Niravam (alprazolam 0.25 mg) Dailymed
Generic: alprazolam
IMPRINT: SP 321 025
SHAPE: round
COLOR: yellow SCORE: 2
All Imprints
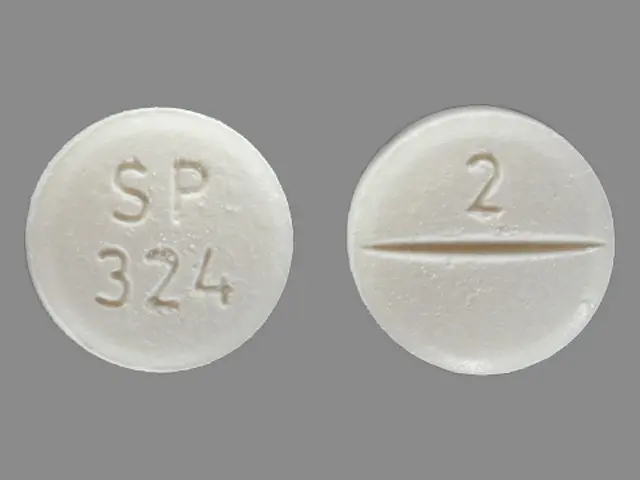
alprazolam 2 mg - sp 324 2 round white
alprazolam 0.5 mg - sp 322 05 round yellow
Go PRO for all pill images
1 Indications And Usage
NIRAVAM® is a benzodiazepine indicated for:
• The treatment of generalized anxiety disorder (1.1 ). The efficacy of alprazolam was demonstrated in 5 short-term, placebo-controlled trials (14 ).• The treatment of panic disorder, with or without agoraphobia (1.2 ). The efficacy of alprazolam in the treatment of panic disorder was established in 2 short-term, placebo-controlled trials (14 ).1.1 Generalized Anxiety Disorder
NIRAVAM® is indicated for the treatment of generalized anxiety disorder.
The efficacy of alprazolam in the treatment of generalized anxiety disorder was demonstrated in 5 short-term, placebo-controlled trials. [see Clinical Studies (14.1)].
1.2 Panic Disorder
NIRAVAM is also indicated for the treatment of panic disorder, with or without agoraphobia.
The efficacy of alprazolam in the treatment of panic disorder was established in 2 short-term, placebo-controlled trials. [see Clinical Studies (14.2)].
Demonstrations of the effectiveness of NIRAVAM by systematic clinical study are limited to 4 months in duration for generalized anxiety disorder and 4 to 10 weeks duration for panic disorder; however, patients with panic disorder have been treated on an open basis for up to 8 months without apparent loss of benefit. The physician should periodically reassess the usefulness of the drug for the individual patient.
2 Dosage And Administration
Dosage should be individualized for maximum beneficial effect. While the usual daily dosages given below will meet the needs of most patients, there will be some who require doses greater than 4 mg per day. In such cases, the dosage should be increased cautiously to avoid adverse reactions. In general, benzodiazepines should be prescribed for short periods. Reevaluate the need for continued therapy before extending the treatment period.
Indication
Recommended Dose
Anxiety Disorder (2.1 )    Â
Initial: 0.25 mg to 0.5 mg given three times daily. Maximum: 4 mg per day given in divided doses
Panic Disorder (2.2 )
Initial: 0.5 mg given three times daily Maximum: Doses up to 10 mg per day may be required to achieve a successful response
• With dry hands, place the tablet on top of the tongue where it will disintegrate and be swallowed with saliva (2.4 ).• Depending on response, the dose may be increased to achieve a maximum therapeutic effect, at intervals of 3 to 4 days (2.1 ,2.2 ).• Use the lowest possible effective dose.• Periodically reassess the need for continued treatment (2.1 ).• In general, benzodiazepines should be prescribed for short periods (2 ). Discontinuation of treatment or dose reduction should be gradual and under close physician supervision. Decrease the dosage by no more than 0.5 mg per day every 3 days. Some patients may require an even slower dosage reduction (2.1 ,2.2 ).• Dosing in elderly: the starting dose is 0.25 mg, given two or three times daily (2.3 ).• Severe hepatic impairment: the starting dose is 0.25 mg, given two or three times daily (2.3 ).2.1 Generalized Anxiety Disorder
Initiate treatment with a dose of 0.25 mg to 0.5 mg three times daily. The dose may be increased to achieve a maximum therapeutic effect, at intervals of 3 to 4 days, to a maximum daily dose of 4 mg, given in divided doses. Use the lowest possible effective dose, and periodically reassess the need for continued treatment. The risk of dependence can increase with dose and duration of treatment.
The dosage should be reduced gradually when discontinuing therapy or when decreasing the daily dosage. Although there are no systematically collected data to support a specific discontinuation schedule, it is suggested that the daily dosage be decreased by no more than 0.5 mg every 3 days. Some patients may require an even slower dosage reduction.
2.2 Panic Disorder
The successful treatment of many panic disorder patients has required the use of alprazolam at doses greater than 4 mg daily. In controlled trials conducted to establish the efficacy of alprazolam in panic disorder, doses in the range of 1 mg to 10 mg daily were used. The mean dosage employed was approximately 5 mg to 6 mg daily. Among the approximately 1700 patients participating in the panic disorder development program, about 300 received alprazolam in dosages of greater than 7 mg per day, including approximately 100 patients who received maximum dosages of greater than 9 mg per day. Occasional patients required as much as 10 mg a day to achieve a successful response.
Dose Titration
Initiate treatment with a dose of 0.5 mg three times daily. Depending on the response, the dose may be increased at intervals of 3 to 4 days in increments of no more than 1 mg per day. Slower titration to the dose levels greater than 4 mg per day may be advisable to allow full expression of the pharmacodynamic effect of NIRAVAM. To lessen the possibility of interdose symptoms, the times of administration should be distributed as evenly as possible throughout the waking hours, (i.e., administered three or four times daily).
