HYDROCORTISONE (hydrocortisone 5 mg) Dailymed
Generic: hydrocortisone is used for the treatment of Adrenal Insufficiency Adrenal Hyperplasia, Congenital Colitis, Ulcerative Dermatitis, Contact Dermatomycoses Facial Dermatoses Foot Dermatoses Hand Dermatoses Hemorrhoids Inflammation Leg Dermatoses Pruritus Ani Rheumatic Diseases Scalp Dermatoses Shock Skin Diseases, Parasitic Status Asthmaticus Tuberculosis, Cutaneous Skin Diseases, Viral
IMPRINT: CP 331
SHAPE: round
COLOR: white SCORE: 2
All Imprints
hydrocortisone 5 mg - cp 331 round white
hydrocortisone 20 mg - cp 333 round white
hydrocortisone 10 mg - cp 332 round white
Go PRO for all pill images
Description
Hydrocortisone Tablets, USP contain hydrocortisone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Hydrocortisone USP is white or practically white, crystalline powder with a melting point of about 215°C. It is very slightly soluble in water and in ether; sparingly soluble in acetone and in alcohol; slightly soluble in chloroform.
The chemical name for hydrocortisone is pregn-4-ene-3, 20-dione, 11,17,21-trihydroxy-, (11β)-. Its molecular weight is 362.5 g/mol and the structural formula is as outlined below.
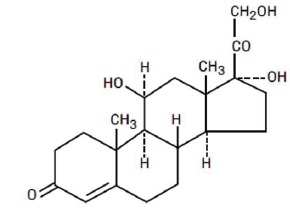
Hydrocortisone Tablets, USP are available for oral administration in three strengths: each tablet contains either 5 mg, 10 mg, or 20 mg of hydrocortisone, USP.
Inactive ingredients: Anhydrous lactose, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium starch glycolate.
Actions
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs are primarily used for their potent anti-inflammatory effects in disorders of many organ systems.
Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body’s immune responses to diverse stimuli.
Indications And Usage
Hydrocortisone Tablets are indicated in the following conditions.
1. Endocrine Disorders
Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice; synthetic analogs may be used in conjunction with mineralocorticoids where applicable; in infancy mineralocorticoid supplementation is of particular importance)Congenital adrenal hyperplasiaNon suppurative thyroiditisHypercalcemia associated with cancer
2. Rheumatic Disorders
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in:Psoriatic arthritisRheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy)Ankylosing spondylitisAcute and subacute bursitisAcute nonspecific tenosynovitisAcute gouty arthritisPost-traumatic osteoarthritisSynovitis of osteoarthritisEpicondylitis
3. Collagen Diseases
During an exacerbation or as maintenance therapy in selected cases of:Systemic lupus erythematosusSystemic dermatomyositis (polymyositis)Acute rheumatic carditis
4. Dermatologic Diseases
PemphigusBullous dermatitis herpetiformisSevere erythema multiforme (Stevens-Johnson syndrome)Exfoliative dermatitisMycosis fungoidesSevere psoriasisSevere seborrheic dermatitis
5. Allergic States
Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment:Seasonal or perennial allergic rhinitisSerum sicknessBronchial asthmaContact dermatitisAtopic dermatitisDrug hypersensitivity reactions
6. Ophthalmic Diseases
Severe acute and chronic allergic and inflammatory processes involving the eye and its adnexa such as:Allergic conjunctivitisKeratitisAllergic corneal marginal ulcersHerpes zoster ophthalmicusIritis and iridocyclitisChorioretinitisAnterior segment inflammationDiffuse posterior uveitis and choroiditisOptic neuritisSympathetic ophthalmia
7. Respiratory Diseases
Symptomatic sarcoidosisLoeffler's syndrome not manageable by other meansBerylliosisFulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapyAspiration pneumonitis
8. Hematologic Disorders
Idiopathic thrombocytopenic purpura in adultsSecondary thrombocytopenia in adultsAcquired (autoimmune) hemolytic anemiaErythroblastopenia (RBC anemia)Congenital (erythroid) hypoplastic anemia
9. Neoplastic Diseases
For palliative management of:Leukemias and lymphomas in adultsAcute leukemia of childhood
10. Edematous States
To induce a diuresis or remission of proteinuria in the nephrotic syndrome, without uremia, of the idiopathic type or that due to lupus erythematosus.
