Placebo-Controlled Trials
Major Depressive Disorder ‚ÄĒ Approximately 8.5% (256/3007) of the patients who received duloxetine in placebo-controlled trials for MDD discontinued treatment due to an adverse reaction, compared with 4.8% (91/1883) of the patients receiving placebo. Nausea (duloxetine 1.2%, placebo 0.4%) was the only common adverse reaction reported as a reason for discontinuation and considered to be drug-related (i.e., discontinuation occurring in at least 1% of the duloxetine-treated patients and at a rate of at least twice that of placebo).
Generalized Anxiety Disorder ‚ÄĒ Approximately 13.8% (126/910) of the patients who received duloxetine in placebo-controlled trials for GAD discontinued treatment due to an adverse reaction, compared with 5.3% (35/665) for placebo. Common adverse reactions reported as a reason for discontinuation and considered to be drug-related (as defined above) included nausea (duloxetine 3.3%, placebo 0.5%), dizziness (duloxetine 1.0%, placebo 0.5%), and vomiting (duloxetine 1.0%, placebo 0.2%).
Diabetic Peripheral Neuropathic Pain ‚ÄĒ Approximately 12.9% (117/906) of the patients who received duloxetine in placebo-controlled trials for DPNP discontinued treatment due to an adverse reaction, compared with 5.1% (23/448) for placebo. Common adverse reactions reported as a reason for discontinuation and considered to be drug-related (as defined above) included nausea (duloxetine 3.5%, placebo 0.7%), dizziness (duloxetine 1.2%, placebo 0.4%), and somnolence (duloxetine 1.1%, placebo 0.0%).
Fibromyalgia ‚ÄĒ Approximately 18.7% (213/1139) of the patients who received duloxetine in 3 to 6 month placebo-controlled trials for FM discontinued treatment due to an adverse reaction, compared with 10.8% (87/802) for placebo. Common adverse reactions reported as a reason for discontinuation and considered to be drug-related (as defined above) included nausea (duloxetine 2.1%, placebo 0.5%), headache (duloxetine 1.2%, placebo 0.2%), somnolence (duloxetine 1.2%, placebo 0.0%), and fatigue (duloxetine 1.1%, placebo 0.1%).
Chronic Pain due to Osteoarthritis ‚ÄĒ Approximately 16.3% (39/239) of the patients who received duloxetine in 13-week, placebo-controlled trials for chronic pain due to OA discontinued treatment due to an adverse reaction, compared with 5.6% (14/248) for placebo. Common adverse reactions reported as a reason for discontinuation and considered to be drug-related (as defined above) included nausea (duloxetine 2.9%, placebo 0.8%) and asthenia (duloxetine 1.3%, placebo 0.0%).
Chronic Low Back Pain ‚ÄĒ Approximately 16.5% (99/600) of the patients who received duloxetine in 13-week, placebo-controlled trials for CLBP discontinued treatment due to an adverse reaction, compared with 6.3% (28/441) for placebo. Common adverse reactions reported as a reason for discontinuation and considered to be drug-related (as defined above) included nausea (duloxetine 3.0%, placebo 0.7%), and somnolence (duloxetine 1.0%, placebo 0.0%).
6.3 Most Common Adverse Reactions
Pooled Trials for all Approved Indications ‚ÄĒ The most commonly observed adverse reactions in Cymbalta-treated patients (incidence of at least 5% and at least twice the incidence in placebo patients) were nausea, dry mouth, somnolence, fatigue, constipation, decreased appetite, and hyperhidrosis.
Diabetic Peripheral Neuropathic Pain ‚ÄĒ The most commonly observed adverse reactions in Cymbalta-treated patients (as defined above) were nausea, somnolence, decreased appetite, constipation, hyperhidrosis, and dry mouth.
Fibromyalgia ‚ÄĒ The most commonly observed adverse reactions in Cymbalta-treated patients (as defined above) were nausea, dry mouth, constipation, somnolence, decreased appetite, hyperhidrosis, and agitation.
Chronic Pain due to Osteoarthritis ‚ÄĒ The most commonly observed adverse reactions in Cymbalta-treated patients (as defined above) were nausea, fatigue, and constipation.
Chronic Low Back Pain ‚ÄĒ The most commonly observed adverse reactions in Cymbalta-treated patients (as defined above) were nausea, dry mouth, insomnia, somnolence, constipation, dizziness, and fatigue.
6.4 Adverse Reactions Occurring at an Incidence of 5% or More Among Duloxetine-Treated Patients in Placebo-Controlled Trials
Table 2 gives the incidence of treatment-emergent adverse reactions in placebo-controlled trials for approved indications that occurred in 5% or more of patients treated with duloxetine and with an incidence greater than placebo.
Table 2: Treatment-Emergent Adverse Reactions: Incidence of 5% or More in Placebo-Controlled Trials of Approved Indicationsa a The inclusion of an event in the table is determined based on the percentages before rounding; however, the percentages displayed in the table are rounded to the nearest integer.
b Also includes asthenia.
c Events for which there was a significant dose-dependent relationship in fixed-dose studies, excluding three MDD studies which did not have a placebo lead-in period or dose titration.
d Also includes middle insomnia, early morning awakening, and initial insomnia.
e Also includes hypersomnia and sedation.
f Also includes anorexia.
g Also includes abdominal discomfort,abdominal pain lower, abdominal pain upper, abdominal tenderness, and gastrointestinal pain.
Adverse Reaction Percentage of Patients Reporting Reaction Cymbalta(N=6801) Placebo(N=4487) Nausea 24 8 Headache 14 13 Dry mouth 13 5 Fatigueb,c 10 5 Somnolencec,e 10 3 Insomniad 10 6 Constipationc 10 4 Dizzinessc 10 5 Diarrhea 9 6 Decreased appetitec,f 7 2 Hyperhidrosis 6 2 Abdominal paing 5 5 6.5 Adverse Reactions Occurring at an Incidence of 2% or More Among Duloxetine-Treated Patients in Placebo-Controlled Trials
Pooled MDD and GAD Trials ‚ÄĒ Table 3 gives the incidence of treatment-emergent adverse reactions in MDD and GAD placebo-controlled trials for approved indications that occurred in 2% or more of patients treated with duloxetine and with an incidence greater than placebo.
Table 3: Treatment-Emergent Adverse Reactions: Incidence of 2% or More in MDD and GAD Placebo-Controlled Trialsa a The inclusion of an event in the table is determined based on the percentages before rounding; however, the percentages displayed in the table are rounded to the nearest integer.
b Events for which there was a significant dose-dependent relationship in fixed-dose studies, excluding three MDD studies which did not have a placebo lead-in period or dose titration.
c Also includes abdominal pain upper, abdominal pain lower, abdominal tenderness, abdominal discomfort, and gastrointestinal pain
d Also includes asthenia
e Also includes anorexia
f Also includes hypersomnia and sedation
g Also includes middle insomnia, early morning awakening, and initial insomnia
h Also includes feeling jittery, nervousness, restlessness, tension, and psychomotor hyperactivity
i Also includes loss of libido
j Also includes anorgasmia
k Also includes nightmare
l Male patients only
m Also includes ejaculation failure and ejaculation dysfunction
n Also includes hot flush
Percentage of Patients Reporting Reaction System Organ Class / Adverse Reaction Cymbalta(N=3917) Placebo(N=2548) Cardiac Disorders Palpitations 2 2 Eye Disorders Vision blurred 3 1 Gastrointestinal Disorders Nausea 24 8 Dry mouth 14 6 Constipationb 9 4 Diarrhea 9 7 Abdominal painc 5 5 Vomiting 4 2 General Disorders and Administration Site Conditions Fatigued 9 5 Metabolism and Nutrition Disorders Decreased appetiteb,e 7 2 Nervous System Disorders Dizzinessb 10 6 Somnolencef 9 3 Tremor 3 <1 Psychiatric Disorders Insomniag 10 6 Agitationh 5 3 Libido decreasedi 3 1 Anxiety 3 2 Orgasm abnormalb,j 2 <1 Abnormal dreamsk 2 1 Reproductive System and Breast Disorders Erectile dysfunctionl 4 1 Ejaculation delayedb,l 3 <1 Ejaculation disorderl,m 2 <1 Respiratory, Thoracic, and Mediastinal Disorders Yawning 2 <1 Skin and Subcutaneous Tissue Disorders Hyperhidrosis 6 2 Vascular Disorders Flushingn 2 1
DPNP, FM, OA, and CLBP ‚ÄĒ Table 4 gives the incidence of treatment-emergent adverse events that occurred in 2% or more of patients treated with Cymbalta (determined prior to rounding) in the premarketing acute phase of DPNP, FM, OA, and CLBP placebo-controlled trials and with an incidence greater than placebo.
