Meclizine Hydrochloride (meclizine hydrochloride 25 mg) Dailymed
Generic: meclizine hydrochloride is used for the treatment of Motion Sickness Vertigo
IMPRINT: AN 442
SHAPE: oval
COLOR: yellow
All Imprints
meclizine hydrochloride tablet - an 442 oval yellow
meclizine hydrochloride 12.5 mg - an 441 oval blue
meclizine hydrochloride 25 mg - an 442 oval yellow
Go PRO for all pill images
Description
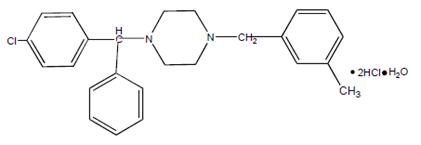
Chemically, meclizine hydrochloride, USP is 1-( p-chloro-α-phenylbenzyl)-4-( m-methylbenzyl) piperazine dihydrochloride monohydrate.
Inactive ingredients for the tablets are: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium starch glycolate and talc. The 12.5 mg tablets also contain FD&C Blue #1 Aluminum Lake. The 25 mg tablets also contain D&C Yellow #10 Aluminum Lake.
Clinical Pharmacology
Meclizine hydrochloride is an antihistamine that shows marked protective activity against nebulized histamine and lethal doses of intravenously injected histamine in guinea pigs. It has a marked effect in blocking the vasodepressor response to histamine, but only a slight blocking action against acetylcholine. Its activity is relatively weak in inhibiting the spasmogenic action of histamine on isolated guinea pig ileum.
Pharmacokinetics
The available pharmacokinetic information for meclizine following oral administration has been summarized from published literature.
Absorption
Meclizine is absorbed after oral administration with maximum plasma concentrations reaching at a median T max value of 3 hours post-dose (range: 1.5 to 6 hours) for the tablet dosage form.
Distribution
Drug distribution characteristics for meclizine in humans are unknown.
Metabolism
The metabolic fate of meclizine in humans is unknown. In an in vitro metabolic study using human hepatic microsome and recombinant CYP enzyme, CYP2D6 was found to be the dominant enzyme for metabolism of meclizine.
The genetic polymorphism of CYP2D6 that results in extensive-, poor-, intermediate- and ultrarapid metabolizer phenotypes could contribute to large inter-individual variability in meclizine exposure.
Elimination
Meclizine has a plasma elimination half-life of about 5 to 6 hours in humans.
Indications And Usage
Meclizine hydrochloride tablets are indicated for the treatment of vertigo associated with diseases affecting the vestibular system.
Contraindications
Meclizine hydrochloride is contraindicated in individuals who have shown a previous hypersensitivity to it.
Warnings
Since drowsiness may, on occasion, occur with use of this drug, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery.
Patients should avoid alcoholic beverages while taking this drug.
Due to its potential anticholinergic action, this drug should be used with caution in patients with asthma, glaucoma, or enlargement of the prostate gland.
Precautions
Pediatric Use
Clinical studies establishing safety and effectiveness in children have not been done; therefore, usage is not recommended in children under 12 years of age.
Pregnancy
Pregnancy Category B. Reproduction studies in rats have shown cleft palates at 25 to 50 times the human dose. Epidemiological studies in pregnant women, however, do not indicate that meclizine increases the risk of abnormalities when administered during pregnancy. Despite the animal findings, it would appear that the possibility of fetal harm is remote. Nevertheless, meclizine, or any other medication, should be used during pregnancy only if clearly necessary.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when meclizine is administered to a nursing woman.
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of meclizine has not been evaluated. As meclizine undergoes metabolism, hepatic impairment may result in increased systemic exposure of the drug. Treatment with meclizine should be administered with caution in patients with hepatic impairment.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of meclizine has not been evaluated. Due to a potential for drug/metabolite accumulation, meclizine should be administered with caution in patients with renal impairment and in the elderly as renal function generally declines with age.
Drug Interactions
There may be increased CNS depression when meclizine is administered concurrently with other CNS depressants, including alcohol, tranquilizers and sedatives (see WARNINGS ).
Based on in vitro evaluation, meclizine is metabolized by CYP2D6. Therefore there is a possibility for a drug interaction between meclizine and CYP2D6 inhibitors.
Adverse Reactions
Anaphylactoid reaction, drowsiness, dry mouth, headache, fatigue, vomiting and, on rare occasions, blurred vision have been reported.
To report SUSPECTED ADVERSE REACTIONS contact AvKARE at 1-855-361-3993; email drugsafety@avkare.com; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Dosage And Administration
For the treatment of vertigo associated with diseases affecting the vestibular system, the recommended dose is 25 mg to 100 mg daily, in divided dosage, depending upon clinical response.
How Supplied
Meclizine Hydrochloride Tablets, USP 12.5 mg, are supplied as light blue colored, oval shaped tablets with “AN 441” debossed on one side and plain on the other side.
Meclizine Hydrochloride Tablets, USP 25 mg, are supplied as light yellow colored, oval shaped tablets with “AN 442” debossed on one side and plain on the other side.
They are available as follows:
Bottles of 1000: NDC 42291-609-10
Meclizine Hydrochloride Tablets, USP 50 mg, are supplied as white, oval shaped, partially bisected tablets with “AN 444” debossed on one side and plain on the other side.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container.
Manufactured for:
AvKARE
Pulaski, TN 38478
Manufactured by:
Amneal Pharmaceuticals of New York, LLC
Brookhaven, NY 11719
Mfg. Rev. 10-2019-05 AV 03/23
Package Label.principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site



