Cyproheptadine Hydrochloride (cyproheptadine hydrochloride 4 mg) Dailymed
Generic: cyproheptadine hydrochloride
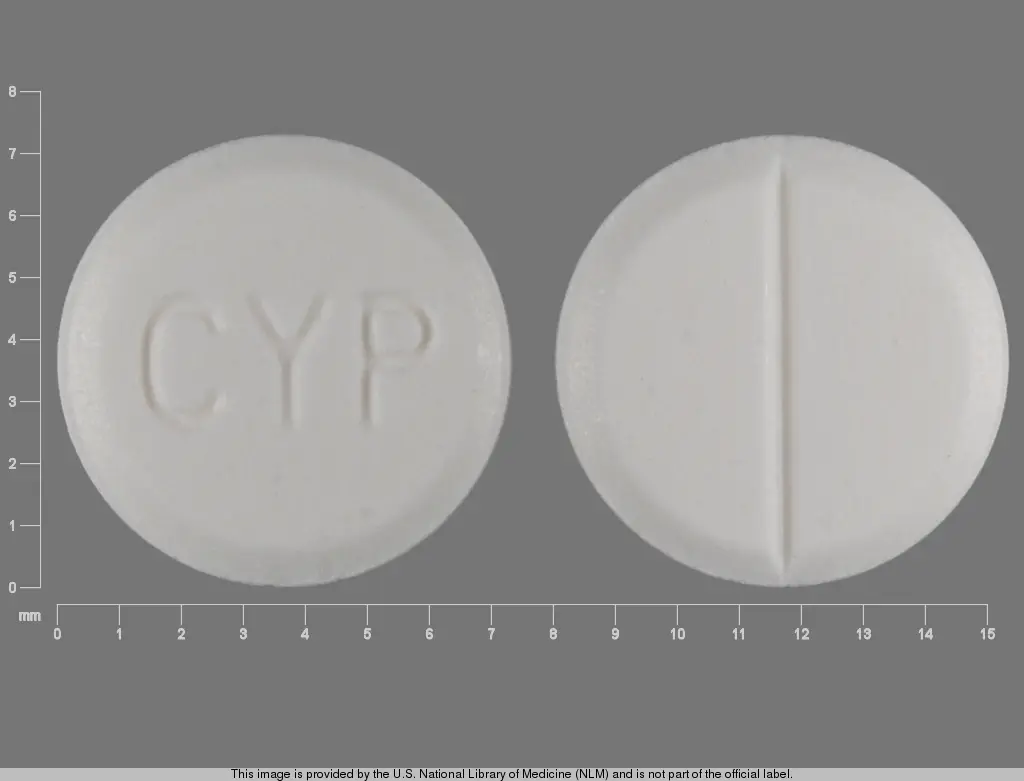
IMPRINT: CYP
SHAPE: round
COLOR: white SCORE: 2
All Imprints
cyproheptadine hydrochloride 4 mg - cyp round white
cyproheptadine hydrochloride 4 mg oral tablet - cyp round white
cyproheptadine hydrochloride - cyproheptadine hydrochloride 4 mg oral tablet - cyp round white
Go PRO for all pill images
Description
Cyproheptadine HCl, is an antihistaminic and antiserotonergic agent. Cyproheptadine hydrochloride is a white to slightly yellowish crystalline solid, with a molecular weight of 350.89, which is soluble in water, freely soluble in methanol, sparingly soluble in ethanol, soluble in chloroform, and practically insoluble in ether. It is the sesquihydrate of 4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine hydrochloride. The molecular formula of the anhydrous salt is C21H21NHCl and the structural formula of the anhydrous salt is: Cyproheptadine hydrochloride is available for oral administration in 4 mg tablets. Inactive ingredients include: lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
Clinical Pharmacology
Cyproheptadine is a serotonin and histamine antagonist with anticholinergic and sedative effects. Antiserotonin and antihistamine drugs appear to compete with serotonin and histamine, respectively, for receptor sites.