Table 4: Treatment-Emergent Adverse Reactions: Incidence of 2% or More in DPNP, FM, OA, and CLBP Placebo-Controlled Trialsa a The inclusion of an event in the table is determined based on the percentages before rounding; however, the percentages displayed in the table are rounded to the nearest integer.
b Incidence of 120 mg/day is significantly greater than the incidence for 60 mg/day.
c Also includes abdominal discomfort, abdominal pain lower, abdominal pain upper, abdominal tenderness and gastrointestinal pain
d Also includes stomach discomfort
e Also includes asthenia
f Also includes anorexia
g Also includes myalgia and neck pain
h Also includes hypersomnia and sedation
i Also includes hypoaesthesia, hypoaesthesia facial, genital hypoaesthesia and paraesthesia oral
j Also includes middle insomnia, early morning awakening, and initial insomnia
k Also includes feeling jittery, nervousness, restlessness, tension and psychomotor hyperactivity
l Male patients only
m Male patients only. Also includes ejaculation failure
n Also includes hot flush
System Organ Class / Adverse Reaction Percentage of Patients Reporting Reaction Cymbalta(N=2884) Placebo(N=1939) Gastrointestinal Disorders Nausea 24 8 Dry Mouthb 11 3 Constipationb 11 3 Diarrhea 9 5 Abdominal Painc 5 4 Vomiting 4 2 Dyspepsiad 2 1 General Disorders and Administration Site Conditions Fatiguee 11 5 Infections and Infestations Nasopharyngitis 4 4 Upper Respiratory Tract Infection 4 4 Influenza 2 2 Metabolism and Nutrition Disorders Decreased Appetiteb,f 9 1 Musculoskeletal and Connective Tissue Musculoskeletal Painb,g 4 4 Muscle Spasms 3 2 Back pain 3 3 Nervous System Disorders Headache 13 9 Somnolenceb,h 12 3 Dizziness 10 5 Paraesthesiai 2 2 Tremorb 2 <1 Psychiatric Disorders Insomniab,j 11 6 Agitationk 4 <1 Reproductive System and Breast Disorders Erectile Dysfunctionb,l 4 <1 Ejaculation Disorderm 2 <1 Respiratory, Thoracic, and Mediastinal Disorders Cough 3 2 Oropharyngeal Painb 2 2 Skin and Subcutaneous Tissue Disorders Hyperhidrosis 6 1 Vascular Disorders Flushingn 3 <1 6.6 Effects on Male and Female Sexual Function
Changes in sexual desire, sexual performance and sexual satisfaction often occur as manifestations of psychiatric disorders or diabetes, but they may also be a consequence of pharmacologic treatment. Because adverse sexual reactions are presumed to be voluntarily underreported, the Arizona Sexual Experience Scale (ASEX), a validated measure designed to identify sexual side effects, was used prospectively in 4 MDD placebo-controlled trials. In these trials, as shown in Table 5 below, patients treated with Cymbalta experienced significantly more sexual dysfunction, as measured by the total score on the ASEX, than did patients treated with placebo. Gender analysis showed that this difference occurred only in males. Males treated with Cymbalta experienced more difficulty with ability to reach orgasm (ASEX Item 4) than males treated with placebo. Females did not experience more sexual dysfunction on Cymbalta than on placebo as measured by ASEX total score. Negative numbers signify an improvement from a baseline level of dysfunction, which is commonly seen in depressed patients. Physicians should routinely inquire about possible sexual side effects.
Table 5: Mean Change in ASEX Scores by Gender in MDD Placebo-Controlled Trials a n=Number of patients with non-missing change score for ASEX total
b p=0.013 versus placebo
c p<0.001 versus placebo
Male Patients a Female Patients a Cymbalta(n=175) Placebo(n=83) Cymbalta(n=241) Placebo(n=126) ASEX Total (Items 1-5) 0.56b -1.07 -1.15 -1.07 Item 1 ‚ÄĒ Sex drive -0.07 -0.12 -0.32 -0.24 Item 2 ‚ÄĒ Arousal 0.01 -0.26 -0.21 -0.18 Item 3 ‚ÄĒ Ability to achieve ¬†¬†erection¬†(men); Lubrication (women) 0.03 -0.25 -0.17 -0.18 Item 4 ‚ÄĒ Ease of reaching orgasm 0.40c -0.24 -0.09 -0.13 Item 5 ‚ÄĒ Orgasm satisfaction 0.09 -0.13 -0.11 -0.17 6.7 Vital Sign Changes
In placebo-controlled clinical trials across approved indications for change from baseline to endpoint, duloxetine treatment was associated with mean increases of 0.17 mm Hg in systolic blood pressure and 0.70 mm Hg in diastolic blood pressure compared to mean decreases of 1.16 mm Hg systolic and 0.59 mm Hg diastolic in placebo-treated patients. There was no significant difference in the frequency of sustained (3 consecutive visits) elevated blood pressure [see Warnings and Precautions (5.3 and 5.9)].
Duloxetine treatment, for up to 26 weeks in placebo-controlled trials across approved indications, typically caused a small increase in heart rate for change from baseline to endpoint compared to placebo of up to 1.36 beats per minute.
6.8 Weight Changes
In placebo-controlled clinical trials, MDD and GAD patients treated with Cymbalta for up to 10 weeks experienced a mean weight loss of approximately 0.5 kg, compared with a mean weight gain of approximately 0.2 kg in placebo-treated patients. In studies of DPNP, FM, OA, and CLBP, patients treated with Cymbalta for up to 26 weeks experienced a mean weight loss of approximately 0.6 kg compared with a mean weight gain of approximately 0.2 kg in placebo-treated patients. In one long-term fibromyalgia 60-week uncontrolled study, duloxetine patients had a mean weight increase of 0.7 kg. In one long-term CLBP 54-week study (13-week, placebo-controlled acute phase and 41-week, uncontrolled extension phase), duloxetine patients had a mean weight decrease of 0.6 kg in 13 weeks of acute phase compared to study entry, then a mean weight increase of 1.4 kg in 41 weeks of extension phase compared to end of acute phase.
6.9 Laboratory Changes
Cymbalta treatment in placebo-controlled clinical trials across approved indications was associated with small mean increases from baseline to endpoint in ALT, AST, CPK, alkaline phosphatase, and potassium; infrequent, modest, transient, abnormal values were observed for these analytes in Cymbalta-treated patients when compared with placebo-treated patients [see Warnings and Precautions (5.2)].
6.10 Electrocardiogram Changes
Electrocardiograms were obtained from duloxetine-treated patients and placebo-treated patients in clinical trials lasting up to 13 weeks. No clinically significant differences were observed for QTc, QT, PR, and QRS intervals between duloxetine-treated and placebo-treated patients. There were no differences in clinically meaningful QTcF elevations between duloxetine and placebo. In a positive-controlled study in healthy volunteers using duloxetine up to 200 mg twice daily, no prolongation of the corrected QT interval was observed.
6.11 Other Adverse Reactions Observed During the Premarketing and Postmarketing Clinical Trial Evaluation of Duloxetine
Following is a ul of treatment-emergent adverse reactions reported by patients treated with duloxetine in clinical trials. In clinical trials of all indications, 31,268 patients were treated with duloxetine. Of these, 29.5% (9222) took duloxetine for at least 6 months, and 13.8% (4317) for at least one year. The following uling is not intended to include reactions (1) already uled in previous tables or elsewhere in labeling, (2) for which a drug cause was remote, (3) which were so general as to be uninformative, (4) which were not considered to have significant clinical implications, or (5) which occurred at a rate equal to or less than placebo.
