Micardis HCT (telmisartan 80 mg hydrochlorothiazide 12.5 mg) Dailymed
Generic: telmisartan and hydrochlorothiazide
Boxed Warning
Warning: Fetal Toxicity
-
When pregnancy is detected, discontinue MICARDIS HCT as soon as possible. -
Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus (seeWARNINGS, Fetal Toxicity ).
Go PRO for all pill images
Prescribing Information
Warning: Fetal Toxicity
When pregnancy is detected, discontinue MICARDIS HCT as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus (seeWARNINGS, Fetal Toxicity ).
Description
MICARDIS HCT tablets are a combination of telmisartan, an orally active angiotensin II antagonist acting on the AT1 receptor subtype, and hydrochlorothiazide, a diuretic.
Telmisartan, a non-peptide molecule, is chemically described as 4'-[(1,4'-dimethyl-2'-propyl[2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid. Its empirical formula is C33H30N4O2, its molecular weight is 514.63, and its structural formula is:
![]()
Telmisartan is a white to slightly yellowish solid. It is practically insoluble in water and in the pH range of 3 to 9, sparingly soluble in strong acid (except insoluble in hydrochloric acid), and soluble in strong base.
Hydrochlorothiazide is a white, or practically white, practically odorless, crystalline powder with a molecular weight of 297.74. It is slightly soluble in water, and freely soluble in sodium hydroxide solution. Hydrochlorothiazide is chemically described as 6-chloro-3,4-dihydro-2H -1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2, and its structural formula is:
![]()
![]()
MICARDIS HCT tablets are formulated for oral administration in three combinations of 40 mg/12.5 mg, 80 mg/12.5 mg, and 80 mg/25 mg telmisartan and hydrochlorothiazide, respectively. The tablets contain the following inactive ingredients: sodium hydroxide, meglumine, povidone, sorbitol, magnesium stearate, lactose monohydrate, microcrystalline cellulose, maize starch, sodium starch glycolate. As coloring agents, the 40 mg/12.5 mg and 80 mg/12.5 mg tablets contain ferric oxide red, and the 80 mg/25 mg tablets contain ferric oxide yellow. Micardis® HCT (telmisartan and hydrochlorothiazide) tablets are hygroscopic and require protection from moisture.
Indications And Usage
Micardis® HCT (telmisartan and hydrochlorothiazide) tablets are indicated for the treatment of hypertension. This fixed dose combination is not indicated for initial therapy (seeDOSAGE AND ADMINISTRATION ).
Contraindications
MICARDIS HCT tablets are contraindicated in patients with known hypersensitivity (e.g., anaphylaxis or angioedema) to telmisartan, hydrochlorothiazide, or any other component of this product (seeADVERSE REACTIONS ).
Because of the hydrochlorothiazide component, this product is contraindicated in patients with anuria or hypersensitivity to other sulfonamide-derived drugs.
Warnings
Fetal Toxicity
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue MICARDIS HCT as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue MICARDIS HCT, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to MICARDIS HCT for hypotension, oliguria, and hyperkalemia (seePRECAUTIONS, Pediatric Use ).
No teratogenic effects were observed when telmisartan was administered to pregnant rats at oral doses of up to 50 mg/kg/day and to pregnant rabbits at oral doses up to 45 mg/kg/day. In rabbits, embryolethality associated with maternal toxicity (reduced body weight gain and food consumption) was observed at 45 mg/kg/day [about 12 times the maximum recommended human dose (MRHD) of 80 mg on a mg/m2 basis]. In rats, maternally toxic (reduction in body weight gain and food consumption) telmisartan doses of 15 mg/kg/day (about 1.9 times the MRHD on a mg/m2 basis), administered during late gestation and lactation, were observed to produce adverse effects in neonates, including reduced viability, low birth weight, delayed maturation, decreased weight gain. Telmisartan has been shown to be present in rat fetuses during late gestation and in rat milk. The no observed effect doses for developmental toxicity in rats and rabbits, 5 and 15 mg/kg/day, respectively, are about 0.64 and 3.7 times, on a mg/m2 basis, the maximum recommended human dose of telmisartan (80 mg/day).
