Lisinopril (lisinopril 2.5 mg) Dailymed
Generic: lisinopril is used for the treatment of Angioedema Diabetic Nephropathies Heart Failure Hyperaldosteronism Hypertension Hypotension Myocardial Infarction Pregnancy Hypertrophy, Left Ventricular Ventricular Dysfunction, Left
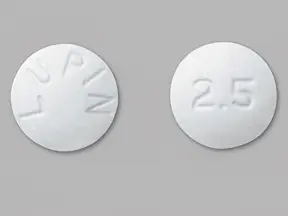
IMPRINT: LUPIN 2 5
SHAPE: round
COLOR: white
All Imprints
lisinopril 2.5 mg - lupin 2 5 round white
lisinopril 5 mg - 5 round pink
lisinopril 20 mg - lupin 20 round pink
lisinopril 30 mg - lupin 30 round red
lisinopril 40 mg - lupin 40 round yellow
lisinopril 10 mg - lupin 10 round pink
Boxed Warning
Boxed Warning Section
Go PRO for all pill images
2.5 mg, 5 mg, 10 mg, 20 mg, 30 mg and 40 mg
Rx only
Boxed Warning Section
USE IN PREGNANCY
When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus. When pregnancy is detected, lisinopril should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
Description
Lisinopril is an oral long-acting angiotensin converting enzyme inhibitor. Lisinopril, a synthetic peptide derivative, is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O5(2H2O and its structural formula is:

Lisinopril is a white to off-white, crystalline powder, with a molecular weight of 441.53. It is soluble in water and sparingly soluble in methanol and practically insoluble in ethanol.
Lisinopril tablets are supplied as 2.5 mg, 5 mg, 10 mg, 20 mg, 30 mg and 40 mg tablets for oral administration.
Inactive Ingredients:
2.5 mg tablets - colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, pre-gelatinized starch, starch.
5 mg, 10 mg, 20 mg and 30 mg tablets ‚Äď colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, pre-gelatinized starch, red iron oxide, starch.
40 mg tablets - colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, pre-gelatinized starch, starch, yellow iron oxide.
Contraindications
Lisinopril is contraindicated in patients who are hypersensitive to this product and in patients with a history of angioedema related to previous treatment with an angiotensin converting enzyme inhibitor and in patients with hereditary or idiopathic angioedema.
Adverse Reactions
Lisinopril has been found to be generally well tolerated in controlled clinical trials involving 1969 patients with hypertension or heart failure. For the most part, adverse experiences were mild and transient.
Hypertension
In clinical trials in patients with hypertension treated with lisinopril, discontinuation of therapy due to clinical adverse experiences occurred in 5.7% of patients. The overall frequency of adverse experiences could not be related to total daily dosage within the recommended therapeutic dosage range.
For adverse experiences occurring in greater than 1% of patients with hypertension treated with lisinopril or lisinopril plus hydrochlorothiazide in controlled clinical trials, and more frequently with lisinopril and/or lisinopril plus hydrochlorothiazide than placebo, comparative incidence data are uled in the table below:
PERCENT OF PATIENTS IN CONTROLLED STUDIES Lisinopril ( n = 1349 ) Incidence ( discontinuation ) Lisinopril /  Hydrochlorothiazide ( n = 629 ) Incidence ( discontinuation ) Placebo ( n = 207 ) Incidence ( discontinuation ) Body  as  a  Whole Fatigue 2.5 (0.3) 4.0 (0.5) 1.0 (0.0) Asthenia 1.3 (0.5) 2.1 (0.2) 1.0 (0.0) Orthostatic Effects 1.2 (0.0) 3.5 (0.2) 1.0 (0.0) Cardiovascular Hypotension 1.2 (0.5) 1.6 (0.5) 0.5 (0.5) Digestive Diarrhea 2.7 (0.2) 2.7 (0.3) 2.4 (0.0) Nausea 2.0 (0.4) 2.5 (0.2) 2.4 (0.0) Vomiting 1.1 (0.2) 1.4 (0.1) 0.5 (0.0) Dyspepsia 0.9 (0.0) 1.9 (0.0) 0.0 (0.0) Musculoskeletal Muscle Cramps 0.5 (0.0) 2.9 (0.8) 0.5 (0.0) Nervous / Psychiatric Headache 5.7 (0.2) 4.5 (0.5) 1.9 (0.0) Dizziness 5.4 (0.4) 9.2 (1.0) 1.9 (0.0) Paresthesia 0.8 (0.1) 2.1 (0.2) 0.0 (0.0) Decreased Libido 0.4 (0.1) 1.3 (0.1) 0.0 (0.0) Vertigo 0.2 (0.1) 1.1 (0.2) 0.0 (0.0) Respiratory Cough 3.5 (0.7) 4.6 (0.8) 1.0 (0.0) Upper Respiratory Infection 2.1 (0.1) 2.7 (0.1) 0.0 (0.0) Common Cold 1.1 (0.1) 1.3 (0.1) 0.0 (0.0) Nasal Congestion 0.4 (0.1) 1.3 (0.1) 0.0 (0.0) Influenza 0.3 (0.1) 1.1 (0.1) 0.0 (0.0) Skin Rash 1.3 (0.4) 1.6 (0.2) 0.5 (0.5) Urogenital Impotence 1.0 (0.4) 1.6 (0.5) 0.0 (0.0)
Chest pain and back pain were also seen, but were more common on placebo than lisinopril.
