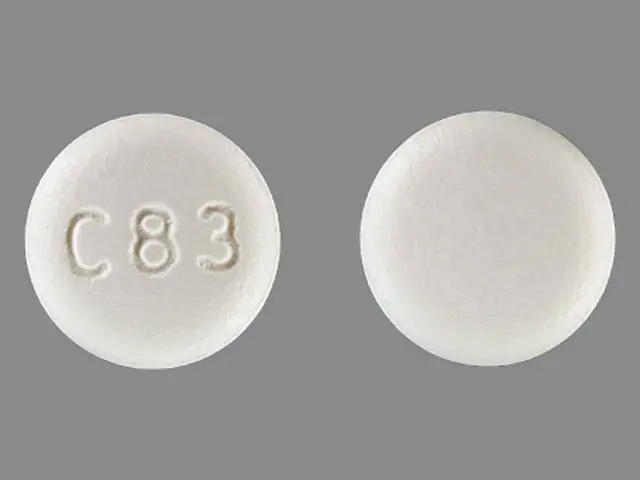
DIPYRIDAMOLE (dipyridamole 75 mg) Dailymed
Generic: dipyridamole
IMPRINT: C83
SHAPE: round
COLOR: yellow
All Imprints
dipyridamole 50 mg - c82 round yellow
dipyridamole 25 mg - c81 round yellow
dipyridamole 75 mg - c83 round yellow
Go PRO for all pill images
Rx only
Description
Dipyridamole tablets are a platelet inhibitor chemically described as 2,2’,2’’,2’’’-[(4,8-Dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. The molecular weight is 504.63 and the molecular formula is C24H40N8O4. The structural formula is represented below:
Dipyridamole is an odorless yellow crystalline powder, having a bitter taste. It is soluble in dilute acids, methanol and chloroform, and practically insoluble in water.
Each tablet, for oral administration, contains 25 mg, 50 mg or 75 mg dipyridamole. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, povidone, pregelatinized starch, sodium starch glycolate, talc, and titanium dioxide.
Clinical Pharmacology
It is believed that platelet reactivity and interaction with prosthetic cardiac valve surfaces, resulting in abnormally shortened platelet survival time, is a significant factor in thromboembolic complications occurring in connection with prosthetic heart valve replacement.
Dipyridamole tablets have been found to lengthen abnormally shortened platelet survival time in a dose-dependent manner.
In three randomized controlled clinical trials involving 854 patients who had undergone surgical placement of a prosthetic heart valve, dipyridamole tablets, in combination with warfarin, decreased the incidence of postoperative thromboembolic events by 62 to 91% compared to warfarin treatment alone. The incidence of thromboembolic events in patients receiving the combination of dipyridamole tablets and warfarin ranged from 1.2 to 1.8%.
In three additional studies involving 392 patients taking dipyridamole tablets and coumarin-like anticoagulants, the incidence of thromboembolic events ranged from 2.3 to 6.9%.
In these trials, the coumarin anticoagulant was begun between 24 hours and 4 days postoperatively, and the dipyridamole tablets were begun between 24 hours and 10 days postoperatively. The length of follow-up in these trials varied from 1 to 2 years.
Dipyridamole tablets do not influence prothrombin time or activity measurements when administered with warfarin.
Mechanism of Action
Dipyridamole inhibits the uptake of adenosine into platelets, endothelial cells and erythrocytes in vitro and in vivo; the inhibition occurs in a dose-dependent manner at therapeutic concentrations (0.5 to 1.9 μg/mL). This inhibition results in an increase in local concentrations of adenosine which acts on the platelet A2-receptor thereby stimulating platelet adenylate cyclase and increasing platelet cyclic-3’,5’-adenosine monophosphate (cAMP) levels. Via this mechanism, platelet aggregation is inhibited in response to various stimuli such as platelet activating factor (PAF), collagen and adenosine diphosphate (ADP).
Dipyridamole inhibits phosphodiesterase (PDE) in various tissues. While the inhibition of cAMP-PDE is weak, therapeutic levels of dipyridamole inhibit cyclic-3’,5’-guanosine monophosphate-PDE (cGMP-PDE), thereby augmenting the increase in cGMP produced by EDRF (endothelium-derived relaxing factor, now identified as nitric oxide).
Hemodynamics
In dogs intraduodenal doses of dipyridamole of 0.5 to 4.0 mg/kg produced dose-related decreases in systemic and coronary vascular resistance leading to decreases in systemic blood pressure and increases in coronary blood flow. Onset of action was in about 24 minutes and effects persisted for about 3 hours.
Similar effects were observed following IV dipyridamole in doses ranging from 0.025 to 2.0 mg/kg.
In man the same qualitative hemodynamic effects have been observed. However, acute intravenous administration of dipyridamole may worsen regional myocardial perfusion distal to partial occlusion of coronary arteries.