Pharmacokinetics and Metabolism:
After a single 4 mg oral dose of 14C-labelled Cyproheptadine HCl in normal subjects, given as tablets, 2-20% of the radioactivity was excreted in the stools. Only about 34% of the stool radioactivity was unchanged drug, corresponding to less than 5.7% of the dose. At least 40% of the administered radioactivity was excreted in the urine. No detectable amounts of unchanged drug were present in the urine of patients on chronic 12-20 mg daily doses. The principle metabolite found in human urine has been identified as a quaternary ammonium glucuronide conjugate of cyproheptadine. Elimination is diminished in renal insufficiency.
Indications & Usage
Perennial and seasonal allergic rhinitis Vasomotor rhinitis Allergic conjunctivitis due to inhalant allergens and foods Mild, uncomplicated allergic skin manifestations of urticaria and angioedema Amelioration of allergic reactions to blood or plasma Cold urticaria Dermatographism As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
Contraindications
Newborn or Premature Infants:
This drug should not be used in newborn or premature infants.
Nursing Mothers:
Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy iscontraindicated in nursing mothers.
Other Conditions:
Hypersensitivity to cyproheptadine and other drugs of similar chemical structure. Monoamine oxidase inhibitor therapy (see DRUG INTERACTIONS.) Angle-closure glaucoma Stenosing peptic ulcer Symptomatic prostatic hypertrophy Bladder neck obstruction Pyloroduodenal obstruction Elderly, debilitated patients
Warnings
Pediatric Patients:
produce excitation.
CNS Depressants:
Antihistamines may have additive effects with alcohol and other CNS depressants, e.g., hypnotics, sedatives, tranquilizers, antianxiety agents.
Activities Requiring Mental Alertness:
Patients should be warned about engaging in activities requiring mental alertness and motor coordination, such as driving a car or operating machinery. Antihistamines are more likely to cause dizziness, sedation, and hypotension in elderly patients. (see PRECAUTIONS, Geriatric Use).
Precautions
General:
Cyproheptadine has an atropine-like action and, therefore, should be used with caution in patients with: History of bronchial asthma Increased intraocular pressure Hyperthyroidism Cardiovascular disease Hypertension
Information For Patients
Antihistamines may diminish mental alertness; conversely, particularly, in the young child, they may occasionally produce excitation. Patients should be warned about engaging in activities requiring metal alertness and motor coordination, such as driving a car or operating machinery.
Drug Interactions
MAO inhibitors prolong and intensify the anticholinergic effects of antihistamines. Antihistamines may have additive effects with alcohol and other CNS depressants, e.g., hypnotics, sedatives, tranquilizers, antianxiety agents.
Carcinogenesis & Mutagenesis & Impairment Of Fertility
Long-term carcinogenic studies have not been done with cyproheptadine. Cyproheptadine had no effect on fertility in a two-litter study in rats or a two generation study in mice at about 10 times the human dose. Cyproheptadine did not produce chromosome damage in human lymphocytes or fibroblasts in vitro; high doses (10-4M) were cytotoxic. Cyproheptadine did not have any mutagenic effect in the Ames microbial mutagen test; concentrations of above 500 mcg/plate inhibited bacterial growth.
Pregnancy
Category B
: Reproduction studies have been performed in rabbits, mice, and rats at oral or subcutaneous doses up to 32 times the maximum recommended human oral dose and have revealed no evidence of impaired fertility or harm to the fetus due to cyproheptadine. Cyproheptadine has been shown to be fetotoxic in rats when given by intraperitoneal injection in doses four times the maximum recommended human oral dose. Two studies in pregnant women, however, have not shown that cyproheptadine increases the risk of abnormalities when administered during the first, second and third trimesters of pregnancy. No teratogenic effects were observed in any of the newborns. Nevertheless, because the studies in humans cannot rule out the possibility of harm, cyproheptadine should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from cyproheptadine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother (see CONTRAINDICATIONS).
Pediatric Use
Safety and effectiveness in pediatric patients below the age of two have not been established. (see CONTRAINDICATIONS, Newborn or Premature Infants, and WARNINGS, Pediatric Patients).