Heart Failure
In patients with heart failure treated with lisinopril for up to four years, discontinuation of therapy due to clinical adverse experiences occurred in 11% of patients. In controlled studies in patients with heart failure, therapy was discontinued in 8.1% of patients treated with lisinopril for 12 weeks, compared to 7.7% of patients treated with placebo for 12 weeks. The following table uls those adverse experiences which occurred in greater than 1% of patients with heart failure treated with lisinopril or placebo for up to 12 weeks in controlled clinical trials, and more frequently on lisinopril than placebo.
Controlled Trials Controlled Trials Lisinopril (n=407)Incidence(discontinuation)12 weeks Placebo (n=155)Incidence(discontinuation)12 weeks Body as a Whole Chest Pain 3.4 (0.2) 1.3 (0.0) Abdominal Pain 2.2 (0.7) 1.9 (0.0) Cardiovascular Hypotension 4.4 (1.7) 0.6 (0.6) Digestive Diarrhea 3.7 (0.5) 1.9 (0.0) Nervous/Psychiatric Dizziness 11.8 (1.2) 4.5 (1.3) Headache 4.4 (0.2) 3.9 (0.0) Respiratory Upper Respiratory Infection 1.5 (0.0) 1.3 (0.0) Skin Rash 1.7 (0.5) 0.6 (0.6)
Also observed at > 1% with lisinopril but more frequent or as frequent on placebo than lisinopril in controlled trials were asthenia, angina pectoris, nausea, dyspnea, cough, and pruritus. Worsening of heart failure, anorexia, increased salivation, muscle cramps, back pain, myalgia, depression, chest sound abnormalities, and pulmonary edema were also seen in controlled clinical trials, but were more common on placebo than lisinopril. In the two-dose ATLAS trial in heart failure patients, withdrawals due to adverse events were not different between the low and high groups, either in total number of discontinuation (17-18%) or in rare specific events (less than 1%).  The following adverse events, mostly related to ACE inhibition, were reported more commonly in the high dose group: 
% of patients Events High Dose(N=1568) Low Dose(N=1596) Dizziness 18.9 12.1 Hypotension 10.8 6.7 Creatinine-increased 9.9 7.0 Hyperkalemia 6.4 3.5 NPN* increased 9.2 6.5 Syncope 7.0 5.1
*NPN = non-protein nitrogen
Acute Myocardial Infarction
In the GISSI-3 trial, in patients treated with lisinopril for six weeks following acute myocardial infarction, discontinuation of therapy occurred in 17.6% of patients.
Patients treated with lisinopril had a significantly higher incidence of hypotension and renal dysfunction compared with patients not taking lisinopril.
In the GISSI-3 trial, hypotension (9.7%), renal dysfunction (2%), cough (0.5%), post infarction angina (0.3%), skin rash and generalized edema (0.01%), and angioedema (0.01%) resulted in withdrawal of treatment. In elderly patients treated with lisinopril, discontinuation due to renal dysfunction was 4.2%.
Other clinical adverse experiences occurring in 0.3% to 1.0% of patients with hypertension or heart failure treated with lisinopril in controlled clinical trials and rarer, serious, possibly drug-related events reported in uncontrolled studies or marketing experience are uled below, and within each category are in order of decreasing severity:
Body as a Whole
Anaphylactoid reactions (see WARNINGS, Anaphylactoid and Possibly Related Reactions ), syncope, orthostatic effects, chest discomfort, pain, pelvic pain, flank pain, edema, facial edema, virus infection, fever, chills, malaise.