Generally, therapy should be initiated at a low dose to minimize the risk of adverse responses in patients especially sensitive to the drug. The dose should be advanced until an acceptable therapeutic response (i.e., a substantial reduction in or total elimination of panic attacks) is achieved, intolerance occurs, or the maximum recommended dose is attained.
Dose Maintenance
For patients receiving doses greater than 4 mg per day, periodically reassess treatment and consider a reduction of dosage. In a controlled postmarketing dose-response study, patients treated with doses of alprazolam greater than 4 mg per day for 3 months were able to taper to 50% of their total daily maintenance dose without apparent loss of clinical benefit. Because of the danger of withdrawal, avoid abrupt discontinuation of treatment. [see Warnings and Precautions (5.3), Drug Abuse and Dependence (9.3)].
The necessary duration of treatment for panic disorder patients responding to NIRAVAM is unknown. After a period of extended freedom from attacks, a carefully supervised tapered discontinuation may be attempted, but there is evidence that this may often be difficult to accomplish without recurrence of symptoms and/or the manifestation of withdrawal phenomena.
Dose Reduction
Because of the danger of withdrawal, abrupt discontinuation of treatment should be avoided [see Warnings and Precautions (5.3), Drug Abuse and Dependence (9.3)].
In all patients, dosage should be reduced gradually when discontinuing therapy or when decreasing the daily dosage. Although there are no systematically collected data to support a specific discontinuation schedule, it is suggested that the daily dosage be decreased by no more than 0.5 mg every three days. Some patients may require an even slower dosage reduction.
In any case, reduction of dose must be undertaken under close supervision and must be gradual. If significant withdrawal symptoms develop, reinstitute the previous stable dosing schedule. After stabilization, consider using a less rapid schedule of discontinuation. In a controlled postmarketing discontinuation study of panic disorder patients which compared this recommended taper schedule with a slower taper schedule, there was no difference between the groups in the proportion of patients who tapered and completely discontinued treatment with alprazolam; however, the slower schedule was associated with a reduction in symptoms associated with a withdrawal syndrome. Reduce the dose by no more than 0.5 mg every 3 days. Some patients may benefit from an even more gradual discontinuation. Some patients may prove resistant to all discontinuation regimens.
2.3 Dosing in Special Populations
In elderly patients, in patients with advanced liver disease, or in patients with debilitating disease (e.g., severe pulmonary disease), the usual starting dose is 0.25 mg, given two or three times daily. This may be gradually increased if needed and tolerated. The elderly may be especially sensitive to the effects of benzodiazepines. If adverse reactions occur at the recommended starting dose, the dose may be lowered.
2.4 Instructions to be Given to Patients for Use/Handling NIRAVAM Tablets
Just prior to administration, with dry hands, remove the tablet from the bottle. Immediately place the NIRAVAM tablet on top of the tongue where it will disintegrate and be swallowed with saliva. Administration with liquid is not necessary.
Discard any cotton that was included in the bottle and reseal the bottle tightly to prevent introducing moisture that might cause the tablets to disintegrate.
3 Dosage Forms And Strengths
0.25 mg, 0.5 mg, 1.0 mg, and 2.0 mg scored orally disintegrating tablets.
0.25 mg, 0.5 mg, 1.0 mg, or 2.0 mg scored orally disintegrating tablets (3 ).
4 Contraindications
NIRAVAM is contraindicated in patients with acute narrow angle glaucoma. NIRAVAM can exacerbate narrow angle closure. NIRAVAM may be used in patients with open angle glaucoma who are receiving appropriate therapy.
NIRAVAM is contraindicated in patients treated with potent CYP3A4 inhibitors (e.g., ketoconazole and itraconazole), because these medications significantly impair the oxidative metabolism mediated by cytochrome P450 3A (CYP3A) and can increase alprazolam exposures [see Clinical Pharmacology (12.3), Warnings and Precautions (5.7), and Drug Interactions (7.4)].
• Acute narrow angle glaucoma. NIRAVAM can exacerbate narrow angle closure (4 ).• Concomitant use with potent CYP3A inhibitors (e.g., ketoconazole and itraconazole) can increase the serum concentration of alprazolam (4 ).
5 Warnings And Precautions
• Suicide: As with other psychotropic medications, use precautions with respect to administration of the drug and size of the prescription, especially in patients who are severely depressed or in patients where there is reason to expect concealed suicidal ideation or plans (5.1 ).• Status Epilepticus and Seizure: can occur during discontinuation of NIRAVAM (5.2 ).• Physical dependence to NIRAVAM can occur, even after relatively short-term use at the recommended doses (5.3 ).• Withdrawal reactions, such as seizures, can occur during dosage reduction. Avoid abrupt discontinuation. Reduce or discontinue the dose gradually (5.3 ).• Fetal Harm: Benzodiazepines can cause fetal harm when administered to pregnant women (5.4 ).• CNS Depression and Impaired Cognitive and Motor Performance: caution patients against engaging in hazardous occupations or activities requiring complete mental alertness, until they are reasonably certain that NIRAVAM treatment does not affect them adversely. Caution patients about the use of alcohol and other CNS depressant drugs during treatment with NIRAVAM (5.5 ).• Interdose anxiety symptoms: can occur at prescribed maintenance doses. Consider dividing the daily dose into more frequent administrations (5.8 ).• Patients with Concomitant Illnes : In the elderly or debilitated patients, the smallest effective dose is recommended to preclude the development of ataxia or oversedation. There have been rare reports of death in patients with severe pulmonary disease shortly after the initiation of treatment with alprazolam (5.11 ).5.1 Suicide and Overdose
As with other psychotropic medications, the usual precautions with respect to administration of the drug and size of the prescription are indicated for severely depressed patients or those in whom there is reason to expect concealed suicidal ideation or plans. Panic disorder has been associated with primary and secondary major depressive disorders and increased reports of suicide among untreated patients.