Reactions are categorized by body system according to the following definitions: frequent adverse reactions are those occurring in at least 1/100 patients; infrequent adverse reactions are those occurring in 1/100 to 1/1000 patients; rare reactions are those occurring in fewer than 1/1000 patients.
Cardiac Disorders ‚ÄĒ Frequent:¬†palpitations; Infrequent: myocardial infarction and tachycardia.
Ear and Labyrinth Disorders ‚ÄĒ Frequent:¬†vertigo; Infrequent:¬†ear pain and tinnitus.
Endocrine Disorders ‚ÄĒ Infrequent:¬†hypothyroidism.
Eye Disorders ‚ÄĒ Frequent:¬†vision blurred; Infrequent:¬†diplopia, dry eye, and visual impairment.
Gastrointestinal Disorders ‚ÄĒ Frequent:¬†flatulence; Infrequent:¬†eructation, gastritis, gastrointestinal hemorrhage, halitosis, and stomatitis; Rare:¬†gastric ulcer.
General Disorders and Administration Site Conditions ‚ÄĒ Frequent:¬†chills/rigors; Infrequent:¬†falls, feeling abnormal, feeling hot and/or cold, malaise, and thirst; Rare: gait disturbance.
Infections and Infestations ‚ÄĒ Infrequent:¬†gastroenteritis and laryngitis.
Investigations ‚ÄĒ Frequent:¬†weight increased, weight decreased; Infrequent:¬†blood cholesterol increased.
Metabolism and Nutrition Disorders ‚ÄĒ Infrequent:¬†dehydration and hyperlipidemia; Rare:¬†dyslipidemia.
Musculoskeletal and Connective Tissue Disorders ‚ÄĒ Frequent:¬†musculoskeletal pain; Infrequent:¬†muscle tightness and muscle twitching.
Nervous System Disorders ‚ÄĒ Frequent:¬†dysgeusia, lethargy, and parasthesia/hypoesthesia; Infrequent:¬†disturbance in attention, dyskinesia, myoclonus, and poor quality sleep; Rare:¬†dysarthria.
Psychiatric Disorders ‚ÄĒ Frequent:¬†abnormal dreams and sleep disorder; Infrequent:¬†apathy, bruxism, disorientation/confusional state, irritability, mood swings, and suicide attempt; Rare:¬†completed suicide.
Renal and Urinary Disorders ‚ÄĒ Infrequent:¬†dysuria, micturition urgency, nocturia, polyuria, and urine odor abnormal.
Reproductive System and Breast Disorders ‚ÄĒ Frequent:¬†anorgasmia/orgasm abnormal; Infrequent:¬†menopausal symptoms and sexual dysfunction; Rare:¬†menstrual disorder.
Respiratory, Thoracic and Mediastinal Disorders ‚ÄĒ Frequent:¬†yawning; Infrequent:¬†throat tightness.
Skin and Subcutaneous Tissue Disorders ‚ÄĒ Frequent:¬†pruritus; Infrequent:¬†cold sweat, dermatitis contact, erythema, increased tendency to bruise, night sweats, and photosensitivity reaction; Rare:¬†ecchymosis.
Vascular Disorders ‚ÄĒ Frequent:¬†hot flush; Infrequent:¬†flushing, orthostatic hypotension, and peripheral coldness.
6.12 Postmarketing Spontaneous Reports
The following adverse reactions have been identified during postapproval use of Cymbalta. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions reported since market introduction that were temporally related to duloxetine therapy and not mentioned elsewhere in labeling include: anaphylactic reaction, aggression and anger (particularly early in treatment or after treatment discontinuation), angioneurotic edema, erythema multiforme, extrapyramidal disorder, galactorrhea, glaucoma, gynecological bleeding, hallucinations, hyperglycemia, hyperprolactinemia, hypersensitivity, hypertensive crisis, muscle spasm, rash, restless legs syndrome, seizures upon treatment discontinuation, supraventricular arrhythmia, tinnitus (upon treatment discontinuation), trismus, and urticaria.
Serious skin reactions including Stevens-Johnson Syndrome that have required drug discontinuation and/or hospitalization have been reported with duloxetine.
7 Drug Interactions
Both CYP1A2 and CYP2D6 are responsible for duloxetine metabolism.
- Potent inhibitors of CYP1A2 should be avoided. (
7.1 )- Potent inhibitors of CYP2D6 may increase duloxetine concentrations. (
7.2 )- Duloxetine is a moderate inhibitor of CYP2D6. (
7.9 )7.1 Inhibitors of CYP1A2
When duloxetine 60 mg was co-administered with fluvoxamine 100 mg, a potent CYP1A2 inhibitor, to male subjects (n=14) duloxetine AUC was increased approximately 6-fold, the Cmax was increased about 2.5-fold, and duloxetine t1/2 was increased approximately 3-fold. Other drugs that inhibit CYP1A2 metabolism include cimetidine and quinolone antimicrobials such as ciprofloxacin and enoxacin [see Warnings and Precautions (5.10)].
7.2 Inhibitors of CYP2D6
Concomitant use of duloxetine (40 mg once daily) with paroxetine (20 mg once daily) increased the concentration of duloxetine AUC by about 60%, and greater degrees of inhibition are expected with higher doses of paroxetine. Similar effects would be expected with other potent CYP2D6 inhibitors (e.g., fluoxetine, quinidine) [see Warnings and Precautions (5.10)].
7.3 Dual Inhibition of CYP1A2 and CYP2D6
Concomitant administration of duloxetine 40 mg twice daily with fluvoxamine 100 mg, a potent CYP1A2 inhibitor, to CYP2D6 poor metabolizer subjects (n=14) resulted in a 6-fold increase in duloxetine AUC and Cmax.
7.4 Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
Serotonin release by platelets plays an important role in hemostasis. Epidemiological studies of the case-control and cohort design that have demonstrated an association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding have also shown that concurrent use of an NSAID or aspirin may potentiate this risk of bleeding. Altered anticoagulant effects, including increased bleeding, have been reported when SSRIs or SNRIs are co-administered with warfarin. Concomitant administration of warfarin (2-9 mg once daily) under steady state conditions with duloxetine 60 or 120 mg once daily for up to 14 days in healthy subjects (n=15) did not significantly change INR from baseline (mean INR changes ranged from 0.05 to +0.07). The total warfarin (protein bound plus free drug) pharmacokinetics (AUCŌĄ,ss, Cmax,ss, or tmax,ss) for both R- and S-warfarin were not altered by duloxetine. Because of the potential effect of duloxetine on platelets, patients receiving warfarin therapy should be carefully monitored when duloxetine is initiated or discontinued [see Warnings and Precautions (5.5)].
7.5 Lorazepam
Under steady-state conditions for duloxetine (60 mg Q 12 hours) and lorazepam (2 mg Q 12 hours), the pharmacokinetics of duloxetine were not affected by co-administration.
7.6 Temazepam
Under steady-state conditions for duloxetine (20 mg qhs) and temazepam (30 mg qhs), the pharmacokinetics of duloxetine were not affected by co-administration.
7.7 Drugs that Affect Gastric Acidity
Cymbalta has an enteric coating that resists dissolution until reaching a segment of the gastrointestinal tract where the pH exceeds 5.5. In extremely acidic conditions, Cymbalta, unprotected by the enteric coating, may undergo hydrolysis to form naphthol. Caution is advised in using Cymbalta in patients with conditions that may slow gastric emptying (e.g., some diabetics). Drugs that raise the gastrointestinal pH may lead to an earlier release of duloxetine. However, co-administration of Cymbalta with aluminum- and magnesium-containing antacids (51 mEq) or Cymbalta with famotidine, had no significant effect on the rate or extent of duloxetine absorption after administration of a 40 mg oral dose. It is unknown whether the concomitant administration of proton pump inhibitors affects duloxetine absorption [see Warnings and Precautions (5.12)].