Studies in which hydrochlorothiazide was administered to pregnant mice and rats during their periods of major organogenesis at doses up to 3000 and 1000 mg/kg/day, respectively, provided no evidence of harm to the fetus.
Thiazides cross the placental barrier and appear in cord blood. There is a risk of fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
Hypotension in Volume-Depleted Patients
Initiation of antihypertensive therapy in patients whose renin-angiotensin system are activated such as patients who are intravascular volume- or sodium-depleted, e.g., in patients treated vigorously with diuretics, should only be approached cautiously. These conditions should be corrected prior to administration of Micardis® HCT (telmisartan and hydrochlorothiazide) tablets. Treatment should be started under close medical supervision (seeDOSAGE AND ADMINISTRATION ). If hypotension occurs, the patients should be placed in the supine position and, if necessary, given an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment which usually can be continued without difficulty once the blood pressure has stabilized.
Hydrochlorothiazide
Hepatic Impairment : Thiazide diuretics should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
Hypersensitivity Reaction : Hypersensitivity reactions to hydrochlorothiazide may occur in patients with or without a history of allergy or bronchial asthma, but are more likely in patients with such a history.
Systemic Lupus Erythematosus : Thiazide diuretics have been reported to cause exacerbation or activation of systemic lupus erythematosus.
Lithium Interaction : Lithium generally should not be given with thiazides (see PRECAUTIONS, Drug Interactions, Hydrochlorothiazide , Lithium ).
Acute Myopia and Secondary Angle-Closure Glaucoma: Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
Adverse Reactions
Micardis® HCT (telmisartan and hydrochlorothiazide) tablets has been evaluated for safety in over 1700 patients, including 716 treated for over six months and 420 for over one year. In clinical trials with MICARDIS HCT tablets, no unexpected adverse events have been observed. Adverse experiences have been limited to those that have been previously reported with telmisartan and/or hydrochlorothiazide. The overall incidence of adverse experiences reported with the combination was comparable to placebo. Most adverse experiences were mild in intensity and transient in nature and did not require discontinuation of therapy.
Adverse events occurring at an incidence of 2% or more in patients treated with telmisartan/hydrochlorothiazide and at a greater rate than in patients treated with placebo, irrespective of their causal association, are presented in Table 1.
TABLE 1 Adverse Events Occurring in ≥ 2% of Telmisartan/Hydrochlorothiazide (HCTZ) Patients* Telm/HCTZ Placebo Telm HCTZ (N=414) (N=74) (N=209) (N=121) (%) (%) (%) (%) * includes all doses of telmisartan (20-160 mg), hydrochlorothiazide (6.25-25 mg), and combinations thereof Body as a whole   Fatigue 3 1 3 3   Influenza-like  symptoms 2 1 2 3 Central/peripheral nervous system   Dizziness 5 1 4 6 Gastrointestinal system   Diarrhea 3 0 5 2   Nausea 2 0 1 2 Respiratory system disorder   Sinusitis 4 3 3 6   Upper respiratory   tract infection 8 7 7 10
The following adverse events were reported at a rate less than 2% in patients treated with telmisartan/hydrochlorothiazide and at a greater rate than in patients treated with placebo: back pain, dyspepsia, vomiting, tachycardia, hypokalemia, bronchitis, pharyngitis, rash, hypotension postural, abdominal pain.
Finally, the following adverse events were reported at a rate of 2% or greater in patients treated with telmisartan/hydrochlorothiazide, but were as, or more common in the placebo group: pain, headache, cough, urinary tract infection.
Adverse events occurred at approximately the same rates in men and women, older and younger patients, and black and non-black patients.
In controlled trials (n=1017), 0.3% of patients treated with Micardis® HCT (telmisartan and hydrochlorothiazide) tablets 40/12.5 mg, 80/12.5 mg or 80/25 mg discontinued due to orthostatic hypotension, and the incidence of dizziness was 4%, 7%, and 1%, respectively.