5.2 Status Epilepticus
Withdrawal seizures have been reported in association with the discontinuation of alprazolam. In most cases, only a single seizure was reported; however, multiple seizures and status epilepticus were reported as well.
5.3 Dependence and Withdrawal Reactions, Including Seizures
NIRAVAM is a Schedule IV controlled substance. The use of benzodiazepines, including NIRAVAM, may lead to physical and psychological dependence. In general, benzodiazepines should be prescribed for short periods. Even after relatively short-term use at the recommended doses, there is some risk of dependence and withdrawal symptoms [see Dependence (9.3)].
Certain adverse clinical events, some life-threatening, are a direct consequence of physical dependence to NIRAVAM. These include a spectrum of withdrawal symptoms; the most important is seizure [see Drug Abuse and Dependence (9.3)]. Spontaneous reporting system data suggest that the risk of dependence and its severity appear to be greater in patients treated with doses greater than 4 mg per day and for long periods (more than 12 weeks). However, in a controlled postmarketing discontinuation study of panic disorder patients, the duration of treatment (3 months compared to 6 months) had no effect on the ability of patients to taper to zero dose. In contrast, patients treated with doses of alprazolam greater than 4 mg per day had more difficulty tapering to zero dose than those treated with less than 4 mg per day.
The importance of dose and the risks of NIRAVAM as a treatment for panic disorder
Because the management of panic disorder often requires the use of average daily doses of NIRAVAM above 4 mg, the risk of dependence among panic disorder patients may be higher than that among those treated for less severe anxiety. Experience in randomized placebo-controlled discontinuation studies of patients with panic disorder showed a high rate of rebound and withdrawal symptoms in patients treated with alprazolam compared to placebo-treated patients.
Relapse or return of illness was defined as a return of symptoms characteristic of panic disorder (primarily panic attacks) to levels approximately equal to those seen at baseline before active treatment was initiated. Rebound refers to a return of symptoms of panic disorder to a level substantially greater in frequency, or more severe in intensity than seen at baseline. Withdrawal symptoms were identified as those which were generally not characteristic of panic disorder and which occurred for the first time more frequently during discontinuation than at baseline.
In a controlled clinical trial in which 63 patients were randomized to alprazolam and where withdrawal symptoms were specifically sought, the following were identified as symptoms of withdrawal: heightened sensory perception, impaired concentration, dysosmia, clouded sensorium, paresthesias, muscle cramps, muscle twitch, diarrhea, blurred vision, appetite decrease, and weight loss. Other symptoms, such as anxiety and insomnia, were frequently seen during discontinuation, but it could not be determined if they were due to return of illness, rebound, or withdrawal.
In two controlled trials of 6 to 8 weeks duration where the ability of patients to discontinue medication was measured, 71% - 93% of patients treated with alprazolam tapered completely off therapy compared to 89% - 96% of placebo-treated patients. In a controlled postmarketing discontinuation study of panic disorder patients, the duration of treatment (3 months compared to 6 months) had no effect on the ability of patients to taper to zero dose.
Seizures attributable to alprazolam were seen after drug discontinuance or dose reduction in 8 of 1980 patients with panic disorder or in patients participating in clinical trials where doses of alprazolam greater than 4 mg/day for over 3 months were permitted. Five of these cases clearly occurred during abrupt dose reduction, or discontinuation from daily doses of 2 mg to 10 mg. Three cases occurred in situations where there was not a clear relationship to abrupt dose reduction or discontinuation. In one instance, seizure occurred after discontinuation from a single dose of 1 mg after tapering at a rate of 1 mg every 3 days from 6 mg daily. In two other instances, the relationship to taper is indeterminate; in both of these cases the patients had been receiving doses of 3 mg daily prior to seizure. The duration of use in the above 8 cases ranged from 4 to 22 weeks. There have been occasional voluntary reports of patients developing seizures while apparently tapering gradually from alprazolam. The risk of seizure seems to be greatest 24 - 72 hours after discontinuation [see Dosage and Administration (2)].
To discontinue treatment in patients taking NIRAVAM, the dosage should be reduced gradually. Decrease the daily dosage of NIRAVAM by no more than 0.5 mg every three days [see Dosage and Administration (2)]. Some patients may benefit from an even slower dosage reduction. In a controlled postmarketing discontinuation study of panic disorder patients which compared this recommended taper schedule with a slower taper schedule, no difference was observed between the groups in the proportion of patients who tapered to zero dose; however, the slower schedule was associated with a reduction in symptoms associated with a withdrawal syndrome.
5.4 Risk of Fetal Harm
Benzodiazepines can potentially cause fetal harm when administered to pregnant women. If NIRAVAM is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Because of experience with other members of the benzodiazepine class, NIRAVAM is assumed to be capable of causing an increased risk of congenital abnormalities when administered to a pregnant woman during the first trimester. Because use of these drugs is rarely a matter of urgency, their use during the first trimester should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physicians about the desirability of discontinuing the drug.
5.5 CNS Depression and Impaired Performance
Because NIRAVAM has CNS depressant effects and has the potential to impair judgment, cognition, and motor performance, caution patients against engaging in hazardous occupations or activities requiring complete mental alertness such as operating machinery or driving a motor vehicle, until they are reasonably certain that NIRAVAM treatment does not affect them adversely. Caution patients about the simultaneous ingestion of alcohol and other CNS depressant drugs during treatment with NIRAVAM.
5.6 Mania
Episodes of hypomania and mania have been reported in association with the use of alprazolam in patients with depression.