7.8 Drugs Metabolized by CYP1A2
In vitro drug interaction studies demonstrate that duloxetine does not induce CYP1A2 activity. Therefore, an increase in the metabolism of CYP1A2 substrates (e.g., theophylline, caffeine) resulting from induction is not anticipated, although clinical studies of induction have not been performed. Duloxetine is an inhibitor of the CYP1A2 isoform in in vitro studies, and in two clinical studies the average (90% confidence interval) increase in theophylline AUC was 7% (1%-15%) and 20% (13%-27%) when co-administered with duloxetine (60 mg twice daily).
7.9 Drugs Metabolized by CYP2D6
Duloxetine is a moderate inhibitor of CYP2D6. When duloxetine was administered (at a dose of 60 mg twice daily) in conjunction with a single 50 mg dose of desipramine, a CYP2D6 substrate, the AUC of desipramine increased 3-fold [see Warnings and Precautions (5.10)].
7.10 Drugs Metabolized by CYP2C9
Results of in vitro studies demonstrate that duloxetine does not inhibit activity. In a clinical study, the pharmacokinetics of S-warfarin, a CYP2C9 substrate, were not significantly affected by duloxetine [see Drug Interactions (7.4)].
7.11 Drugs Metabolized by CYP3A
Results of in vitro studies demonstrate that duloxetine does not inhibit or induce CYP3A activity. Therefore, an increase or decrease in the metabolism of CYP3A substrates (e.g., oral contraceptives and other steroidal agents) resulting from induction or inhibition is not anticipated, although clinical studies have not been performed.
7.12 Drugs Metabolized by CYP2C19
Results of in vitro studies demonstrate that duloxetine does not inhibit CYP2C19 activity at therapeutic concentrations. Inhibition of the metabolism of CYP2C19 substrates is therefore not anticipated, although clinical studies have not been performed.
7.13 Monoamine Oxidase Inhibitors
[see Dosage and Administration (2.5), Contraindications (4.1), and Warnings and Precautions (5.4)].
7.14 Serotonergic Drugs
Based on the mechanism of action of SNRIs and SSRIs, including Cymbalta, and the potential for serotonin syndrome, caution is advised when Cymbalta is co-administered with other drugs that may affect the serotonergic neurotransmitter systems, such as triptans, linezolid (an antibiotic which is a reversible non-selective MAOI), lithium, tramadol, or St. John's Wort. The concomitant use of Cymbalta with other SSRIs, SNRIs or tryptophan is not recommended [see Warnings and Precautions (5.4)].
7.15 Triptans
There have been rare postmarketing reports of serotonin syndrome with use of an SSRI and a triptan. If concomitant treatment of Cymbalta with a triptan is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases [see Warnings and Precautions (5.4)].
7.16 Alcohol
When Cymbalta and ethanol were administered several hours apart so that peak concentrations of each would coincide, Cymbalta did not increase the impairment of mental and motor skills caused by alcohol.
In the Cymbalta clinical trials database, three Cymbalta-treated patients had liver injury as manifested by ALT and total bilirubin elevations, with evidence of obstruction. Substantial intercurrent ethanol use was present in each of these cases, and this may have contributed to the abnormalities seen [see Warnings and Precautions (5.2 and 5.10)].
7.17 CNS Drugs
[See Warnings and Precautions (5.10)].
7.18 Drugs Highly Bound to Plasma Protein
Because duloxetine is highly bound to plasma protein, administration of Cymbalta to a patient taking another drug that is highly protein bound may cause increased free concentrations of the other drug, potentially resulting in adverse reactions. However, co-administration of duloxetine (60 or 120 mg) with warfarin (2-9 mg), a highly protein-bound drug, did not result in significant changes in INR and in the pharmacokinetics of either total S-or total R-warfarin (protein bound plus free drug) [see Drug Interactions (7.4)].
8 Use In Specific Populations
- Pregnancy and Nursing Mothers: Use only if the potential benefit justifies the potential risk to the fetus or child. (
2.3 ,8.1 ,8.3 )8.1 Pregnancy
TERATOGENIC EFFECTS SECTION
Teratogenic Effects, Pregnancy Category C ‚ÄĒ In animal reproduction studies, duloxetine has been shown to have adverse effects on embryo/fetal and postnatal development.
When duloxetine was administered orally to pregnant rats and rabbits during the period of organogenesis, there was no evidence of teratogenicity at doses up to 45¬†mg/kg/day (7¬†times the maximum recommended human dose¬†[MRHD, 60¬†mg/day] and 4¬†times the human dose of 120¬†mg/day on a mg/m2 basis, in rat; 15¬†times the MRHD and 7¬†times the human dose of 120¬†mg/day on a mg/m2 basis in rabbit). However, fetal weights were decreased at this dose, with a no-effect dose of 10¬†mg/kg/day (2¬†times the MRHD and ‚Čą1¬†times the human dose of 120¬†mg/day on a mg/m2 basis in rat; 3¬†times the MRHD and 2¬†times the human dose of 120¬†mg/day on a mg/m2 basis in rabbits).
When duloxetine was administered orally to pregnant rats throughout gestation and lactation, the survival of pups to 1 day postpartum and pup body weights at birth and during the lactation period were decreased at a dose of 30 mg/kg/day (5 times the MRHD and 2 times the human dose of 120 mg/day on a mg/m2 basis); the no-effect dose was 10 mg/kg/day. Furthermore, behaviors consistent with increased reactivity, such as increased startle response to noise and decreased habituation of locomotor activity, were observed in pups following maternal exposure to 30 mg/kg/day. Post-weaning growth and reproductive performance of the progeny were not affected adversely by maternal duloxetine treatment.
There are no adequate and well-controlled studies in pregnant women; therefore, duloxetine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
NONTERATOGENIC EFFECTS SECTION
Nonteratogenic Effects ‚ÄĒ Neonates exposed to SSRIs or serotonin and norepinephrine reuptake inhibitors (SNRIs), late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These features are consistent with either a direct toxic effect of SSRIs and SNRIs or, possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome [see Warnings and Precautions (5.4)].
When treating pregnant women with Cymbalta during the third trimester, the physician should carefully consider the potential risks and benefits of treatment. The physician may consider tapering Cymbalta in the third trimester [see Dosage and Administration (2.3)].
Lilly maintains a pregnancy registry to monitor the pregnancy outcomes of women exposed to Cymbalta while pregnant. Healthcare providers are encouraged to register any patient who is exposed to Cymbalta during pregnancy by calling the Cymbalta Pregnancy Registry at 1-866-814-6975 or by visiting www.cymbaltapregnancyregistry.com
8.2 Labor and Delivery
The effect of duloxetine on labor and delivery in humans is unknown. Duloxetine should be used during labor and delivery only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Duloxetine is excreted into the milk of lactating women. The estimated daily infant dose on a mg/kg basis is approximately 0.14% of the maternal dose. Because the safety of duloxetine in infants is not known, nursing while on Cymbalta is not recommended. However, if the physician determines that the benefit of duloxetine therapy for the mother outweighs any potential risk to the infant, no dosage adjustment is required as lactation did not influence duloxetine pharmacokinetics.
The disposition of duloxetine was studied in 6 lactating women who were at least 12 weeks postpartum. Duloxetine 40 mg twice daily was given for 3.5 days. Like many other drugs, duloxetine is detected in breast milk, and steady state concentrations in breast milk are about one-fourth those in plasma. The amount of duloxetine in breast milk is approximately 7 őľg/day while on 40 mg BID dosing. The excretion of duloxetine metabolites into breast milk was not examined. Because the safety of duloxetine in infants is not known, nursing while on Cymbalta is not recommended [see Dosage and Administration (2.3)].
8.4 Pediatric Use
Safety and effectiveness in the pediatric population have not been established [see Boxed Warning and Warnings and Precautions (5.1)]. Anyone considering the use of Cymbalta in a child or adolescent must balance the potential risks with the clinical need.
