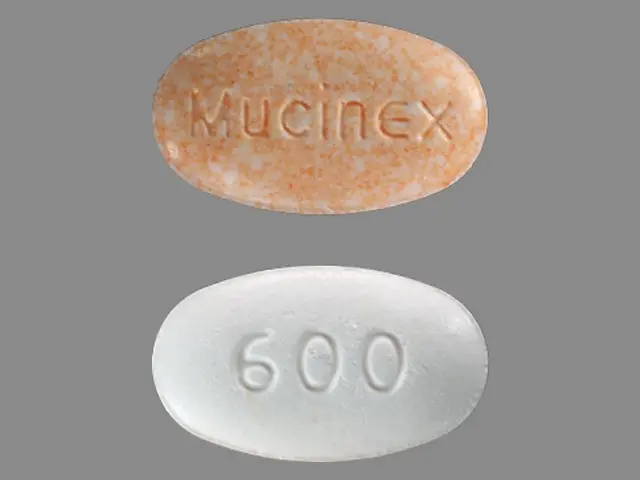
Mucinex D (guaifenesin 600 mg pseudoephedrine hydrochloride 60 mg) Dailymed
Generic: guaifenesin and pseudoephedrine hydrochloride is used for the treatment of Bronchitis Common Cold Cough Laryngitis Pharyngitis Rhinitis, Allergic, Perennial Sinusitis Whooping Cough Asthma
Go PRO for all pill images
Drug Facts
Otc - Active Ingredient Section
OTC - PURPOSE SECTION
Active ingredients (in each extended-release bi-layer tablet) Purposes Guaifenesin 600 mg Expectorant Pseudoephedrine HCl 60 mg Nasal Decongestant
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
- temporarily relieves sinus congestion and pressure
Warnings
OTC - DO NOT USE SECTION
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
OTC - ASK DOCTOR SECTION
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
OTC - WHEN USING SECTION
When using this product
- do not use more than directed
OTC - STOP USE SECTION
Stop use and ask a doctor if
- you get nervous, dizzy, or sleepless
- symptoms do not get better within 7 days, come back or occur with a fever, rash, or persistent headache. These could be signs of a serious illness.
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding, ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for timing of meals
- adults and children 12 years and older: 2 tablets every 12 hours; not more than 4 tablets in 24 hours
- children under 12 years of age: do not use
Other Information
- tamper evident: do not use if carton is open or if printed seal on buler is broken or missing
- store at 20-25°C (68-77°F)
Inactive Ingredients
carbomer homopolymer type B; FD&C yellow #6 aluminum lake; hypromellose, USP; magnesium stearate, NF; microcrystalline cellulose, NF; sodium starch glycolate, NF
Questions?
1-866-MUCINEX (1-866-682-4639) You may also report side effects to this phone number.
Dist. by: RB Health (US) Parsippany, NJ 07054-0224 Made in England
Principal Display Panel - 18 Tablet Carton
NDC 63824-057-18
Mucinex®D600 mg guaifenesin & 60 mg pseudoephedrine HCl extended-release bi-layer tablets
EXPECTORANT & NASAL DECONGESTANT
12 HOUR
✓ Clears Nasal/Sinus Congestion✓ Thins and Loosens Mucus✓ Immediate and Extended Release
18 EXTENDED-RELEASE BI-LAYER TABLETS

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site