Geriatric Use
Clinical studies of Cyproheptadine HCl tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see WARNINGS, Activities Requiring Mental Alertness). Adverse reactions which have been reported with the use of antihistamines are as follows:
Central Nervous System
: Sedation and sleepiness (often transient), dizziness, disturbed coordination, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, paresthesias, neuritis, convulsions, euphoria, hallucinations, hysteria, faintness.
Integumentary:
Allergic manifestation of rash and edema, excessive perspiration, urticaria, photosensitivity.
Special Senses:
Acute labyrinthitis, blurred vision, diplopia, vertigo, tinnitus.
Cardiovascular:
Hypotension, palpitation, tachycardia, extrasystoles, anaphylactic shock.
Hematologic:
Hemolytic anemia, leukopenia, agranulocytosis, thrombocytopenia.
Digestive System:
Cholestasis, hepatic failure, hepatitis, hepatic function abnormality, dryness of mouth, epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation, jaundice.
Genitourinary:
Urinary frequency, difficult urination, urinary retention, early menses.
Respiratory:
Dryness of nose and throat, thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness.
Miscellaneous:
Fatigue, chills, headache, increased appetite/weight gain.
Overdosage
If vomiting has not occurred spontaneously, the patient should be induced to vomit with syrup of ipecac. If patient is unable to vomit, perform gastric lavage followed by activated charcoal. Isotonic or 1isotonic saline is the lavage of choice. Precautions against aspiration must be taken especially in infants and children. When life threatening CNS signs and symptoms are present, intravenous physostigmine salicylate may be considered. Dosage and frequency of administration are dependent on age, clinical response and recurrence after response. (See package circulars for physostigmine products.) Saline cathartics, as milk of magnesia, by osmosis draw water into the bowel and, therefore, are valuable, for their action in rapid dilution of bowel content. Stimulants should not be used. Vasopressors may be used to treat hypotension. The oral LD50 of Cyproheptadine is 123 mg/kg, and 295 mg/kg in the mouse and rat, respectively.
Dosage & Administration
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT. Each tablet contains 4 mg of cyproheptadine hydrochloride.
Pediatric Patients: Age 2 to 6 years:
The total daily dosage for pediatric patients may be calculated on the basis of body weight or body area using approximately 0.25 mg/kg/day or 8 mg per square meter of body surface (8 mg/m2). The usual dose is 2 mg (1tablet) two or three times a day, adjusted as necessary to the size and response of the patient. The dose is not to exceed 12 mg a day.
Age 7 to 14 years:
The usual dose is 4 mg (1 tablet) two or three times a day adjusted as necessary to the size and response of the patient. The dose is not to exceed 16 mg a day.
Adults:
The total daily dose for adults should not exceed 0.5 mg/kg/day. The therapeutic range is 4 to 20 mg a day, with the majority of patients requiring 12 to 16 mg a day. An occasional patient may require as much as 32 mg a day for adequate relief. It is suggested that dosage be initiated with 4 mg (1 tablet) three times a day and adjusted according to the size and response of the patient.
How Supplied
Cyproheptadine Hydrochloride Tablets USP are available as white to off white, flat-faced, bevel-edged, round shaped tablets, one side debossed withCYP, the other side bisected, containing 4 mg of Cyproheptadine HCl packaged in bottles of 100 tablets, NDC 60258-850-01, and 1000 tablets, NDC 60258-850-10.
Storage And Handling
Store at 20- 25C (68- 77F) excursions permitted to 15- 30C (59- 86F) [see USP Controlled Room Temperature] Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. PHARMACIST: Dispense in a well-closed container as defined in the USP. Use child-resistant closure (as required).
Package Label.principal Display Panel Section
DRUG: Cyproheptadine Hydrochloride GENERIC: Cyproheptadine Hydrochloride DOSAGE: TABLET ADMINSTRATION: ORAL NDC: 49349-781-02 STRENGTH:4 mg COLOR: white SHAPE: ROUND SCORE: Two even pieces SIZE: 7 mm IMPRINT: 30 QTY: 30![]()
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site