8.5 Geriatric Use
Of the 2,418 patients in premarketing clinical studies of Cymbalta for MDD, 5.9% (143) were 65 years of age or over. Of the 1041 patients in CLBP premarketing studies, 21.2% (221) were 65 years of age or over. Of the 487 patients in OA premarketing studies, 40.5% (197) were 65 years of age or over. Of the 1,074 patients in the DPNP premarketing studies, 33% (357) were 65 years of age or over. Of the 1,761 patients in FM premarketing studies, 7.9% (140) were 65 years of age or over. Premarketing clinical studies of GAD did not include sufficient numbers of subjects age 65 or over to determine whether they respond differently from younger subjects. In the MDD, DPNP, FM, OA, and CLBP studies, no overall differences in safety or effectiveness were generally observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. SSRIs and SNRIs, including Cymbalta have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse event [see Warnings and Precautions (5.11)]. In a subgroup analysis of patients 65 years of age and older (N=3278) from all placebo-controlled trials, 1.1% of patients treated with duloxetine reported one or more falls, compared with 0.4% of patients treated with placebo. While many patients with falls had underlying potential risk factors for falls (e.g., medications; medical comorbidities; gait disturbances), the impact of these factors on falls is unclear. Fall with serious consequences including bone fractures and hospitalizations have been reported [see Other Adverse Reactions Observed During the Premarketing and Postmarketing Clinical Trial Evaluation of Duloxetine (6.11)].
The pharmacokinetics of duloxetine after a single dose of 40 mg were compared in healthy elderly females (65 to 77 years) and healthy middle-age females (32 to 50 years). There was no difference in the Cmax, but the AUC of duloxetine was somewhat (about 25%) higher and the half-life about 4 hours longer in the elderly females. Population pharmacokinetic analyses suggest that the typical values for clearance decrease by approximately 1% for each year of age between 25 to 75 years of age; but age as a predictive factor only accounts for a small percentage of between-patient variability. Dosage adjustment based on the age of the patient is not necessary [see Dosage and Administration (2.3)].
8.6 Gender
Duloxetine's half-life is similar in men and women. Dosage adjustment based on gender is not necessary.
8.7 Smoking Status
Duloxetine bioavailability (AUC) appears to be reduced by about one-third in smokers. Dosage modifications are not recommended for smokers.
8.8 Race
No specific pharmacokinetic study was conducted to investigate the effects of race.
8.9 Hepatic Insufficiency
Patients with clinically evident hepatic insufficiency have decreased duloxetine metabolism and elimination. After a single 20 mg dose of Cymbalta, 6 cirrhotic patients with moderate liver impairment (Child-Pugh Class B) had a mean plasma duloxetine clearance about 15% that of age- and gender-matched healthy subjects, with a 5-fold increase in mean exposure (AUC). Although Cmax was similar to normals in the cirrhotic patients, the half-life was about 3 times longer [see Dosage and Administration (2.3) and Warnings and Precautions (5.12)].
8.10 Severe Renal Impairment
Limited data are available on the effects of duloxetine in patients with end-stage renal disease (ESRD). After a single 60 mg dose of duloxetine, Cmax and AUC values were approximately 100% greater in patients with end-stage renal disease receiving chronic intermittent hemodialysis than in subjects with normal renal function. The elimination half-life, however, was similar in both groups. The AUCs of the major circulating metabolites, 4-hydroxy duloxetine glucuronide and 5-hydroxy, 6-methoxy duloxetine sulfate, largely excreted in urine, were approximately 7- to 9-fold higher and would be expected to increase further with multiple dosing. Population PK analyses suggest that mild to moderate degrees of renal dysfunction (estimated CrCl 30-80 mL/min) have no significant effect on duloxetine apparent clearance [see Dosage and Administration (2.3) and Warnings and Precautions (5.12)].
9 Drug Abuse And Dependence
9.2 Abuse
In animal studies, duloxetine did not demonstrate barbiturate-like (depressant) abuse potential.
While Cymbalta has not been systematically studied in humans for its potential for abuse, there was no indication of drug-seeking behavior in the clinical trials. However, it is not possible to predict on the basis of premarketing experience the extent to which a CNS active drug will be misused, diverted, and/or abused once marketed. Consequently, physicians should carefully evaluate patients for a history of drug abuse and follow such patients closely, observing them for signs of misuse or abuse of Cymbalta (e.g., development of tolerance, incrementation of dose, drug-seeking behavior).
9.3 Dependence
In drug dependence studies, duloxetine did not demonstrate dependence-producing potential in rats.
10 Overdosage
10.1 Signs and Symptoms
In postmarketing experience, fatal outcomes have been reported for acute overdoses, primarily with mixed overdoses, but also with duloxetine only, at doses as low as 1000 mg. Signs and symptoms of overdose (duloxetine alone or with mixed drugs) included somnolence, coma, serotonin syndrome, seizures, syncope, tachycardia, hypotension, hypertension, and vomiting.
10.2 Management of Overdose
There is no specific antidote to Cymbalta, but if serotonin syndrome ensues, specific treatment (such as with cyproheptadine and/or temperature control) may be considered. In case of acute overdose, treatment should consist of those general measures employed in the management of overdose with any drug.
An adequate airway, oxygenation, and ventilation should be assured, and cardiac rhythm and vital signs should be monitored. Induction of emesis is not recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion or in symptomatic patients.
Activated charcoal may be useful in limiting absorption of duloxetine from the gastrointestinal tract. Administration of activated charcoal has been shown to decrease AUC and Cmax by an average of one-third, although some subjects had a limited effect of activated charcoal. Due to the large volume of distribution of this drug, forced diuresis, dialysis, hemoperfusion, and exchange transfusion are unlikely to be beneficial.
In managing overdose, the possibility of multiple drug involvement should be considered. A specific caution involves patients who are taking or have recently taken Cymbalta and might ingest excessive quantities of a TCA. In such a case, decreased clearance of the parent tricyclic and/or its active metabolite may increase the possibility of clinically significant sequelae and extend the time needed for close medical observation [see Warnings and Precautions (5.4) and Drug Interactions (7)]. The physician should consider contacting a poison control center for additional information on the treatment of any overdose. Telephone numbers for certified poison control centers are uled in the Physicians' Desk Reference (PDR).
11 Description
Cymbalta¬ģ (duloxetine hydrochloride) is a selective serotonin and norepinephrine reuptake inhibitor¬†(SSNRI) for oral administration. Its chemical designation is (+)-(S)-N-methyl-ő≥-(1-naphthyloxy)-2-thiophenepropylamine hydrochloride. The empirical formula is C18H19NOS‚ÄĘHCl, which corresponds to a molecular weight of¬†333.88. The structural formula is:
![]()
Duloxetine hydrochloride is a white to slightly brownish white solid, which is slightly soluble in water.
Each capsule contains enteric-coated pellets of 22.4, 33.7, or 67.3 mg of duloxetine hydrochloride equivalent to 20, 30, or 60 mg of duloxetine, respectively. These enteric-coated pellets are designed to prevent degradation of the drug in the acidic environment of the stomach. Inactive ingredients include FD&C Blue No. 2, gelatin, hypromellose, hydroxypropyl methylcellulose acetate succinate, sodium lauryl sulfate, sucrose, sugar spheres, talc, titanium dioxide, and triethyl citrate. The 20 and 60 mg capsules also contain iron oxide yellow.
12 Clinical Pharmacology
12.1 Mechanism of Action
Although the exact mechanisms of the antidepressant, central pain inhibitory and anxiolytic actions of duloxetine in humans are unknown, these actions are believed to be related to its potentiation of serotonergic and noradrenergic activity in the CNS.
12.2 Pharmacodynamics
Preclinical studies have shown that duloxetine is a potent inhibitor of neuronal serotonin and norepinephrine reuptake and a less potent inhibitor of dopamine reuptake. Duloxetine has no significant affinity for dopaminergic, adrenergic, cholinergic, histaminergic, opioid, glutamate, and GABA receptors in vitro. Duloxetine does not inhibit monoamine oxidase (MAO).