Telmisartan
Other adverse experiences that have been reported with telmisartan, without regard to causality, are uled below:
Autonomic Nervous System : impotence, increased sweating, flushing
Body as a Whole : allergy, fever, leg pain, malaise, chest pain
Cardiovascular : palpitation, dependent edema, angina pectoris, leg edema, abnormal ECG, hypertension, peripheral edema
CNS : insomnia, somnolence, migraine, vertigo, paresthesia, involuntary muscle contractions, hypoaesthesia
Gastrointestinal : flatulence, constipation, gastritis, dry mouth, hemorrhoids, gastroenteritis, enteritis, gastroesophageal reflux, toothache, non-specific gastrointestinal disorders
Metabolic : gout, hypercholesterolemia, diabetes mellitus
Musculoskeletal : arthritis, arthralgia, leg cramps, myalgia
Psychiatric : anxiety, depression, nervousness
Resistance Mechanism : infection, fungal infection, abscess, otitis media
Respiratory : asthma, rhinitis, dyspnea, epistaxis
Skin : dermatitis, eczema, pruritus
Urinary : micturition frequency, cystitis
Vascular : cerebrovascular disorder
Special Senses : abnormal vision, conjunctivitis, tinnitus, earache
A single case of angioedema was reported (among a total of 3781 patients treated with telmisartan).
Hydrochlorothiazide
Other adverse experiences that have been reported with hydrochlorothiazide, without regard to causality, are uled below:
Body as a whole : weakness
Digestive : pancreatitis, jaundice (intrahepatic cholestatic jaundice), sialadenitis, cramping, gastric irritation
Hematologic : aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia
Hypersensitivity : purpura, photosensitivity, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), fever, respiratory distress including pneumonitis and pulmonary edema, anaphylactic reactions
Metabolic : hyperglycemia, glycosuria, hyperuricemia
Musculoskeletal : muscle spasm
Nervous System/Psychiatric : restlessness
Renal : renal failure, renal dysfunction, interstitial nephritis
Skin : erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis
Special Senses : transient blurred vision, xanthopsia
Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of Micardis® (telmisartan) tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to MICARDIS tablets. The most frequently spontaneously reported events include: headache, dizziness, asthenia, coughing, nausea, fatigue, weakness, edema, face edema, lower limb edema, angioneurotic edema, urticaria, hypersensitivity, sweating increased, erythema, chest pain, atrial fibrillation, congestive heart failure, myocardial infarction, blood pressure increased, hypertension aggravated, hypotension (including postural hypotension), hyperkalemia, syncope, dyspepsia, diarrhea, pain, urinary tract infection, erectile dysfunction, back pain, abdominal pain, muscle cramps (including leg cramps), myalgia, bradycardia, eosinophilia, thrombocytopenia, uric acid increased, abnormal hepatic function/liver disorder, renal impairment including acute renal failure, anemia, increased CPK, anaphylactic reaction, tendon pain (including tendonitis, tenosynovitis), drug eruption (toxic skin eruption mostly reported as toxicoderma, rash, and urticaria), hypoglycemia (in diabetic patients), and angioedema (with fatal outcome).
Rare cases of rhabdomyolysis have been reported in patients receiving angiotensin II receptor blockers, including MICARDIS tablets.
Clinical Laboratory Findings
In controlled trials, clinically relevant changes in standard laboratory test parameters were rarely associated with administration of Micardis® HCT (telmisartan and hydrochlorothiazide) tablets.
Hemoglobin and Hematocrit : Decreases in hemoglobin (≥ 2 g/dL) and hematocrit (≥ 9%) were observed in 1.2% and 0.6% of telmisartan/hydrochlorothiazide patients, respectively, in controlled trials. Changes in hemoglobin and hematocrit were not considered clinically significant and there were no discontinuations due to anemia.
Creatinine, Blood Urea Nitrogen (BUN) : Increases in BUN (≥ 11.2 mg/dL) and serum creatinine (≥ 0.5 mg/dL) were observed in 2.8% and 1.4%, respectively, of patients with essential hypertension treated with MICARDIS HCT tablets in controlled trials. No patient discontinued treatment with MICARDIS HCT tablets due to an increase in BUN or creatinine.