Cardiovascular: Cardiac arrest; myocardial infarction or cerebrovascular accident possibly secondary to excessive hypotension in high risk patients (see WARNINGS, Hypotension ); pulmonary embolism and infarction, arrhythmias (including ventricular tachycardia, atrial tachycardia, atrial fibrillation, bradycardia and premature ventricular contractions), palpitations, transient ischemic attacks, paroxysmal nocturnal dyspnea, orthostatic hypotension, decreased blood pressure, peripheral edema, vasculitis.
Digestive: Pancreatitis, hepatitis (hepatocellular or cholestatic jaundice) (see WARNINGS, Hepatic Failure ), vomiting, gastritis, dyspepsia, heartburn, gastrointestinal cramps, constipation, flatulence, dry mouth.
Hematologic: Rare cases of bone marrow depression, hemolytic anemia, leukopenia/neutropenia and thrombocytopenia.
Endocrine: Diabetes mellitus, inappropriate antidiuretic hormone secretion.
Metabolic: Weight loss, dehydration, fluid overload, gout, weight gain. Cases of hypoglycemia in diabetic patients on oral antidiabetic agents or insulin have been reported in post-marketing experience (See PRECAUTIONS, Drug Interactions ).
Musculoskeletal: Arthritis, arthralgia, neck pain, hip pain, low back pain, joint pain, leg pain, knee pain, shoulder pain, arm pain, lumbago.
Nervous System/Psychiatric: Stroke, ataxia, memory impairment, tremor, peripheral neuropathy (e.g., dysesthesia), spasm, paresthesia, confusion, insomnia, somnolence, hypersomnia, irritability, nervousness and mood alterations (including depressive symptoms).
Respiratory System: Malignant lung neoplasms, hemoptysis, pulmonary infiltrates, bronchospasm, asthma, pleural effusion, pneumonia, eosinophilic pneumonitis, bronchitis, wheezing, orthopnea, painful respiration, epistaxis, laryngitis, sinusitis, pharyngeal pain, pharyngitis, rhinitis, rhinorrhea.
Skin: Urticaria, alopecia, herpes zoster, photosensitivity, skin lesions, skin infections, pemphigus, erythema, flushing, diaphoresis, cutaneous pseudolymphoma. Other severe skin reactions have been reported rarely, including toxic epidermal necrolysis and Stevens-Johnson syndrome; causal relationship has not been established.
Special Senses: Visual loss, diplopia, blurred vision, tinnitus, photophobia, taste disturbances.
Urogenital System: Acute renal failure, oliguria, anuria, uremia, progressive azotemia, renal dysfunction (see PRECAUTIONS and DOSAGE AND ADMINISTRATION ), pyelonephritis, dysuria, urinary tract infection, breast pain.
Miscellaneous: A symptom complex has been reported which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia, fever, vasculitis, eosinophilia and leukocytosis. Rash, photosensitivity or other dermatological manifestations may occur alone or in combination with these symptoms.
Angioedema: Angioedema has been reported in patients receiving lisinopril (0.1%) with an incidence higher in Black than in non-Black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with lisinopril should be discontinued and appropriate therapy instituted immediately (See WARNINGS).
In rare cases, intestinal angioedema has been reported in post marketing experience.
Hypotension: In hypertensive patients, hypotension occurred in 1.2% and syncope occurred in 0.1% of patients with an incidence higher in Black than in non-Black patients. Hypotension or syncope was a cause of discontinuation of therapy in 0.5% of hypertensive patients. In patients with heart failure, hypotension occurred in 5.3% and syncope occurred in 1.8% of patients. These adverse experiences were possibly dose-related (see above data from ATLAS Trial) and caused discontinuation of therapy in 1.8% of these patients in the symptomatic trials. In patients treated with lisinopril for six weeks after acute myocardial infarction, hypotension (systolic blood pressure (100 mmHg) resulted in discontinuation of therapy in 9.7% of the patients. (See WARNINGS).
Fetal/Neonatal Morbidity and Mortality: See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
Cough: See PRECAUTIONS, Cough
Pediatric Patients: No relevant differences between the adverse experience profile for pediatric patients and that previously reported for adult patients were identified.
CLINICAL LABORATORY TEST FINDINGS
Serum Electrolytes: Hyperkalemia (See PRECAUTIONS ), hyponatremia.