Cymbalta is in a class of drugs known to affect urethral resistance. If symptoms of urinary hesitation develop during treatment with Cymbalta, consideration should be given to the possibility that they might be drug-related.
12.3 Pharmacokinetics
Duloxetine has an elimination half-life of about 12 hours (range 8 to 17 hours) and its pharmacokinetics are dose proportional over the therapeutic range. Steady-state plasma concentrations are typically achieved after 3 days of dosing. Elimination of duloxetine is mainly through hepatic metabolism involving two P450 isozymes, CYP1A2 and CYP2D6.
Absorption and Distribution ‚ÄĒ Orally administered duloxetine hydrochloride is well absorbed. There is a median 2 hour lag until absorption begins¬†(Tlag), with maximal plasma concentrations¬†(Cmax) of duloxetine occurring 6¬†hours post dose. Food does not affect the Cmax of duloxetine, but delays the time to reach peak concentration from 6¬†to 10¬†hours and it marginally decreases the extent of absorption¬†(AUC) by about¬†10%. There is a 3 hour delay in absorption and a one-third¬†increase in apparent clearance of duloxetine after an evening dose as compared to a morning dose.
The apparent volume of distribution averages about 1640¬†L. Duloxetine is highly bound¬†(>90%) to proteins in human plasma, binding primarily to albumin and őĪ1-acid glycoprotein. The interaction between duloxetine and other highly protein bound drugs has not been fully evaluated. Plasma protein binding of duloxetine is not affected by renal or hepatic impairment.
Metabolism and Elimination ‚ÄĒ Biotransformation and disposition of duloxetine in humans have been determined following oral administration of 14C-labeled duloxetine. Duloxetine comprises about 3% of the total radiolabeled material in the plasma, indicating that it undergoes extensive metabolism to numerous metabolites. The major biotransformation pathways for duloxetine involve oxidation of the naphthyl ring followed by conjugation and further oxidation. Both CYP1A2 and CYP2D6 catalyze the oxidation of the naphthyl ring in vitro. Metabolites found in plasma include 4-hydroxy duloxetine glucuronide and 5-hydroxy, 6-methoxy duloxetine sulfate. Many additional metabolites have been identified in urine, some representing only minor pathways of elimination. Only trace (<1% of the dose) amounts of unchanged duloxetine are present in the urine. Most (about 70%) of the duloxetine dose appears in the urine as metabolites of duloxetine; about 20% is excreted in the feces. Duloxetine undergoes extensive metabolism, but the major circulating metabolites have not been shown to contribute significantly to the pharmacologic activity of duloxetine.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis ‚ÄĒ Duloxetine was administered in the diet to mice and rats for 2 years.
In female mice receiving duloxetine at 140 mg/kg/day (11 times the maximum recommended human dose [MRHD, 60 mg/day] and 6 times the human dose of 120 mg/day on a mg/m2 basis), there was an increased incidence of hepatocellular adenomas and carcinomas. The no-effect dose was 50 mg/kg/day (4 times the MRHD and 2 times the human dose of 120 mg/day on a mg/m2 basis). Tumor incidence was not increased in male mice receiving duloxetine at doses up to 100 mg/kg/day (8 times the MRHD and 4 times the human dose of 120 mg/day on a mg/m2 basis).
In rats, dietary doses of duloxetine up to 27 mg/kg/day in females (4 times the MRHD and 2 times the human dose of 120 mg/day on a mg/m2 basis) and up to 36 mg/kg/day in males (6 times the MRHD and 3 times the human dose of 120 mg/day on a mg/m2 basis) did not increase the incidence of tumors.
Mutagenesis ‚ÄĒ Duloxetine was not mutagenic in the in vitro bacterial reverse mutation assay (Ames test) and was not clastogenic in an in vivo chromosomal aberration test in mouse bone marrow cells. Additionally, duloxetine was not genotoxic in an in vitro mammalian forward gene mutation assay in mouse lymphoma cells or in an in vitro unscheduled DNA synthesis (UDS) assay in primary rat hepatocytes, and did not induce sister chromatid exchange in Chinese hamster bone marrow in vivo.
Impairment of Fertility ‚ÄĒ Duloxetine administered orally to either male or female rats prior to and throughout mating at doses up to 45 mg/kg/day (7 times the maximum recommended human dose of 60 mg/day and 4 times the human dose of 120 mg/day on a mg/m2 basis) did not alter mating or fertility.
14 Clinical Studies
14.1 Major Depressive Disorder
The efficacy of Cymbalta as a treatment for depression was established in 4 randomized, double-blind, placebo-controlled, fixed-dose studies in adult outpatients (18 to 83 years) meeting DSM-IV criteria for major depression. In 2 studies, patients were randomized to Cymbalta 60 mg once daily (N=123 and N=128, respectively) or placebo (N=122 and N=139, respectively) for 9 weeks; in the third study, patients were randomized to Cymbalta 20 or 40 mg twice daily (N=86 and N=91, respectively) or placebo (N=89) for 8 weeks; in the fourth study, patients were randomized to Cymbalta 40 or 60 mg twice daily (N=95 and N=93, respectively) or placebo (N=93) for 8 weeks. There is no evidence that doses greater than 60 mg/day confer additional benefits.
In all 4 studies, Cymbalta demonstrated superiority over placebo as measured by improvement in the 17-li Hamilton Depression Rating Scale (HAMD-17) total score.
In all of these clinical studies, analyses of the relationship between treatment outcome and age, gender, and race did not suggest any differential responsiveness on the basis of these patient characteristics.
In another study, 533 patients meeting DSM-IV criteria for MDD received Cymbalta 60 mg once daily during an initial 12-week open-label treatment phase. Two hundred and seventy-eight patients who responded to open label treatment (defined as meeting the following criteria at weeks 10 and 12: a HAMD-17 total score ‚ȧ9, Clinical Global Impressions of Severity (CGI-S) ‚ȧ2, and not meeting the DSM-IV criteria for MDD) were randomly assigned to continuation of Cymbalta at the same dose (N=136) or to placebo (N=142) for 6 months. Patients on Cymbalta experienced a statistically significantly longer time to relapse of depression than did patients on placebo. Relapse was defined as an increase in the CGI‚ÄďS score of ‚Č•2 points compared with that obtained at week 12, as well as meeting the DSM-IV criteria for MDD at 2 consecutive visits at least 2 weeks apart, where the 2-week temporal criterion had to be satisfied at only the second visit. The effectiveness of Cymbalta in hospitalized patients with major depressive disorder has not been studied.
14.2 Generalized Anxiety Disorder
The efficacy of Cymbalta in the treatment of generalized anxiety disorder (GAD) was established in 1 fixed-dose randomized, double-blind, placebo-controlled trial and 2 flexible-dose randomized, double-blind, placebo-controlled trials in adult outpatients between 18 and 83 years of age meeting the DSM-IV criteria for GAD.
In 1 flexible-dose study and in the fixed-dose study, the starting dose was 60 mg once daily where down titration to 30 mg once daily was allowed for tolerability reasons before increasing it to 60 mg once daily. Fifteen percent of patients were down titrated. One flexible-dose study had a starting dose of 30 mg once daily for 1 week before increasing it to 60 mg once daily.
The 2 flexible-dose studies involved dose titration with Cymbalta doses ranging from 60 mg once daily to 120 mg once daily (N=168 and N=162) compared to placebo (N=159 and N=161) over a 10-week treatment period. The mean dose for completers at endpoint in the flexible-dose studies was 104.75 mg/day. The fixed-dose study evaluated Cymbalta doses of 60 mg once daily (N=168) and 120 mg once daily (N=170) compared to placebo (N=175) over a 9-week treatment period. While a 120 mg/day dose was shown to be effective, there is no evidence that doses greater than 60 mg/day confer additional benefit.