Liver Function Tests : Occasional elevations of liver enzymes and/or serum bilirubin have occurred. No telmisartan/hydrochlorothiazide treated patients discontinued therapy due to abnormal hepatic function.
Serum Electrolytes : See PRECAUTIONS .
Overdosage
Telmisartan
Limited data are available with regard to overdosage in humans. The most likely manifestations of overdosage with telmisartan would be hypotension, dizziness and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. If symptomatic hypotension should occur, supportive treatment should be instituted. Telmisartan is not removed by hemodialysis.
Hydrochlorothiazide
The most common signs and symptoms observed in patients are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias. The degree to which hydrochlorothiazide is removed by hemodialysis has not been established. The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in both mice and rats.
Dosage And Administration
The usual starting dose of telmisartan is 40 mg once a day; blood pressure response is dose related over the range of 20-80 mg. Patients with depletion of intravascular volume should have the condition corrected or telmisartan tablets should be initiated under close medical supervision (seeWARNINGS, Hypotension in Volume-Depleted Patients ). Patients with biliary obstructive disorders or hepatic insufficiency should have treatment started under close medical supervision (seePRECAUTIONS ).
Hydrochlorothiazide is effective in doses of 12.5 mg to 50 mg once daily.
To minimize dose-independent side effects, it is usually appropriate to begin combination therapy only after a patient has failed to achieve the desired effect with monotherapy. The side effects (seeWARNINGS ) of telmisartan are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent phenomena (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter. Therapy with any combination of telmisartan and hydrochlorothiazide will be associated with both sets of dose-independent side effects.
Micardis® HCT (telmisartan and hydrochlorothiazide) tablets may be administered with other antihypertensive agents.
MICARDIS HCT tablets may be administered with or without food.
Replacement Therapy
The combination may be substituted for the titrated components.
Dose Titration by Clinical Effect
MICARDIS HCT tablets are available as tablets containing either telmisartan 40 mg and hydrochlorothiazide 12.5 mg, or telmisartan 80 mg and hydrochlorothiazide 12.5 mg or 25 mg. A patient whose blood pressure is not adequately controlled with telmisartan monotherapy 80 mg (see above) may be switched to MICARDIS HCT tablets, telmisartan 80 mg/hydrochlorothiazide 12.5 mg once daily, and finally titrated up to 160/25 mg, if necessary.
A patient whose blood pressure is inadequately controlled by 25 mg once daily of hydrochlorothiazide may be switched to MICARDIS HCT (telmisartan 80 mg/hydrochlorothiazide 12.5 mg or telmisartan 80 mg/hydrochlorothiazide 25 mg) tablets once daily. The clinical response to MICARDIS HCT tablets should be subsequently evaluated and if blood pressure remains uncontrolled after 2-4 weeks of therapy, the dose may be titrated up to 160/25 mg, if necessary. Those patients controlled by 25 mg hydrochlorothiazide but who experience hypokalemia with this regimen, may be switched to MICARDIS HCT (telmisartan 80 mg/hydrochlorothiazide 12.5 mg) tablets once daily, reducing the dose of hydrochlorothiazide without reducing the overall expected antihypertensive response.
Patients with Renal Impairment
The usual regimens of therapy with Micardis® HCT (telmisartan and hydrochlorothiazide) tablets may be followed as long as the patient’s creatinine clearance is >30 mL/min. In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so MICARDIS HCT tablets are not recommended.
Patients with Hepatic Impairment
MICARDIS HCT tablets are not recommended for patients with severe hepatic impairment. Patients with biliary obstructive disorders or hepatic insufficiency should have treatment started under close medical supervision using the 40/12.5 mg combination (seePRECAUTIONS ).