11. Gastrointestinal Diseases
To tide the patient over a critical period of the disease in:Ulcerative colitisRegional enteritis
12. Miscellaneous
Tuberculous meningitis with subarachnoid block or impending block when used concurrently with appropriate antituberculous chemotherapyTrichinosis with neurologic or myocardial involvement
Contraindications
Systemic fungal infections and known hypersensitivity to components.
Warnings
In patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during and after the stressful situation is indicated.
Immunosuppression and Increased Risk of Infection
Corticosteroids, including hydrocortisone, suppress the immune system and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan or helminthic pathogens. Corticosteroids can:
- Reduce resistance to new infections
- Exacerbate existing infections
- Increase the risk of disseminated infections
- Increase the risk of reactivation or exacerbation of latent infections
- Mask some signs of infection
Corticosteroid-associated infections can be mild but can be severe and at times fatal. The rate of infectious complications increases with increasing corticosteroid dosages.
Monitor for the development of infection and consider hydrocortisone withdrawal or dosage reduction as needed.
Tuberculosis
If hydrocortisone is used to treat a condition in patients with latent tuberculosis or tuberculin reactivity, reactivation of tuberculosis may occur. Closely monitor such patients for reactivation. During prolonged hydrocortisone therapy, patients with latent tuberculosis or tuberculin reactivity should receive chemoprophylaxis.
Varicella Zoster and Measles Viral Infections
Varicella and measles can have a serious or even fatal course in non-immune patients taking corticosteroids, including hydrocortisone. In corticosteroid-treated patients who have not had these diseases or are non-immune, particular care should be taken to avoid exposure to varicella and measles:
- If a hydrocortisone-treated patient is exposed to varicella, prophylaxis with varicella zoster immune globulin may be indicated. If varicella develops, treatment with antiviral agents may be considered.
- If a hydrocortisone-treated patient is exposed to measles, prophylaxis with immunoglobulin may be indicated.
Hepatitis B Virus Reactivation
Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers treated with immunosuppressive dosages of corticosteroids, including hydrocortisone. Reactivation can also occur infrequently in corticosteroid-treated patients who appear to have resolved hepatitis B infection.
Screen patients for hepatitis B infection before initiating immunosuppressive (e.g., prolonged) treatment with hydrocortisone. For patients who show evidence of hepatitis B infection, recommend consultation with physicians with expertise in managing hepatitis B regarding monitoring and consideration for hepatitis B antiviral therapy.
Fungal Infections
Corticosteroids, including hydrocortisone, may exacerbate systemic fungal infections; therefore, avoid hydrocortisone use in the presence of such infections unless hydrocortisone is needed to control drug reactions. For patients on chronic hydrocortisone therapy who develop systemic fungal infections, hydrocortisone withdrawal or dosage reduction is recommended.
Amebiasis
Corticosteroids, including hydrocortisone, may activate latent amebiasis. Therefore, it is recommended that latent amebiasis or active amebiasis be ruled out before initiating hydrocortisone in patients who have spent time in the tropics or patients with unexplained diarrhea.
Strongyloides Infestation
Corticosteroids, including hydrocortisone, should be used with great care in patients with known or suspected Strongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
Cerebral Malaria
Avoid corticosteroids, including hydrocortisone, in patients with cerebral malaria.
Ophthalmic Effects
Prolonged use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to fungi or viruses.
Kaposi’s Sarcoma
Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement of Kaposi’s sarcoma.