Creatinine, Blood Urea Nitrogen: Minor increases in blood urea nitrogen and serum creatinine, reversible upon discontinuation of therapy, were observed in about 2% of patients with essential hypertension treated with lisinopril alone. Increases were more common in patients receiving concomitant diuretics and in patients with renal artery stenosis (See PRECAUTIONS). Reversible minor increases in blood urea nitrogen and serum creatinine were observed in approximately 11.6% of patients with heart failure on concomitant diuretic therapy.  Frequently, these abnormalities resolved when the dosage of the diuretic was decreased.
Hemoglobin and Hematocrit: Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.4 g% and 1.3 vol%, respectively) occurred frequently in patients treated with lisinopril but were rarely of clinical importance in patients without some other cause of anemia. In clinical trials, less than 0.1% of patients discontinued therapy due to anemia. Hemolytic anemia has been reported; a causal relationship to lisinopril cannot be excluded.
Liver Function Tests: Rarely, elevations of liver enzymes and/or serum bilirubin have occurred (See WARNINGS, Hepatic Failure).
In hypertensive patients, 2.0% discontinued therapy due to laboratory adverse experiences, principally elevations in blood urea nitrogen (0.6%), serum creatinine (0.5%) and serum potassium (0.4%).
In the heart failure trials, 3.4% of patients discontinued therapy due to laboratory adverse experiences; 1.8% due to elevations in blood urea nitrogen and/or creatinine and 0.6% due to elevations in serum potassium.
In the myocardial infarction trial, 2.0% of patients receiving lisinopril discontinued therapy due to renal dysfunction (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration); less than 1.0% of patients discontinued therapy due to other laboratory adverse experiences: 0.1% with hyperkalemia and less than 0.1% with hepatic enzyme alterations.
Overdosage
Following a single oral dose of 20 g/kg no lethality occurred in rats, and death occurred in one of 20 mice receiving the same dose. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution.
Lisinopril can be removed by hemodialysis (See WARNINGS, Anaphylactoid Reactions During Membrane Exposure).
How Supplied
Lisinopril Tablets, USP are available as:
2.5 mg tablet is a white to off-white, round, biconvex uncoated tablet with ‚ÄėLUPIN" debossed on one side and "2.5" on other side. They are available as follows:
Bottles of 90              NDC 68180-512-09
Bottles of 100            NDC 68180-512-01
Bottles of 500            NDC 68180-512-02
Bottles of 1000          NDC 68180-512-03
5 mg tablet is a pink coloured, round, biconvex uncoated tablet with "5" debossed on one side and breakline on other side. They are available as follows:
Bottles of 90              NDC 68180-513-09
Bottles of 100            NDC 68180-513-01
Bottles of 500            NDC 68180-513-02
Bottles of 1000          NDC 68180-513-03
Bottles of 5000          NDC 68180-513-05
10 mg tablet is a pink coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "10" on other side. They are available as follows:
Bottles of 90              NDC 68180-514-09
Bottles of 100            NDC 68180-514-01
Bottles of 500            NDC 68180-514-02
Bottles of 1000          NDC 68180-514-03
Bottles of 5000          NDC 68180-514-05
20 mg tablet is a pink coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "20" on other side. They are available as follows:
Bottles of 90              NDC 68180-515-09
Bottles of 100            NDC 68180-515-01
Bottles of 500            NDC 68180-515-02
Bottles of 1000          NDC 68180-515-03
Bottles of 5000          NDC 68180-515-05
30 mg tablet is a red coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "30" on other side. They are available as follows:
Bottles of 90              NDC 68180-516-09
Bottles of 100            NDC 68180-516-01
Bottles of 500            NDC 68180-516-02
Bottles of 1000          NDC 68180-516-03
40 mg tablet is a yellow coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "40" on other side. They are available as follows:
Bottles of 90              NDC 68180-517-09
Bottles of 100            NDC 68180-517-01
Bottles of 500            NDC 68180-517-02
Bottles of 1000          NDC 68180-517-03
Bottles of 2000          NDC 68180-517-04
Storage:
Store at 20¬ļ to 25¬ļC (68¬ļ to 77¬ļF)[see USP Controlled Room Temperature]. Protect from moisture, freezing and excessive heat. Dispense in a tight container.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States.  
Manufactured by:
Lupin Limited
Goa 403 722
INDIA.
Or
Lupin Limited
Pithampur (M.P.) 454 775
INDIA.
Principal Display Panel

NDC 68258-6036-XXNDC 68258-6036-03
NDC 68258-6036-09
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site