5.7 NIRAVAM Interaction with Drugs that Inhibit Metabolism via Cytochrome P450 3A
The initial step in NIRAVAM metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP3A). Drugs that inhibit this metabolic pathway may have a profound effect on the clearance of NIRAVAM. Consequently, NIRAVAM should be avoided in patients receiving potent inhibitors of CYP3A. With drugs inhibiting CYP3A to a lesser but still significant degree, NIRAVAM should be used only with caution and consideration of appropriate dosage reduction. For some drugs, an interaction with NIRAVAM has been quantified with clinical data; for other drugs, interactions are predicted from in vitro data and/or experience with similar drugs in the same pharmacologic class.
The following are examples of drugs known to inhibit the metabolism of NIRAVAM and/or related benzodiazepines, presumably through inhibition of CYP3A.
Potent CYP3A Inhibitors
Azole antifungal agents — Ketoconazole and itraconazole are potent CYP3A inhibitors and have been shown in vivo to increase plasma alprazolam concentrations 3.98 fold and 2.70 fold, respectively. The coadministration of alprazolam with these agents is not recommended. Other azole-type antifungal agents should also be considered potent CYP3A inhibitors and the coadministration of alprazolam with them is not recommended [see CONTRAINDICATIONS (4)].
Drugs demonstrated to be CYP3A inhibitors on the basis of clinical studies involving alprazolam
Consider dose reduction of NIRAVAM during coadministration with the following drugs:
• Nefazodone — Coadministration of nefazodone increased alprazolam concentration two-fold.• Fluvoxamine — Coadministration of fluvoxamine approximately doubled the maximum plasma concentration of alprazolam, decreased clearance by 49%, increased half-life by 71%, and decreased measured psychomotor performance.• Cimetidine — Coadministration of cimetidine increased the maximum plasma concentration of alprazolam by 86%, decreased clearance by 42%, and increased half-life by 16%.
Other drugs possibly affecting alprazolam metabolism
Other drugs possibly affect alprazolam metabolism by inhibition of CYP3A [see Drug Interactions (7.6)].
5.8 Interdose Symptoms
Early morning anxiety and emergence of anxiety symptoms between doses of alprazolam have been reported in patients with panic disorder taking prescribed maintenance doses of alprazolam. These symptoms may reflect the development of tolerance or a time interval between doses which is longer than the duration of clinical action of the administered dose. In either case, it is presumed that the prescribed dose is not sufficient to maintain plasma levels above those needed to prevent relapse, rebound or withdrawal symptoms over the entire course of the interdosing interval. In these situations, it is recommended that the same total daily dose be given divided as more frequent administrations [see Dosage and Administration (2)].
5.9 Risk of Dose Reduction
Withdrawal reactions may occur when dosage reduction occurs for any reason. This includes purposeful tapering, but also inadvertent reduction of dose (e.g., the patient forgets, the patient is admitted to a hospital). Therefore, the dosage of NIRAVAM should be reduced or discontinued gradually [see Dosage and Administration (2)].
5.10 Uricosuric Effect
Alprazolam has a weak uricosuric effect. Although other medications with weak uricosuric effect have been reported to cause acute renal failure, there have been no reported instances of acute renal failure attributable to therapy with alprazolam.
5.11 Use in Patients with Concomitant Illness
It is recommended that the dosage be limited to the smallest effective dose to preclude the development of ataxia or oversedation which may be a particular problem in elderly or debilitated patients [see Dosage and Administration (2)]. The usual precautions in treating patients with impaired renal, hepatic or pulmonary function should be observed. There have been rare reports of death in patients with severe pulmonary disease shortly after the initiation of treatment with alprazolam. A decreased systemic alprazolam elimination rate (e.g., increased plasma half-life) has been observed in both alcoholic liver disease patients and obese patients receiving alprazolam [see Clinical Pharmacology (12)].
6 Adverse Reactions
• Anxiety Disorder: The most common adverse reactions (≥ 5% and ~twice the rate of placebo) were sedation, and hypotension.• Panic Disorder: The most common adverse reactions included sedation, impaired coordination, dysarthria, and increased libido (6.1 ).
To report SUSPECTED ADVERSE REACTIONS, contact Jazz Pharmaceuticals at (800)-890-3098 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
6.1 Clinical Trial Experience
The most commonly reported (≥5% and ~ twice the rate of placebo) adverse reactions with NIRAVAM treatment are: sedation, impaired coordination, dysarthria, and increased libido.
The data cited in the two tables below are estimates of adverse reactions occurring in patients who participated in clinical trials under the following conditions: relatively short duration (four weeks) placebo-controlled clinical studies with dosages up to 4 mg per day of alprazolam (for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety) and short-term (up to ten weeks) placebo-controlled clinical studies with dosages up to 10 mg per day of alprazolam in patients with panic disorder, with or without agoraphobia.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Comparison of the cited figures, however, can provide the prescriber with some basis for estimating the relative contributions of drug and non-drug factors to the adverse reaction incidence in the population studied. Even this use must be approached cautiously, as a drug may relieve a symptom in one patient but induce it in others. (For example, an anxiolytic drug may relieve dry mouth [a symptom of anxiety] in some subjects but induce dry mouth in others.)
Table 1: Adverse Reactions Reported in Placebo-Controlled Trials of Alprazolam in Generalized Anxiety Disorder (>2% and at a rate greater than placebo) a Events reported by 1% or more of alprazolam patients are included.