In all 3 studies, Cymbalta demonstrated superiority over placebo as measured by greater improvement in the Hamilton Anxiety Scale (HAM-A) total score and by the Sheehan Disability Scale (SDS) global functional impairment score. The SDS is a widely used and well-validated scale that measures the extent emotional symptoms disrupt patient functioning in 3 life domains: work/school, social life/leisure activities, and family life/home responsibilities.
In another study, 887 patients meeting DSM-IV-TR criteria for GAD received Cymbalta 60 mg to 120 mg once daily during an initial 26-week open-label treatment phase. Four hundred and twenty-nine patients who responded to open-label treatment (defined as meeting the following criteria at weeks 24 and 26: a decrease from baseline HAM-A total score by at least 50% to a score no higher than 11, and a Clinical Global Impressions of Improvement [CGI-Improvement] score of 1 or 2) were randomly assigned to continuation of Cymbalta at the same dose (N=216) or to placebo (N=213) and were observed for relapse. Of the patients randomized, 73% had been in a responder status for at least 10 weeks. Relapse was defined as an increase in CGI-Severity score at least 2 points to a score ‚Č•4 and a MINI (Mini-International Neuropsychiatric Interview) diagnosis of GAD (excluding duration), or discontinuation due to lack of efficacy. Patients taking Cymbalta experienced a statistically significantly longer time to relapse of GAD than did patients taking placebo.
Subgroup analyses did not indicate that there were any differences in treatment outcomes as a function of age or gender.
14.3 Diabetic Peripheral Neuropathic Pain
The efficacy of Cymbalta for the management of neuropathic pain associated with diabetic peripheral neuropathy¬†was established in 2¬†randomized, 12-week, double-blind, placebo-controlled, fixed-dose studies in adult patients having diabetic peripheral neuropathic pain for at least 6¬†months. Study¬†DPNP-1 and¬†Study DPNP-2 enrolled a total of 791¬†patients of whom 592¬†(75%) completed the studies. Patients enrolled had Type¬†I or¬†II diabetes mellitus with a diagnosis of painful distal symmetrical sensorimotor polyneuropathy for at least 6¬†months. The patients had a baseline pain score of¬†‚Č•4 on an 11-point scale ranging from 0¬†(no pain) to 10¬†(worst possible pain). Patients were permitted up to 4¬†g of acetaminophen per day as needed for pain, in addition to Cymbalta. Patients recorded their pain daily in a diary.
Both studies compared Cymbalta 60 mg once daily or 60 mg twice daily with placebo. DPNP-1 additionally compared Cymbalta 20 mg with placebo. A total of 457 patients (342 Cymbalta, 115 placebo) were enrolled in DPNP-1 and a total of 334 patients (226 Cymbalta, 108 placebo) were enrolled in DPNP-2. Treatment with Cymbalta 60 mg one or two times a day statistically significantly improved the endpoint mean pain scores from baseline and increased the proportion of patients with at least a 50% reduction in pain scores from baseline. For various degrees of improvement in pain from baseline to study endpoint, Figures 1 and 2 show the fraction of patients achieving that degree of improvement. The figures are cumulative, so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study were assigned 0% improvement. Some patients experienced a decrease in pain as early as week 1, which persisted throughout the study.
![]()
Figure 1: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity - DPNP-1 Figure 2: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity - DPNP-2 14.4 Fibromyalgia
The efficacy of Cymbalta for the management of fibromyalgia was established in two randomized, double-blind, placebo-controlled, fixed-dose studies in adult patients meeting the American College of Rheumatology criteria for fibromyalgia (a history of widespread pain for 3 months, and pain present at 11 or more of the 18 specific tender point sites). Study FM-1 was three months in duration and enrolled female patients only. Study FM-2 was six months in duration and enrolled male and female patients. Approximately 25% of participants had a comorbid diagnosis of major depressive disorder (MDD). FM-1 and FM-2 enrolled a total of 874 patients of whom 541 (62%) completed the studies. The patients had a baseline pain score of 6.5 on an 11-point scale ranging from 0 (no pain) to 10 (worse possible pain).
Both studies compared Cymbalta 60 mg once daily or 120 mg daily (given in divided doses in FM-1 and as a single daily dose in FM-2) with placebo. FM-2 additionally compared Cymbalta 20 mg with placebo during the initial three months of a six-month study. A total of 354 patients (234 Cymbalta, 120 placebo) were enrolled in FM-1 and a total of 520 patients (376 Cymbalta, 144 placebo) were enrolled in FM-2 (5% male, 95% female). Treatment with Cymbalta 60 mg or 120 mg daily statistically significantly improved the endpoint mean pain scores from baseline and increase the proportion of patients with at least a 50% reduction in pain score from baseline. Pain reduction was observed in patients both with and without comorbid MDD. However, the degree of pain reduction may be greater in patients with comorbid MDD. For various degrees of improvement in pain from baseline to study endpoint, Figures 3 and 4 show the fraction of patients achieving that degree of improvement. The figures are cumulative so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study were assigned 0% improvement. Some patients experienced a decrease in pain as early as week 1, which persisted throughout the study. Improvement was also demonstrated on measures of function (Fibromyalgia Impact Questionnaires) and patient global impression of change (PGI). Neither study demonstrated a benefit of 120 mg compared to 60 mg, and a higher dose was associated with more adverse reactions and premature discontinuations of treatment.
![]()
Figure 3: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity - FM-1 Figure 4: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity - FM-2
Additionally, the benefit of up-titration in non-responders to Cymbalta at 60 mg/day was evaluated in a separate study. Patients were initially treated with Cymbalta 60 mg once daily for eight weeks in open-label fashion. Subsequently, completers of this phase were randomized to double-blind treatment with Cymbalta at either 60 mg once daily or 120 mg once daily. Those patients who were considered non-responders, where response was defined as at least a 30% reduction in pain score from baseline at the end of the 8-week treatment, were no more likely to meet response criteria at the end of 60 weeks of treatment if blindly titrated to Cymbalta 120 mg as compared to those who were blindly continued on Cymbalta 60 mg.
14.5 Chronic Musculoskeletal Pain
Cymbalta is indicated for the management of chronic musculoskeletal pain. This has been established in studies in patients with chronic low back pain and chronic pain due to osteoarthritis.
Studies in Chronic Low Back Pain ‚ÄĒ The efficacy of Cymbalta in chronic low back pain (CLBP) was assessed in two double-blind, placebo-controlled, randomized clinical trials of 13-weeks duration (Study CLBP-1 and Study CLBP-2), and one of 12-weeks duration (CLBP-3). CLBP-1 and CLBP-3 demonstrated efficacy of Cymbalta in the treatment of chronic low back pain. Patients in all studies had no signs of radiculopathy or spinal stenosis.
Study CLBP-1: Two hundred thirty-six adult patients (N=115 on Cymbalta, N=121 on placebo) enrolled and 182 (77%) completed 13-week treatment phase. After 7 weeks of treatment, Cymbalta patients with less than 30% reduction in average daily pain and who were able to tolerate duloxetine 60 mg once daily had their dose of Cymbalta, in a double-blinded fashion, increased to 120 mg once daily for the remainder of the study. Patients had a mean baseline pain rating of 6 on a numerical rating scale ranging from 0 (no pain) to 10 (worst possible pain). After 13 weeks of treatment, patients taking Cymbalta 60-120 mg daily had a significantly greater pain reduction compared to placebo. Randomization was stratified by the patients' baseline NSAIDs-use status. Subgroup analyses did not indicate that there were differences in treatment outcomes as a function of NSAIDs use.
Study CLBP-2: Four hundred and four patients were randomized to receive fixed doses of Cymbalta daily or a matching placebo (N=59 on Cymbalta 20 mg, N=116 on Cymbalta 60 mg, N=112 on Cymbalta 120 mg, N=117 on placebo) and 267 (66%) completed the entire 13-week study. After 13 weeks of treatment, none of the three Cymbalta doses showed a statistically significant difference in pain reduction compared to placebo.