How Supplied
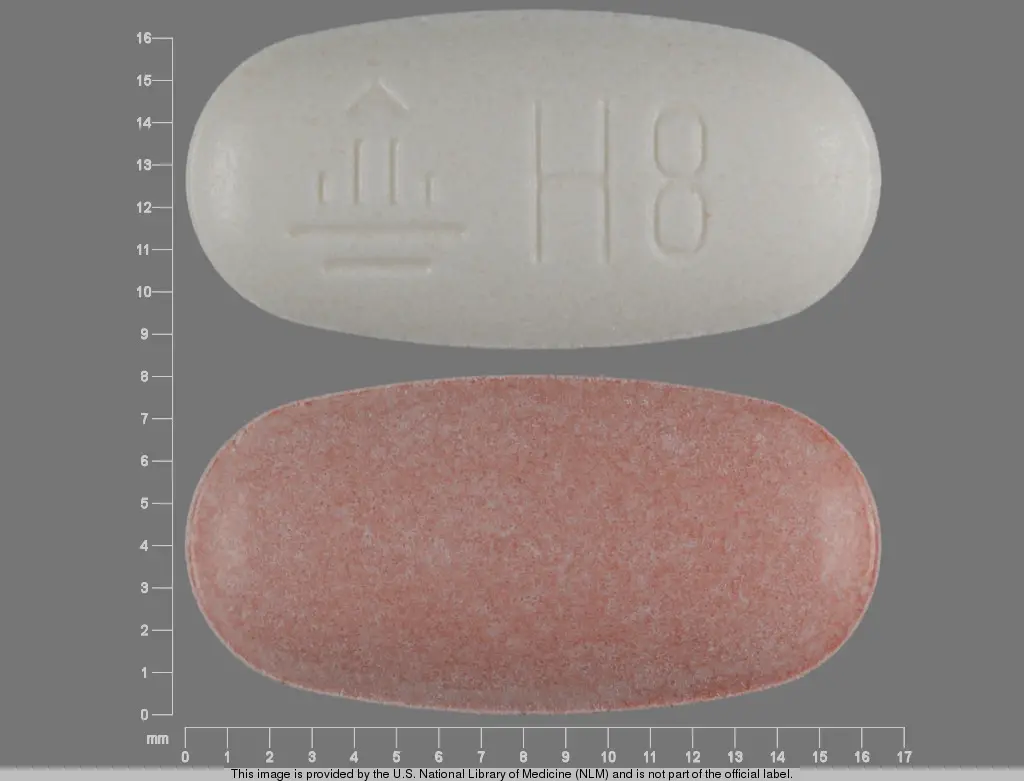
MICARDIS HCT tablets are available in three strengths as biconvex two-layered, oblong-shaped, uncoated tablets in three combinations of 40 mg/12.5 mg, 80 mg/12.5 mg and 80 mg/25 mg telmisartan and hydrochlorothiazide, respectively. The hydrochlorothiazide layer is red in the 40 mg/12.5 mg and 80 mg/12.5 mg tablets, and yellow in the 80 mg/25 mg tablets, and all are unmarked. The telmisartan layer for all three strengths is white, but may contain red specks in the 40 mg/12.5 mg and 80 mg/12.5 mg tablets and yellow specks in the 80 mg/25 mg tablets. The telmisartan layer is marked with the Boehringer Ingelheim logo and H4 for the 40 mg/12.5 mg dose strength, H8 for the 80 mg/12.5 mg dose strength and H9 for the 80 mg/25 mg dose strength.
Tablets are provided as follows:
MICARDIS HCT tablets 40 mg/12.5 mg are individually buler-sealed in cartons of 30 tablets as 3 x 10 cards (NDC 0597-0043-37).
MICARDIS HCT tablets 80 mg/12.5 mg are individually buler-sealed in cartons of 30 tablets as 3 x 10 cards (NDC 0597-0044-37).
MICARDIS HCT tablets 80 mg/25 mg are individually buler-sealed in cartons of 30 tablets as 3 x 10 cards (NDC 0597-0042-37).
Storage
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Tablets should not be removed from bulers until immediately before administration.
Address medical inquiries to (800) 542-6257 or (800) 459-9906 TTY.
Distributed by: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA
Licensed from: Boehringer Ingelheim International GmbH, Ingelheim, Germany
Copyright 2012 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED
Revised: January 2012
IT13006NA252012 10004142/09
Image Of 80mg/12.5mg Label
Image Of 80mg/12.5mg Label
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site