GENERALIZED ANXIETY DISORDER
Body System/Adverse Reaction    Â
Treatment-Emergent Symptom Incidencea
    ALPRAZOLAM (%)    N = 565
    PLACEBO (%)    N = 505
Central Nervous System
  Sedation
41
22
  Lightheadedness
21
19
  Dizziness
2
1
  Akathisia
2
1
Gastrointestinal
  Dry Mouth
15
13
  Increased Salivation
4
2
Cardiovascular
  Hypotension
5
2
Cutaneous
  Dermatitis/Allergy
4
3
In addition to the relatively common (i.e., greater than 1%) adverse reactions described in the table above, the following adverse reactions have been reported in association with the use of benzodiazepines: dystonia, irritability, concentration difficulties, anorexia, transient amnesia or memory impairment, loss of coordination, fatigue, seizures, sedation, slurred speech, jaundice, musculoskeletal weakness, pruritus, diplopia, dysarthria, changes in libido, menstrual irregularities, incontinence and urinary retention.
Table 2: Adverse Reactions Reported in Placebo-Controlled Trials of Alprazolam in Panic Disorder (>2% and greater than placebo) a Events reported by 1% or more of alprazolam patients are included.
PANIC DISORDER
Body System/Adverse Reaction    Â
Treatment-Emergent Symptom Incidencea
    ALPRAZOLAM (%)    N = 1388
    PLACEBO (%)    N = 1231
Central Nervous System
  Sedation
77
43
  Fatigue and Tiredness
49
42
  Impaired Coordination
40
18
  Irritability
33
30
  Memory Impairment
33
22
  Cognitive Disorder
29
21
  Dysarthria
23
6
  Decreased Libido
14
8
  Confusional State
10
8
  Increased Libido
8
4
  Change in Libido (Not Specified)
7
6
  Disinhibition
3
2
  Talkativeness
2
1
  Derealization
2
1
Gastrointestinal
  Constipation
26
15
  Increased Salivation
6
4
Cutaneous
  Rash
11
8
Other
  Increased Appetite
33
23
  Decreased Appetite
28
24
  Weight Gain
27
18
  Weight Loss
23
17
  Micturition Difficulties
12
9
  Menstrual Disorders
10
9
  Sexual Dysfunction
7
4
  Incontinence
2
1
In addition to the relatively common (i.e., greater than 1%) adverse reactions described in the table above, the following adverse reactions have been reported in association with the use of alprazolam: seizures, hallucinations, depersonalization, taste alterations, diplopia, elevated bilirubin, elevated hepatic enzymes, and jaundice.
Panic disorder has been associated with primary and secondary major depressive disorders and increased reports of suicide among untreated patients [see Warnings and Precautions, (5.1)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of NIRAVAM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Reported events include: liver enzyme elevations, hepatitis, hepatic failure, Stevens-Johnson syndrome, hyperprolactinemia, gynecomastia, and galactorrhea.
7 Drug Interactions
• NIRAVAM produces additive CNS depressant effects when co-administered with other psychotropic medications, anticonvulsants, antihistaminics, alcohol and other drugs that produce CNS depression (7.1 ).• The formulation requires an acidic environment to dissolve; therefore, drugs or diseases that cause dry mouth or raise stomach pH may slow disintegration or dissolution, resulting in decreased absorption (7.2 ).• Drugs which inhibit the hydroxylation catalyzed by cytochrome P450 3A (CYP3A) metabolic pathway can decrease the clearance of NIRAVAM and increase the serum concentration (7.4 ).7.1 Use with Other CNS Depressants
If NIRAVAM is coadministered with other psychotropic agents or anticonvulsant drugs, carefully consider the pharmacology of the agents to be employed, particularly with compounds which might potentiate the action of benzodiazepines. The benzodiazepines, including NIRAVAM, produce additive CNS depressant effects when co-administered with other psychotropic medications, anticonvulsants, antihistaminics, alcohol and other drugs which themselves produce CNS depression.
7.2 Drugs Effecting Salivary Flow and Stomach pH
Because NIRAVAM disintegrates in the presence of saliva, and the formulation requires an acidic environment to dissolve, concomitant drugs or diseases that cause dry mouth or raise stomach pH might slow disintegration or dissolution, resulting in slowed or decreased absorption.
7.3 Use with Imipramine and Desipramine
The steady state plasma concentrations of imipramine and desipramine can increase by approximately 30% and 20%, respectively, when administered concomitantly with NIRAVAM in doses up to 4 mg per day. The clinical significance of these changes is unknown.
7.4 Drugs that Inhibit NIRAVAM Metabolism via Cytochrome P450 3A
The initial step in NIRAVAM metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP3A). Drugs which inhibit this metabolic pathway can have a profound effect on the clearance of NIRAVAM [see Contraindications (4) and Warnings and Precautions (5.7)].
7.5 Drugs Demonstrated to be CYP3A Inhibitors of Possible Clinical Significance on the Basis of Clinical Studies Involving Alprazolam
Use caution during coadministration of NIRAVAM and the following drugs:
Fluoxetine — Coadministration of fluoxetine with alprazolam increased the maximum plasma concentration of alprazolam by 46%, decreased clearance by 21%, increased half-life by 17%, and decreased measured psychomotor performance.
Propoxyphene — Coadministration of propoxyphene decreased the maximum plasma concentration of alprazolam by 6%, decreased clearance by 38%, and increased half-life by 58%.
Oral Contraceptives — Coadministration of oral contraceptives increased the maximum plasma concentration of alprazolam by 18%, decreased clearance by 22%, and increased half-life by 29%.