Study CLBP-3: Four hundred and one patients were randomized to receive fixed doses of Cymbalta 60 mg daily or placebo (N=198 on Cymbalta, N=203 on placebo), and 303 (76%) completed the study. Patients had a mean baseline pain rating of 6 on a numerical rating scale ranging from 0 (no pain) to 10 (worst possible pain). After 12 weeks of treatment, patients taking Cymbalta 60 mg daily had significantly greater pain reduction compared to placebo.
For various degrees of improvement in pain from baseline to study endpoint, Figures 5 and 6 show the fraction of patients in CLBP-1 and CLBP-3 achieving that degree of improvement. The figures are cumulative, so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study were assigned the value of 0% improvement.
Figure¬†5: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity ‚Äď CLBP-1 Figure¬†6: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity ‚Äď CLBP-3
Studies in Chronic Pain Due to Osteoarthritis ‚ÄĒ The efficacy of Cymbalta in chronic pain due to osteoarthritis was assessed in 2 double-blind, placebo-controlled, randomized clinical trials of 13-weeks duration (Study OA-1 and Study OA-2). All patients in both studies fulfilled the ACR clinical and radiographic criteria for classification of idiopathic osteoarthritis of the knee. Randomization was stratified by the patients' baseline NSAIDs-use status. Patients assigned to Cymbalta started treatment in both studies at a dose of 30 mg once daily for one week. After the first week, the dose of Cymbalta was increased to 60 mg once daily. After 7¬†weeks of treatment with Cymbalta 60 mg once daily, in OA-1 patients with sub-optimal response to treatment (<30% pain reduction) and tolerated duloxetine 60 mg once daily had their dose increased to 120 mg. However, in OA-2, all patients, regardless of their response to treatment after 7 weeks, were re-randomized to either continue receiving Cymbalta 60 mg once daily or have their dose increased to 120 mg once daily for the remainder of the study. Patients in the placebo treatment groups in both studies received a matching placebo for the entire duration of studies. For both studies, efficacy analyses were conducted using 13-week data from the combined Cymbalta 60 mg and 120 mg once daily treatment groups compared to the placebo group.
Study OA-1: Two hundred fifty-six patients (N=128 on Cymbalta, N=128 on placebo) enrolled and 204 (80%) completed the study. Patients had a mean baseline pain rating of 6 on a numerical rating scale ranging from 0 (no pain) to 10 (worst possible pain). After 13 weeks of treatment, patients taking Cymbalta had significantly greater pain reduction. Subgroup analyses did not indicate that there were differences in treatment outcomes as a function of NSAIDs use.
Study OA-2: Two hundred thirty-one patients (N=111 on Cymbalta, N=120 on placebo) enrolled and 173 (75%) completed the study. Patients had a mean baseline pain of 6 on a numerical rating scale ranging from 0 (no pain) to 10 (worst possible pain). After 13 weeks of treatment, patients taking Cymbalta did not show a significantly greater pain reduction.
In Study OA-1, for various degrees of improvement in pain from baseline to study endpoint, Figure 7 shows the fraction of patients achieving that degree of improvement. The figure is cumulative, so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study were assigned the value of 0% improvement.
Figure¬†7:¬† Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity ‚Äď OA-1
16 How Supplied/storage And Handling
16.1 How Supplied
Cymbalta is available as delayed release capsules in the following strengths, colors, imprints, and presentations:
a equivalent to duloxetine base
‚Ć Identi-Dose¬ģ (unit dose medication, Lilly)
Features Strengths 20 mga 30 mga 60 mga Body color Opaque green Opaque white Opaque green Cap color Opaque green Opaque blue Opaque blue Cap imprint Lilly 3235 Lilly 3240 Lilly 3237 Lilly 3270 Body imprint 20mg 30mg 60mg 60mg Capsule number PU3235 PU3240 PU3237 PU3270 Presentations and NDC Codes Bottles of 30 NA 0002-3240-30 0002-3237-30 0002-3270-30 Bottles of 60 0002-3235-60 NA NA NA Bottles of 90 NA 0002-3240-90 NA NA Bottles of 1000 NA 0002-3240-04 0002-3237-04 0002-3270-04 Bulers ID†100 NA 0002-3240-33 0002-3237-33 0002-3270-33 16.2 Storage
Store at 25¬įC (77¬įF); excursions permitted to 15-30¬įC (59-86¬įF) [see USP Controlled Room Temperature].
17 Patient Counseling Information
See FDA-approved Medication Guide
17.1 Information on Medication Guide
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with Cymbalta and should counsel them in its appropriate use. A patient Medication Guide is available for Cymbalta. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide before starting Cymbalta and each time their prescription is renewed, and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking Cymbalta.
17.2 Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to observe for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication [see Boxed Warning, and Warnings and Precautions (5.1)].
17.3 Medication Administration
Cymbalta should be swallowed whole and should not be chewed or crushed, nor should the capsule be opened and its contents be sprinkled on food or mixed with liquids. All of these might affect the enteric coating.
17.4 Continuing the Therapy Prescribed
While patients may notice improvement with Cymbalta therapy in 1 to 4 weeks, they should be advised to continue therapy as directed.
17.5 Abnormal Bleeding
Patients should be cautioned about the concomitant use of duloxetine and NSAIDs, aspirin, warfarin, or other drugs that affect coagulation since combined use of psychotropic drugs that interfere with serotonin reuptake and these agents has been associated with an increased risk of bleeding [see Warnings and Precautions (5.5)].
17.6 Concomitant Medications
Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter medications, since there is a potential for interactions [see Dosage and Administration (2.5), Contraindications (4.1), Warnings and Precautions (5.4 and 5.10), and Drug Interactions (7)].
17.7 Serotonin Syndrome
Patients should be cautioned about the risk of serotonin syndrome with the concomitant use of Cymbalta and triptans, tramadol or other serotonergic agents [see Warnings and Precautions (5.4) and Drug Interactions (7.14)].
17.8 Pregnancy and Breast Feeding
Patients should be advised to notify their physician if they
- become pregnant during therapy
- intend to become pregnant during therapy
- are breast feeding [see Dosage and Administration (2.3) and Use in Specific Populations (8.1, 8.2, and 8.3)].
Lilly maintains a pregnancy registry to monitor the pregnancy outcomes of women exposed to Cymbalta while pregnant. Healthcare providers are encouraged to register any patient who is exposed to Cymbalta during pregnancy by calling the Cymbalta Pregnancy Registry at 1-866-814-6975 or by visiting www.cymbaltapregnancyregistry.com
17.9 Alcohol
Although Cymbalta does not increase the impairment of mental and motor skills caused by alcohol, use of Cymbalta concomitantly with heavy alcohol intake may be associated with severe liver injury. For this reason, Cymbalta should not be prescribed for patients with substantial alcohol use [see Warnings and Precautions (5.2) and Drug Interactions (7.16)].
17.10 Orthostatic Hypotension and Syncope
Patients should be advised of the risk of orthostatic hypotension and syncope, especially during the period of initial use and subsequent dose escalation, and in association with the use of concomitant drugs that might potentiate the orthostatic effect of duloxetine [see Warnings and Precautions (5.3)].
17.11 Interference with Psychomotor Performance
Any psychoactive drug may impair judgment, thinking, or motor skills. Although in controlled studies Cymbalta has not been shown to impair psychomotor performance, cognitive function, or memory, it may be associated with sedation and dizziness. Therefore, patients should be cautioned about operating hazardous machinery including automobiles, until they are reasonably certain that Cymbalta therapy does not affect their ability to engage in such activities.
Literature revised May 6, 2011
Eli Lilly and CompanyIndianapolis, IN 46285, USA
Copyright © 2004, 2011, Eli Lilly and Company. All rights reserved.
PV 7218 AMP
Spl Medguide Section
Medication Guide
Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Read the Medication Guide that comes with your or your family member's antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines. Talk to your, or your family member's, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a ul of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
These are not all the possible side effects. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the US Food and Drug Administration for all antidepressants.
Patient Information revised December 4, 2008
PV 7090 AMP
Package Label.principal Display Panel
image of label![]()
Package Label.principal Display Panel
image of label![]()
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site