Pharmacokinetics and Metabolism
Following an oral dose of dipyridamole tablets, the average time to peak concentration is about 75 minutes. The decline in plasma concentration following a dose of dipyridamole tablets fits a two-compartment model. The alpha half-life (the initial decline following peak concentration) is approximately 40 minutes. The beta half-life (the terminal decline in plasma concentration) is approximately 10 hours. Dipyridamole is highly bound to plasma proteins. It is metabolized in the liver where it is conjugated as a glucuronide and excreted with the bile.
Indications And Usage
Dipyridamole tablets are indicated as an adjunct to coumarin anticoagulants in the prevention of postoperative thromboembolic complications of cardiac valve replacement.
Contraindications
Hypersensitivity to dipyridamole and any of the other components.
Precautions
General
Coronary Artery Disease: Dipyridamole has a vasodilatory effect and should be used with caution in patients with severe coronary artery disease (e.g., unstable angina or recently sustained myocardial infarction). Chest pain may be aggravated in patients with underlying coronary artery disease who are receiving dipyridamole.
Hepatic Insufficiency: Elevations of hepatic enzymes and hepatic failure have been reported in association with dipyridamole administration.
Hypotension: Dipyridamole should be used with caution in patients with hypotension since it can produce peripheral vasodilation.
Laboratory Tests
Dipyridamole has been associated with elevated hepatic enzymes.
Drug Interactions
No pharmacokinetic drug-drug interaction studies were conducted with dipyridamole tablets. The following information was obtained from the literature.
Adenosine: Dipyridamole has been reported to increase the plasma levels and cardiovascular effects of adenosine. Adjustment of adenosine dosage may be necessary.
Cholinesterase Inhibitors: Dipyridamole may counteract the anticholinesterase effect of cholinesterase inhibitors, thereby potentially aggravating myasthenia gravis.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In studies in which dipyridamole was administered in the feed to mice (up to 111 weeks in males and females) and rats (up to 128 weeks in males and up to 142 weeks in females), there was no evidence of drug-related carcinogenesis. The highest dose administered in these studies (75 mg/kg/day) was, on a mg/m2 basis, about equivalent to the maximum recommended daily human oral dose (MRHD) in mice and about twice the MRHD in rats. Mutagenicity tests of dipyridamole with bacterial and mammalian cell systems were negative. There was no evidence of impaired fertility when dipyridamole was administered to male and female rats at oral doses up to 500 mg/kg/day (about 12 times the MRHD on a mg/m2 basis). A significant reduction in number of corpora lutea with consequent reduction in implantations and live fetuses was, however, observed at 1250 mg/kg(more than 30 times the MRHD on a mg/m2 basis).
Pregnancy
PREGNANCY CATEGORY B
Reproduction studies have been performed in mice, rabbits and rats at oral dipyridamole doses of up to 125 mg/kg, 40 mg/kg and 1000 mg/kg, respectively (about 1 1/2, 2 and 25 times the maximum recommended daily human oral dose, respectively, on a mg/m2 basis) and have revealed no evidence of harm to the fetus due to dipyridamole. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, dipyridamole tablets should be used during pregnancy only if clearly needed.
Nursing Mothers
As dipyridamole is excreted in human milk, caution should be exercised when dipyridamole tablets are administered to a nursing woman.
Pediatric Use
Safety and effectiveness in the pediatric population below the age of 12 years have not been established.
Adverse Reactions
Adverse reactions at therapeutic doses are usually minimal and transient. On long-term use of dipyridamole tablets initial side effects usually disappear. The following reactions in Table 1 were reported in two heart valve replacement trials comparing dipyridamole tablets and warfarin therapy to either warfarin alone or warfarin and placebo:
Â
Table 1 Adverse Reactions Reported in 2 Heart Valve Replacement Trials AdverseReaction DipyridamoleTablets /Warfarin Placebo/Warfarin Number of patients 147 170 Dizziness 13.6% 8.2% Abdominal distress 6.1% 3.5% Headache 2.3% 0.0% Rash 2.3% 1.1%
Other reactions from uncontrolled studies include diarrhea, vomiting, flushing and pruritus. In addition, angina pectoris has been reported rarely and there have been rare reports of liver dysfunction. On those uncommon occasions when adverse reactions have been persistent or intolerable, they have ceased on withdrawal of the medication.
When dipyridamole tablets were administered concomitantly with warfarin, bleeding was no greater in frequency or severity than that observed when warfarin was administered alone. In rare cases, increased bleeding during or after surgery has been observed.