7.6 Drugs and Other Substances Demonstrated to be CYP3A Inhibitors on the Basis of Clinical Studies Involving Benzodiazepines Metabolized Similarly to Alprazolam or on the Basis of Studies with Alprazolam or Other Benzodiazepines
Use caution during the coadministration of NIRAVAM and the following:
Available data from clinical studies of benzodiazepines other than alprazolam suggest a possible drug interaction between alprazolam and the following: diltiazem, isoniazid, macrolide antibiotics such as erythromycin and clarithromycin, and grapefruit juice. Data from in vitro studies of alprazolam suggest a possible drug interaction between alprazolam and the following: sertraline and paroxetine. However, data from an in vivo drug interaction study involving a single dose of alprazolam 1 mg and steady state doses of sertraline (50 mg to 150 mg per day) did not reveal any clinically significant changes in the pharmacokinetics of alprazolam. Data from in vitro studies of benzodiazepines other than alprazolam suggest a possible drug interaction between benzodiazepines and the following: ergotamine, cyclosporine, amiodarone, nicardipine, and nifedipine. [see Warnings and Precautions (5.7)].
7.7 Inducers of CYP3A
Carbamazepine can increase NIRAVAM metabolism and therefore can decrease plasma levels of NIRAVAM.
8 Use In Specific Populations
• Teratogenic Effects: Pregnancy Category D. NIRAVAM can cause fetal harm (8.1 ).• Nonteratogenic Effects: A neonate born to a mother who is treated with NIRAVAM may be at risk for withdrawal, flaccidity, and respiratory problems (8.1 ).• Nursing mothers: Chronic administration of diazepam to nursing mothers has been reported to cause their infants to become lethargic and to lose weight (8.3 ).• Geriatric use: The elderly exhibit higher plasma concentrations due to reduced clearance, compared with a younger population receiving the same doses (8.5 ).• Pediatric use: Safety and effectiveness of NIRAVAM in individuals below 18 years of age have not been established (8.4 ).8.1 Pregnancy
Benzodiazepines can potentially cause fetal harm when administered to a pregnant woman. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. If NIRAVAM is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Because of experience with other members of the benzodiazepine class, NIRAVAM is assumed to be capable of causing an increased risk of congenital abnormalities when administered to a pregnant woman during the first trimester. Because use of these drugs is rarely a matter of urgency, their use during the first trimester should almost always be avoided [see Warnings and Precautions (5.4)].
Nonteratogenic Effects: It should be considered that the child born of a mother who is receiving benzodiazepines may be at some risk for withdrawal symptoms from the drug during the postnatal period. Also, neonatal flaccidity and respiratory problems have been reported in children born of mothers who have been receiving benzodiazepines.
8.2 Labor and Delivery
The potential effect of NIRAVAM in labor and delivery in humans has not been studied. However, perinatal complications have been reported in neonates exposed to benzodiazepines late in pregnancy. The findings are suggestive of excess benzodiazepine exposure or withdrawal phenomena.
8.3 Nursing Mothers
Benzodiazepines are excreted in human milk. It should be assumed that NIRAVAM is excreted in human milk. Chronic administration of diazepam to nursing mothers has been reported to cause their infants to become lethargic and to lose weight. Because of the potential for serious adverse reactions in nursing infants from NIRAVAM, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. As a general rule, nursing should not be undertaken by mothers who must use NIRAVAM.
8.4 Pediatric Use
Safety and effectiveness of NIRAVAM in individuals below 18 years of age have not been studied.
8.5 Geriatric Use
The elderly may be more sensitive to the effects of benzodiazepines. They exhibit higher plasma alprazolam concentrations due to reduced clearance of the drug, compared with a younger population receiving the same doses. The smallest effective dose of NIRAVAM should be used in the elderly to preclude the development of ataxia and oversedation [see Clinical Pharmacology (12) and Dosage and Administration (2)].
Changes in the absorption, distribution, metabolism and excretion of benzodiazepines have been demonstrated in geriatric patients. A mean half-life of NIRAVAM of 16.3 hours has been observed in healthy elderly subjects (range: 9.0 - 26.9 hours, n=16) compared to 11.0 hours (range: 6.3 - 15.8 hours, n=16) in healthy adult subjects.
9 Drug Abuse And Dependence
9.1 Controlled Substance
NIRAVAM is a Schedule IV controlled substance.
9.3 Dependence
Withdrawal symptoms similar in character to those noted with sedative/hypnotics and alcohol have occurred following discontinuance of benzodiazepines, including NIRAVAM. The symptoms can range from mild dysphoria and insomnia to a major syndrome that may include abdominal and muscle cramps, vomiting, sweating, tremors and convulsions. Distinguishing between withdrawal emergent signs and symptoms and the recurrence of illness is often difficult in patients undergoing dose reduction. The long term strategy for treatment of these phenomena will vary with their cause and the therapeutic goal. When necessary, immediate management of withdrawal symptoms requires re-institution of treatment at doses of NIRAVAM sufficient to suppress symptoms. There have been reports of failure of other benzodiazepines to fully suppress these withdrawal symptoms. These failures have been attributed to incomplete cross-tolerance but may also reflect the use of an inadequate dosing regimen of the substituted benzodiazepine or the effects of concomitant medications.
While it is difficult to distinguish withdrawal from recurrence of anxiety symptoms, the time course and the nature of the symptoms may be helpful. A withdrawal syndrome typically includes the occurrence of new symptoms, tends to appear toward the end of taper or shortly after discontinuation, and will decrease with time. In recurring panic disorder, symptoms similar to those observed before treatment may recur either early or late, and they will persist.
While the severity and incidence of withdrawal phenomena appear to be related to dose and duration of treatment, withdrawal symptoms, including seizures, have been reported after only brief therapy with alprazolam at doses within the recommended range for the treatment of anxiety (e.g., 0.75 mg to 4 mg per day). Signs and symptoms of withdrawal are often more prominent after rapid decrease of dosage or abrupt discontinuance. The risk of withdrawal seizures may be increased at doses above 4 mg per day [see Warnings and Precautions (5.3)].
Avoid abrupt discontinuation of NIRAVAM, especially in individuals with a history of seizures or epilepsy. It is recommended that all patients on NIRAVAM who require a dosage reduction be gradually tapered under close supervision [see Warnings and Precautions (5.3) and Dosage and Administration (2)].