Hypertension, Volume Overload, and Hypokalemia
Average and large doses of hydrocortisone or cortisone can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
Vaccinations
Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids. Killed or inactivated vaccines may be administered to patients receiving immunosuppressive doses of corticosteroids; however, the response to such vaccines may be diminished. Indicated immunization procedures may be undertaken in patients receiving nonimmunosuppressive doses of corticosteroids.
WARNINGS SECTION
Usage in Pregnancy
Since adequate human reproduction studies have not been done with corticosteroids, the use of these drugs in pregnancy, nursing mothers or women of child bearing potential requires that the possible benefits of the drug be weighed against the potential hazards to the mother and embryo or fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy, should be carefully observed for signs of hypoadrenalism.
Corticosteroids have been shown to impair fertility in male rats.
Precautions
General Precautions
Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted.
There is an enhanced effect of corticosteroids on patients with hypothyroidism and in those with cirrhosis.
Corticosteroids should be used cautiously in patients with ocular herpes simplex because of possible corneal perforation.
The lowest possible dose of corticosteroid should be used to control the condition under treatment and when reduction in dosage is possible, the reduction should be gradual.
Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Steroids should be used with caution in nonspecific ulcerative colitis, if there is a probability of impending perforation, abscess or other pyogenic infection; diverticulitis; fresh intestinal anastomoses; active or latent peptic ulcer; renal insufficiency; hypertension; osteoporosis; and myasthenia gravis.
Growth and development of infants and children on prolonged corticosteroid therapy should be carefully observed.
Since complications of treatment with glucocorticoids are dependent on the size of the dose and the duration of treatment, a risk/benefit decision must be made in each individual case as to dose and duration of treatment and as to whether daily or intermittent therapy should be used.
Pheochromocytoma crisis, which can be fatal, has been reported after administration of systemic corticosteroids. In patients with suspected pheochromocytoma, consider the risk of pheochromocytoma crisis prior to administering corticosteroids.
In post-marketing experience tumor lysis syndrome (TLS) has been reported in patients with malignancies, including hematological malignancies and solid tumors, following the use of systemic corticosteroids alone or in combination with other chemotherapeutic agents. Patients at high risk of TLS, such as patients with tumors that have a high proliferative rate, high tumor burden and high sensitivity to cytotoxic agents, should be monitored closely and appropriate precautions should be taken.
Drug Interactions
The pharmacokinetic interactions uled below are potentially clinically important. Drugs that induce hepatic enzymes such as phenobarbital, phenytoin and rifampin may increase the clearance of corticosteroids and may require increases in corticosteroid dose to achieve the desired response. Drugs such as troleandomycin and ketoconazole may inhibit the metabolism of corticosteroids and thus decrease their clearance. Therefore, the dose of corticosteroid should be titrated to avoid steroid toxicity. Corticosteroids may increase the clearance of chronic high dose aspirin. This could lead to decreased salicylate serum levels or increase the risk of salicylate toxicity when corticosteroid is withdrawn. Aspirin should be used cautiously in conjunction with corticosteroids in patients suffering from hypoprothrombinemia. The effect of corticosteroids on oral anticoagulants is variable. There are reports of enhanced as well as diminished effects of anticoagulants when given concurrently with corticosteroids. Therefore, coagulation indices should be monitored to maintain the desired anticoagulant effect.
Information for the Patient
Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chicken pox or measles. Patients should also be advised that if they are exposed, medical advice should be sought without delay.
Adverse Reactions
Fluid and Electrolyte Disturbances
Sodium retentionFluid retentionCongestive heart failure in susceptible patientsPotassium lossHypokalemic alkalosisHypertension
Musculoskeletal
Muscle weaknessSteroid myopathyLoss of muscle massOsteoporosisTendon rupture, particularly of the Achilles tendonVertebral compression fracturesAseptic necrosis of femoral and humeral headsPathologic fracture of long bones
Gastrointestinal
Peptic ulcer with possible perforation and hemorrhagePancreatitisAbdominal distentionUlcerative esophagitisIncreases in alanine transaminase (ALT, SGPT), aspartate transaminase (AST, SGOT) and alkaline phosphatase have been observed following corticosteroid treatment. These changes are usually small, not associated with any clinical syndrome and are reversible upon discontinuation.
