Methocarbamol (methocarbamol 500 mg) Dailymed
Generic: methocarbamol is used for the treatment of Muscle Cramp Muscle Rigidity Myositis Pain Spasm Tetanus Renal Insufficiency
All Imprints
methocarbamol 750 mg - 115 h oval white
methocarbamol 500 mg - 114 h oval white
Go PRO for all pill images
Description
Methocarbamol tablets USP a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties.
The chemical name of methocarbamol is 3-(2-methoxyphenoxy)-1,2-propanediol 1-carbamate and has the empirical formula C 11H 15NO 5. Its molecular weight is 241.24. The structural formula is shown below.
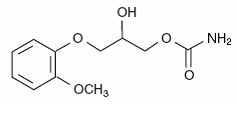
Methocarbamol is a white powder, sparingly soluble in water and chloroform, soluble in alcohol (only with heating) and propylene glycol, and insoluble in benzene and n-hexane.
Methocarbamol tablets USP are available as 500 mg and 750 mg tablets for oral administration. Methocarbamol tablets USP 500 mg and 750 mg contain the following inactive ingredients: sodium lauryl sulfate, sodium starch glycolate, povidone K 90, polyethylene glycol, magnesium stearate, colloidal silicon dioxide, low substituted hydroxypropyl cellulose and stearic acid.
Clinical Pharmacology
The mechanism of action of methocarbamol in humans has not been established, but may be due to general central nervous system (CNS) depression. It has no direct action on the contractile mechanism of striated muscle, the motor end plate or the nerve fiber.
Pharmacokinetics
In healthy volunteers, the plasma clearance of methocarbamol ranges between 0.20 and 0.80 L/h/kg, the mean plasma elimination half-life ranges between 1 and 2 hours, and the plasma protein binding ranges between 46% and 50%.
Methocarbamol is metabolized via dealkylation and hydroxylation. Conjugation of methocarbamol also is likely. Essentially all methocarbamol metabolites are eliminated in the urine. Small amounts of unchanged methocarbamol also are excreted in the urine.
Special populations
Elderly The mean (±SD) elimination half-life of methocarbamol in elderly healthy volunteers (mean (±SD) age, 69 (±4) years) was slightly prolonged compared to a younger (mean (±SD) age, 53.3 (±8.8) years), healthy population (1.5 (±0.4) hours versus 1.1 (±0.27) hours, respectively). The fraction of bound methocarbamol was slightly decreased in the elderly versus younger volunteers (41 to 43% versus 46 to 50%, respectively).
Renally impaired The clearance of methocarbamol in 8 renally-impaired patients on maintenance hemodialysis was reduced about 40% compared to 17 normal subjects, although the mean (±SD) elimination half-life in these two groups was similar: 1.2 (± 0.6) versus 1.1 (±0.3) hours, respectively.
Hepatically impaired In 8 patients with cirrhosis secondary to alcohol abuse, the mean total clearance of methocarbamol was reduced approximately 70% compared to that obtained in 8 age- and weight-matched normal subjects. The mean (±SD) elimination half-life in the cirrhotic patients and the normal subjects was 3.38 (±1.62) hours and 1.11 (±0.27) hours, respectively. The percent of methocarbamol bound to plasma proteins was decreased to approximately 40 to 45% compared to 46 to 50% in the normal subjects.
Indications & Usage
Methocarbamol tablets USP are indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of methocarbamol has not been clearly identified, but may be related to its sedative properties. Methocarbamol does not directly relax tense skeletal muscles in man.
Contraindications
Methocarbamol tablets USP are contraindicated in patients hypersensitive to methocarbamol or to any of the tablet components.
Warnings
Since methocarbamol may possess a general CNS depressant effect, patients receiving methocarbamol tablets USP should be cautioned about combined effects with alcohol and other CNS depressants.
Safe use of methocarbamol tablets USP has not been established with regard to possible adverse effects upon fetal development. There have been reports of fetal and congenital abnormalities following in utero exposure to methocarbamol. Therefore, methocarbamol tablets USP should not be used in women who are or may become pregnant and particularly during early pregnancy unless in the judgment of the physician the potential benefits outweigh the possible hazards (see PRECAUTIONS, Pregnancy ).
Use In Activities Requiring Mental Alertness
Methocarbamol may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle. Patients should be cautioned about operating machinery, including automobiles, until they are reasonably certain that methocarbamol therapy does not adversely affect their ability to engage in such activities.
Precautions
Information for Patients
Patients should be cautioned that methocarbamol may cause drowsiness or dizziness, which may impair their ability to operate motor vehicles or machinery.
Because methocarbamol may possess a general CNS-depressant effect, patients should be cautioned about combined effects with alcohol and other CNS depressants.
Drug Interactions
See WARNINGS and PRECAUTIONS for interaction with CNS drugs and alcohol.
Methocarbamol may inhibit the effect of pyridostigmine bromide. Therefore, methocarbamol should be used with caution in patients with myasthenia gravis receiving anticholinesterase agents.
Drug/Laboratory Test Interactions
Methocarbamol may cause a color interference in certain screening tests for 5-hydroxyindoleacetic acid (5-HIAA) using nitrosonaphthol reagent and in screening tests for urinary vanillylmandelic acid (VMA) using the Gitlow method.
Carcinogenesis, Mutagenesis. Impairment of Fertility