In post-marketing reporting experience, there have been rare reports of hypersensitivity reactions (such as rash, urticaria, severe bronchospasm, and angioedema), larynx edema, fatigue, malaise, myalgia, arthritis, nausea, dyspepsia, paresthesia, hepatitis, thrombocytopenia, alopecia, cholelithiasis, hypotension, palpitation, and tachycardia.
Overdosage
In case of real or suspected overdose, seek medical attention or contact a Poison Control Center immediately. Careful medical management is essential. Based upon the known hemodynamic effects of dipyridamole, symptoms such as warm feeling, flushes, sweating, restlessness, feeling of weakness and dizziness may occur. A drop in blood pressure and tachycardia might also be observed.
Symptomatic treatment is recommended, possibly including a vasopressor drug. Gastric lavage should be considered. Administration of xanthine derivatives (e.g., aminophylline) may reverse the hemodynamic effects of dipyridamole overdose. Since dipyridamole is highly protein bound, dialysis is not likely to be of benefit.
Dosage And Administration
Adjunctive Use in Prophylaxis of Thromboembolism after Cardiac Valve Replacement. The recommended dose is 75 to 100 mg four times daily as an adjunct to the usual warfarin therapy. Please note that aspirin is not to be administered concomitantly with coumarin anticoagulants.
How Supplied
Dipyridamole Tablets USP, 25 mg are white to pale yellow, round, standard convex film-coated tablets debossed with “C81” on one side and plain on the other side. 10 Tablets per card, 5 cards per carton...................NDC 50268-263-15
Dipyridamole Tablets USP, 50 mg are white to pale yellow, round, standard convex film-coated tablets debossed with “C82” on one side and plain on the other side. 10 Tablets per card, 5 cards per carton...................NDC 50268-264-15
Dipyridamole Tablets USP, 75 mg are white to pale yellow, round, standard convex film-coated tablets debossed with “C83” on one side and plain on the other side. 10 Tablets per card, 5 cards per carton....................NDC 50268-265-15
STORAGE AND HANDLING SECTION
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tightly-closed, light-resistant container (USP). Keep out of reach of children. Dispensed in a buler punch material for Institutional Use Only. Keep out of reach of children.
Rx only
Mfg. by: IMPAX Laboratories, Inc. Hayward, CA 94544
Distributed by: AvPAK Pulaski, TN 38478
657-01 Iss. 06/2007
Principal Display Panel
NDC 50268-263-15 Dipyridamole Tablets, USP 25 mgRx Only
50 Tablets (5 X 10) Unit Dose 5026826315
NDC 50268-263-15 Dipyridamole Tablets, USP 25 mgRx Only
50 Tablets (5 X 10) Unit Dose 5026826315
USUAL DOSAGE: See accompaning outsert for additional dosing information. Each tablet contains 25 mg of dipyridamole.
Storage: Store at 20oCÂ to 25oC (68oFÂ to 77oF) (See USP Controlled Toom Temperature). Keep this and all medication out of reach of children. Protect from light.
Manufactured for: AvKARE, Inc.Pulaski, TN 38478
AvPAK A DIVISION OF AvKARE, Inc. Mfg. Iss. 06/07Â Â Â Â Â Â Â Â Â AV Rev. 12/11
NDC 50268-264-15 Dipyridamole Tablets, USP 50 mgRx Only
50 Tablets (5 X 10) Unit Dose 5026826415
NDC 50268-264-15 Dipyridamole Tablets, USP 50 mgRx Only
50 Tablets (5 X 10) Unit Dose 5026826415
USUAL DOSAGE: See accompaning outsert for additional dosing information. Each tablet contains 50 mg of dipyridamole.
Storage: Store at 20oCÂ to 25oC (68oFÂ to 77oF) (See USP Controlled Toom Temperature). Keep this and all medication out of reach of children. Protect from light.
Manufactured for: AvKARE, Inc.Pulaski, TN 38478
AvPAK A DIVISION OF AvKARE, Inc. Mfg. Iss. 06/07Â Â Â Â Â Â Â Â Â AV Rev. 12/11
NDC 50268-265-15 Dipyridamole Tablets, USP 75 mgRx Only
50 Tablets (5 X 10) Unit Dose 5026826515
NDC 50268-265-15 Dipyridamole Tablets, USP 75 mgRx Only
50 Tablets (5 X 10) Unit Dose 5026826515
USUAL DOSAGE: See accompaning outsert for additional dosing information.
Each tablet contains 75mg of dipyridamole. Storage: Store at 20oCÂ to 25oC (68oFÂ to 77oF) (See USP Controlled Toom Temperature). Keep this and all medication out of reach of children. Protect from light.
Manufactured for:Â AvKARE, Inc.Pulaski, TN 38478
AvPAK A DIVISION OF AvKARE, Inc. Mfg. Iss. 06/07Â Â Â Â Â Â Â Â Â AV Rev. 12/11
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site