Dermatologic
Impaired wound healingThin fragile skinPetechiae and ecchymosesFacial erythemaIncreased sweatingMay suppress reactions to skin tests
Neurological
Increased intracranial pressure with papilledema (pseudotumor cerebri) usually after treatmentConvulsionsVertigoHeadacheEpidural lipomatosis
Endocrine
Development of Cushingoid stateSuppression of growth in childrenSecondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illnessMenstrual irregularitiesDecreased carbohydrate toleranceManifestations of latent diabetes mellitusIncreased requirements for insulin or oral hypoglycemic agents in diabetics
Ophthalmic
Central serous chorioretinopathyPosterior subcapsular cataractsIncreased intraocular pressureGlaucomaExophthalmos
Metabolic
Negative nitrogen balance due to protein catabolism
Blood and lymphatic system disorders
Leukocytosis
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Dosage And Administration
The initial dosage of hydrocortisone tablets may vary from 20 mg to 240 mg of hydrocortisone per day depending on the specific disease entity being treated. In situations of less severity lower doses will generally suffice while in selected patients higher initial doses may be required. The initial dosage should be maintained or adjusted until a satisfactory response is noted. If after a reasonable period of time there is a lack of satisfactory clinical response, hydrocortisone tablets should be discontinued and the patient transferred to other appropriate therapy. IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT. After a favorable response is noted, the proper maintenance dosage should be determined by decreasing the initial drug dosage in small decrements at appropriate time intervals until the lowest dosage which will maintain an adequate clinical response is reached. It should be kept in mind that constant monitoring is needed in regard to drug dosage. Included in the situations which may make dosage adjustments necessary are changes in clinical status secondary to remissions or exacerbations in the disease process, the patient’s individual drug responsiveness and the effect of patient exposure to stressful situations not directly related to the disease entity under treatment; in this latter situation it may be necessary to increase the dosage of hydrocortisone tablets for a period of time consistent with the patient’s condition. If after long-term therapy the drug is to be stopped, it is recommended that it be withdrawn gradually, rather than abruptly.
How Supplied
Hydrocortisone Tablets, USP 5 mg are white, round tablets bisected, debossed “CP” score “331” on one side and plain on the other side.
They are available as follows:
Bottles of 50: NDC 0115-1696-06
Hydrocortisone Tablets, USP 10 mg are white, round tablets bisected, debossed “CP” score “332” on one side and plain on the other side.
They are available as follows:
Bottles of 100: NDC 0115-1697-01
Hydrocortisone Tablets, USP 20 mg are white, round tablets bisected, debossed “CP” score “333” on one side and plain on the other side.
They are available as follows:
Bottles of 100: NDC 0115-1700-01
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
References
1Fekety R. Infections associated with corticosteroids and immunosuppressive therapy. In: Gorbach SL, Bartlett JG, Blacklow NR, eds. Infectious Diseases. Philadelphia: WB Saunders Company 1992:1050-1.
2Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticoids. Rev Infect Dis 1989:11(6):954-63.
Manufactured by: Amneal Pharmaceuticals Pvt. Ltd. Ahmedabad 382220, INDIA
Distributed by: Amneal Pharmaceuticals LLC Bridgewater, NJ 08807
Rev. 07-2024-02
Principal Display Panel
NDC 0115-1696-06
Hydrocortisone Tablets USP, 5 mg
Rx only
50 Tablets
Bottle Label
Amneal Pharmaceuticals LLC

NDC 0115-1697-01
Hydrocortisone Tablets USP, 10 mg
Rx only
100 Tablets
Bottle Label
Amneal Pharmaceuticals LLC

NDC 0115-1700-01
Hydrocortisone Tablets USP, 20 mg
Rx only
100 Tablets
Bottle Label
Amneal Pharmaceuticals LLC

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site




