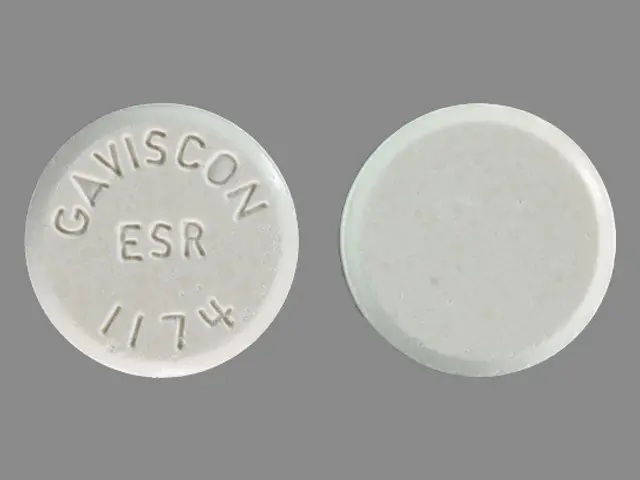
Gaviscon (aluminum hydroxide 160 mg magnesium carbonate 105 mg) Dailymed
Generic: aluminum hydroxide and magnesium trisilicate is used for the treatment of Diarrhea Dyspepsia Gastroesophageal Reflux Heart Block Heartburn Hepatitis Kidney Failure, Chronic Magnesium Deficiency Peptic Ulcer Renal Insufficiency Duodenal Ulcer Esophagitis, Peptic Gastrointestinal Hemorrhage Stomach Ulcer
Go PRO for all pill images
Active Ingredients (in Each Tablet) (extra Strength)
Aluminum hydroxide 160mg
Magnesium carbonate 105mg
Active Ingredients (in Each Tablet) (regular Strength)
Dried aluminum hydroxide gel 80mg
Magnesium trisilicate 14.2mg
Purpose
Antacid
Antacid
Uses (extra Strength)
relieves
- • acid indigestion
- • heartburn
- • sour stomach
- • upset stomach associated with these symptoms
Uses (regular Strength)
temporarily relieves symptoms of:
- •heartburn and acid indigestion due to acid reflux
Warnings (extra Strength)
Ask a doctor or pharmacist before use if you are
- •taking a prescription drug. Antacids may interact with certain prescription drugs.
- •if you are on a sodium-restricted diet
When using this product
- •do not take more than 16 tablets in 24 hours
- •do not use the maximum dosage for more than 2 weeks
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Warnings (regular Strength)
Do not use
- •for peptic ulcers
- •if you have trouble swallowing
Ask a doctor before use if you have
- •kidney disease
- •a sodium restricted diet
Ask a doctor or pharmacist if you
- •are taking a prescription drug. Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if
- •heartburn or stomach pain continues
- •you need to take this product for more than 14 days
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center.
Directions (extra Strength)
- •chew 2-4 tablets four times a day or as directed by a doctor
- •take after meals and at bedtime or as needed
- •for best results follow by a half glass of water or other liquid
- •DO NOT SWALLOW WHOLE
Directions (regular Strength)
- • do not swallow tablets whole
- •chew 2 to 4 tablets after meals and at bedtime as needed (up to 4 times a day) or as directed by a doctor. For best results, drink a half glass of water or other liquid after each dose.
- •do not take more than 16 tablets in 24 hours
Other Information (extra Strength)
- • Each tablet contains: magnesium 35mg, sodium 20mg
- •Store at up to 25°C (77°F) in a dry place
Other Information (regular Strength)
- • Each tablet contains: magnesium 5mg, sodium 21 mg
- •Store at up to 25°C (77°F) in a dry place
Inactive Ingredients (extra Strength)
alginic acid, calcium stearate, flavor, sodium bicarbonate, and sucrose. May contain stearic acid. Contains sorbitol or mannitol. May contain starch.
Inactive Ingredients (extra Strength Cherry)
acesulfame k, alginic acid, artificial flavor, calcium stearate, corn starch, corn syrup solids, mannitol, sodium bicarbonate, stearic acid, sucrose
Inactive Ingredients (regular Strength)
alginic acid, calcium stearate, flavor, sodium bicarbonate, starch (may contain corn starch) and sucrose
Questions Or Comments?
call toll-free 1-888-367-6471 (English/Spanish) weekdays
Principal Display Panel
NDC 0135-0098-26
Gaviscon ®
EXTRA STRENGTH
ANTACID
- • Fast-Acting Heartburn Relief
- • Helps Keep Acid Down for Hours
ORIGINAL FLAVOR
100 Chewable Tablets
IMPORTANT: Do not use if foil inner seal printed "SEALED for YOUR PROTECTION" is disturbed or missing.
GAVISCON® is a registered trademark of the Sanofi group of companies and licensed by the GlaxoSmithKline group of companies and TORSO device is a registered trademark of the GlaxoSmithKline group of companies.
Distributed by:
GlaxoSmithKline
Consumer Healthcare, L.P.
Moon Twp, PA 15108
Made in France
©2012 GlaxoSmithKline
102597XA

Principal Display Panel
NDC 0135-0430-03
Gaviscon ®
EXTRA STRENGTH
ANTACID
- • Fast-Acting Heartburn Relief
- • Helps Keep Acid Down for Hours
CHERRY FLAVOR
100 Chewable Tablets
IMPORTANT: Do not use if foil inner seal printed "SEALED for YOUR PROTECTION" is disturbed or missing.
GAVISCON® is a registered trademark of the Sanofi group of companies and licensed by the GlaxoSmithKline group of companies and TORSO device is a registered trademark of the GlaxoSmithKline group of companies.
Distributed by:
GlaxoSmithKline
Consumer Healthcare, L.P.
Moon Twp, PA 15108
Made in France
©2012 GlaxoSmithKline
102599XA

Principal Display Panel
NDC 0135-0096-26
Gaviscon ®
REGULAR STRENGTH
Alumina & Magnesium Trisilicate Tablets/ANTACID
- • Relieves Heartburn Caused by Acid Reflux
- • Unique Antacid Barrier
ORIGINAL FLAVOR
100 Chewable Tablets
IMPORTANT: Do not use if foil inner seal printed "SEALED for YOUR PROTECTION" is disturbed or missing.
GAVISCON® is a registered trademark of the Sanofi group of companies and licensed by the GlaxoSmithKline group of companies and TORSO device is a registered trademark of the GlaxoSmithKline group of companies.
Distributed by:
GlaxoSmithKline
Consumer Healthcare, L.P.
Moon Twp, PA 15108
Made in France
©2012 GlaxoSmithKline
102598XA

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site