Long-term studies to evaluate the carcinogenic potential of methocarbamol have not been performed. No studies have been conducted to assess the effect of methocarbamol on mutagenesis or its potential to impair fertility.
Pregnancy
Teratogenic effects -Pregnancy Category C Animal reproduction studies have not been conducted with methocarbamol. It is also not known whether methocarbamol can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methocarbamol tablets USP should be given to a pregnant woman only if clearly needed.
Safe use of methocarbamol tablets USP has not been established with regard to possible adverse effects upon fetal development. There have been reports of fetal and congenital abnormalities following in utero exposure to methocarbamol. Therefore, methocarbamol tablets USP should not be used in women who are or may become pregnant and particularly during early pregnancy unless in the judgment of the physician the potential benefits outweigh the possible hazards ( see WARNINGS ).
Nursing Mothers
Methocarbamol and/or its metabolites are excreted in the milk of dogs; however, it is not known whether methocarbamol or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when methocarbamol tablets USP are administered to a nursing woman.
Pediatric Use
Safety and effectiveness of methocarbamol tablets USP in pediatric patients below the age of 16 have not been established.
Adverse Reactions
Adverse reactions reported coincident with the administration of methocarbamol include: Body as a whole: Anaphylactic reaction, angioneurotic edema, fever, headache Cardiovascular system: Bradycardia, flushing, hypotension, syncope, thrombophlebitis Digestive system: Dyspepsia, jaundice (including cholestatic jaundice), nausea and vomiting Hemic and lymphatic system: Leukopenia Immune system: Hypersensitivity reactions Nervous system: Amnesia, confusion, diplopia, dizziness or lightheadedness, drowsiness, insomnia, mild muscular incoordination, nystagmus, sedation, seizures (including grand mal), vertigo Skin and special senses: Blurred vision, conjunctivitis, nasal congestion, metallic taste, pruritus, rash, urticarial
Overdosage
Limited information is available on the acute toxicity of methocarbamol. Overdose of methocarbamol is frequently in conjunction with alcohol or other CNS depressants and includes the following symptoms: nausea, drowsiness, blurred vision, hypotension, seizures, and coma.
In post-marketing experience, deaths have been reported with an overdose of methocarbamol alone or in the presence of other CNS depressants, alcohol or psychotropic drugs.
Treatment
Management of overdose includes symptomatic and supportive treatment. Supportive measures include maintenance of an adequate airway, monitoring urinary output and vital signs, and administration of intravenous fluids if necessary. The usefulness of hemodialysis in managing overdose is unknown.
Dosage & Administration
Methocarbamol Tablets USP 500 mg – Adults: Initial dosage: 3 tablets q.i.d. Maintenance dosage: 2 tablets q.i.d.
Methocarbamol Tablets USP 750 mg – Adults: Initial dosage: 2 tablets q.i.d. Maintenance dosage: 1 tablet q.4h. or 2 tablets t.i.d.
Six grams a day are recommended for the first 48 to 72 hours of treatment. (For severe conditions 8 grams a day may be administered). Thereafter, the dosage can usually be reduced to approximately 4 grams a day.
How Supplied
Methocarbamol tablets USP 500 mg are white to off white, capsule shaped, tablets debossed with ‘H’ on scored side and ‘114’ on unscored side. They are supplied as follows: Unit dose packages of 100 (10 x 10) NDC 60687-559-01
Methocarbamol tablets USP 750 mg are white to off white, capsule shaped, tablets debossed with ‘H’ on one side and ‘115’ on other side. They are supplied as follows: Unit dose packages of 100 (10 x 10) NDC 60687-568-01
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
FOR YOUR PROTECTION: Do not use if buler is torn or broken.
Packaging Information
American Health Packaging unit dose bulers (see How Supplied section) contain drug product from Camber Pharmaceuticals, Inc. as follows: (500 mg/ 100 UD) NDC 60687-559-01 packaged from NDC 31722-533 (750 mg/ 100 UD) NDC 60687-568-01 packaged from NDC 31722-534
Distributed by: American Health Packaging Columbus, OH 43217
8455901/0320
Package/label Display Panel Carton 500 Mg

NDC 60687- 559-01
Methocarbamol Tablets USP
500 mg
100 Tablets (10 x 10)                 Rx Only
Each Uncoated Tablet Contains: Methocarbamol USP ..................................................500 mg
Usual Dosage: Two to four tablets four times daily. See full prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
FOR YOUR PROTECTION: Do not use if buler is torn or broken.
The drug product contained in this package is from NDC # 31722-533, Camber Pharmaceuticals, Inc.
Distributed by: American Health Packaging, Columbus, Ohio 43217
755901 0455901/0824
Package/label Display Panel Blister 500 Mg

Methocarbamol Tablet USP
500 mg
Package/label Display Panel Carton 750 Mg

NDC 60687- 568-01
Methocarbamol Tablets USP
750 mg
100 Tablets (10 x 10)                 Rx Only
Each Uncoated Tablet Contains: Methocarbamol USP ..................................................750 mg
Usual Dosage: Two tablets three times daily. See full prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if buler is torn or broken.
The drug product contained in this package is from NDC # 31722-534, Camber Pharmaceuticals, Inc.
Distributed by: American Health Packaging, Columbus, Ohio 43217
756801 0456801/0824
Package/label Display Panel Blister 750 Mg

Methocarbamol Tablet USP
750 mg
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