Psychological dependence is a risk with all benzodiazepines, including NIRAVAM. The risk of psychological dependence may also be increased at doses greater than 4 mg per day and with longer term use, and this risk is further increased in patients with a history of alcohol or drug abuse. Some patients have experienced considerable difficulty in tapering and discontinuing from NIRAVAM, especially those receiving higher doses for extended periods. Addiction-prone individuals should be under careful surveillance when receiving NIRAVAM. As with all anxiolytics, repeat prescriptions should be limited to those who are under medical supervision.
10 Overdosage
10.1 Human Clinical Experience
Manifestations of alprazolam overdosage include somnolence, confusion, impaired coordination, diminished reflexes and coma. Death has been reported in association with overdoses of alprazolam by itself, as it has with other benzodiazepines. In addition, fatalities have been reported in patients who have overdosed with a combination of a single benzodiazepine, including alprazolam, and alcohol; alcohol levels seen in some of these patients have been lower than those usually associated with alcohol-induced fatality.
10.2 Management of Overdose
For the most up to date information on management of alprazolam overdose, contact a certified poison center in your area (1-800-222-1222 or www.poison.org). In case of an overdose, provide supportive care, including close medical supervision and monitoring. Treatment should consist of those general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdose. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. Use supportive and symptomatic measures.
Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for re-sedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS and PRECAUTIONS should be consulted prior to use.
11 Description
NIRAVAM (alprazolam orally disintegrating tablets) contains alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.
NIRAVAM is an orally administered formulation of alprazolam which rapidly disintegrates on the tongue and does not require water to aid dissolution or swallowing.
The chemical name of alprazolam is 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-α] [1,4] benzodiazepine. The empirical formula is C17H13CIN4 and the molecular weight is 308.76.
The structural formula is:
![]()
Alprazolam is a white crystalline powder, which is soluble in methanol or ethanol but which has no appreciable solubility in water at physiological pH.
11.1 NIRAVAM Tablets
Each orally disintegrating tablet contains either 0.25 mg, 0.5 mg, 1 mg, or 2 mg of alprazolam and the following inactive ingredients: colloidal silicon dioxide, corn starch, crospovidone, magnesium stearate, mannitol, methacrylic acid copolymer, microcrystalline cellulose, natural and artificial orange flavor, sucralose and sucrose. In addition, the 0.25 mg and 0.5 mg tablets contain yellow iron oxide.
12 Clinical Pharmacology
12.1 Mechanism of Action
The exact mechanism of action of alprazolam is unknown. Benzodiazepines bind to gamma aminobutyric acid (GABA) receptors in the brain and enhance GABA-mediated synaptic inhibition; such actions may be responsible for the efficacy of alprazolam in anxiety disorder and panic disorder.
12.3 Pharmacokinetics
Absorption
Following oral administration, alprazolam is readily absorbed. The peak plasma concentration is reached about 1.5 to 2 hours after administration of NIRAVAM given with or without water. When taken with water, mean Tmax occurs about 15 minutes earlier than when taken without water with no change in Cmax or AUC. Plasma levels are proportional to the dose given; over the dose range of 0.5 mg to 3.0 mg, peak levels of 8.0 to 37 ng/mL are observed. The elimination half-life of alprazolam is approximately 12.5 hours (range 7.9 - 19.2 hours) after administration of NIRAVAM in healthy adults.
Food decreased the mean Cmax by about 25% and increased the mean Tmax by 2 hours from 2.2 hours to 4.4 hours after the ingestion of a high-fat meal. Food did not affect the extent of absorption (AUC) or the elimination half-life.
Distribution
In vitro, alprazolam is bound (80 percent) to human serum protein. Serum albumin accounts for the majority of the binding.
Metabolism/Elimination
Alprazolam is extensively metabolized in humans, primarily by cytochrome P450 3A4 (CYP3A4), to two major metabolites in the plasma: 4-hydroxyalprazolam and α-hydroxyalprazolam. A benzophenone derived from alprazolam is also found in humans. Their half-lives appear to be similar to that of alprazolam. The plasma concentrations of 4-hydroxyalprazolam and α-hydroxyalprazolam relative to unchanged alprazolam concentration were always less than 4%. The reported relative potencies in benzodiazepine receptor binding experiments and in animal models of induced seizure inhibition are 0.20 and 0.66, respectively, for 4-hydroxyalprazolam and α-hydroxyalprazolam. Such low concentrations and the lesser potencies of 4-hydroxyalprazolam and α-hydroxyalprazolam suggest that they are unlikely to contribute much to the pharmacological effects of alprazolam. The benzophenone metabolite is essentially inactive.
Alprazolam and its metabolites are excreted primarily in the urine.
Special Populations
Changes in the absorption, distribution, metabolism and excretion of benzodiazepines have been reported in a variety of disease states including alcoholism, impaired hepatic function and impaired renal function. Changes have also been demonstrated in geriatric patients. A mean half-life of alprazolam of 16.3 hours has been observed in healthy elderly subjects (range: 9.0 - 26.9 hours, n=16) compared to 11.0 hours (range: 6.3 - 15.8 hours, n=16) in healthy adult subjects. In patients with alcoholic liver disease, the half-life of alprazolam ranged between 5.8 and 65.3 hours (mean: 19.7 hours, n=17) as compared to between 6.3 and 26.9 hours (mean=11.4 hours, n=17) in healthy subjects. In an obese group of subjects, the half-life of alprazolam ranged between 9.9 and 40.4 hours (mean=21.8 hours, n=12) as compared to between 6.3 and 15.8 hours (mean=10.6 hours, n=12) in healthy subjects.
Because of its similarity to other benzodiazepines, it is assumed that alprazolam undergoes transplacental passage and that it is excreted in human milk.
Race — Maximal concentrations (Cmax) and half-life of alprazolam are approximately 15% and 25% higher in Asians compared to Caucasians.
Pediatrics — The pharmacokinetics of alprazolam in pediatric patients have not been studied.Â
Gender — Gender has no effect on the pharmacokinetics of alprazolam.
Cigarette Smoking — Alprazolam concentrations may be reduced by up to 50% in smokers compared to non-smokers.
Drug-Drug Interactions
Alprazolam is primarily eliminated by metabolism via cytochrome P450 3A (CYP3A). Most of the interactions that have been documented with alprazolam are with drugs that inhibit or induce CYP3A.
Compounds that are potent inhibitors of CYP3A would be expected to increase plasma alprazolam concentrations. Drug products that have been studied in vivo, along with their effect on increasing alprazolam AUC, are as follows: ketoconazole, 3.98 fold; itraconazole, 2.70 fold; nefazodone, 1.98 fold; fluvoxamine, 1.96 fold; and erythromycin, 1.61 fold [see Contraindications (4), Warnings and Precautions (5.7), and Drug Interactions (7)].
CYP3A inducers would be expected to decrease alprazolam concentrations and this has been observed in vivo. The oral clearance of alprazolam (given in a 0.8 mg single dose) was increased from 0.90 ± 0.21 mL/min/kg to 2.13 ± 0.54 mL/min/kg and the elimination t1/2 was shortened (from 17.1 ± 4.9 to 7.7 ± 1.7 h) following administration of 300 mg/day carbamazepine for 10 days [see Drug Interactions (7)]. However, the carbamazepine dose used in this study was fairly low compared to the recommended doses (1000 mg - 1200 mg/day); the effect at usual carbamazepine doses is unknown.
The ability of alprazolam to induce or inhibit human hepatic enzyme systems has not been determined. However, this is not a property of benzodiazepines in general. Further, alprazolam did not affect the prothrombin or plasma warfarin levels in male volunteers administered sodium warfarin orally.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenic potential was observed during 2-year bioassay studies of alprazolam in rats at doses up to 30 mg/kg per day (30 times the maximum recommended human dose of 10 mg per day on a mg/m2 basis) and in mice at doses up to 10 mg/kg per day (5 times the maximum recommended human dose on a mg/m2).
Alprazolam also was not mutagenic in vitro in the DNA Damage/Alkaline Elution Assay or the Ames Assay, and was negative in the rat micronucleus test.
Alprazolam produced no impairment of fertility in rats at doses up to 5 mg/kg per day, which is 5 times the maximum recommended human dose of 10 mg per day on a mg/m2 basis.
13.2 Animal Toxicology and/or Pharmacology
When rats were treated with oral alprazolam doses of 3, 10, and 30 mg/kg per day (3 to 30 times the maximum recommended human dose of 10 mg per day on a mg/m2 basis) for 2 years, a tendency for a dose related increase in the number of cataracts was observed in females, and a tendency for a dose related increase in corneal vascularization was observed in males. These lesions did not appear until after 11 months of treatment.
14 Clinical Studies
14.1 Anxiety Disorders
The efficacy of alprazolam in the treatment of anxiety symptoms was demonstrated in five short-term (4 weeks), randomized, double-blind, placebo-controlled studies. The studies included patients with a diagnosis of anxiety or anxiety with associated depressive symptomatology. Alprazolam doses ranged from 0.5 to 4 mg per day. The mean daily doses ranged from 1.6 to 2.4 mg. Treatment with alprazolam was statistically significantly superior to placebo treatment, as measured by the following psychometric instruments: Hamilton Anxiety Rating Scale, Physician’s Global Impressions, Target Symptoms, Patient’s Global Impressions and Self-Rating Symptom Scale.
14.2 Panic Disorder
The efficacy of alprazolam in the treatment of panic disorder was demonstrated in three short-term (up to 10 weeks), randomized, double-blind, placebo-controlled studies. Patients in the studies had diagnoses corresponding closely to DSM-III-R criteria for panic disorder (with or without agoraphobia).
The average dose of alprazolam was 5 mg to 6 mg per day in two of the studies, and the doses of alprazolam were fixed at 2 mg and 6 mg per day in the third study. In all three studies, alprazolam was superior to placebo on a variable defined as "the number of patients with zero panic attacks" (range, 37 - 83% met this criterion), as well as on a global improvement score. In two of the three studies, alprazolam was superior to placebo on a variable defined as "change from baseline on the number of panic attacks per week" (range, 3.3 - 5.2), and also on a phobia rating scale. A subgroup of patients who were improved on alprazolam during short-term treatment in one of these trials was continued on an open basis up to 8 months, without apparent loss of benefit.
16 How Supplied/storage And Handling
NIRAVAM (alprazolam orally disintegrating tablets) 0.25 mg are yellow, round, orange-flavored, scored and engraved “SP 321” on the unscored side and “0.25” on the scored side. They are supplied as follows: Bottles of 100 NDC 18860-321-01
NIRAVAM (alprazolam orally disintegrating tablets) 0.5 mg are yellow, round, orange-flavored, scored and engraved “SP 322” on the unscored side and “0.5” on the scored side. They are supplied as follows: Bottles of 100 NDC 18860-322-01
NIRAVAM (alprazolam orally disintegrating tablets) 1 mg are white, round, orange-flavored, scored and engraved “SP 323” on the unscored side and “1” on the scored side. They are supplied as follows: Bottles of 100 NDC 18860-323-01
NIRAVAM (alprazolam orally disintegrating tablets) 2 mg are white, round, orange-flavored, scored and engraved “SP 324” on the unscored side and “2” on the scored side. They are supplied as follows: Bottles of 100 NDC 18860-324-01
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86ºF) [See USP Controlled Room Temperature]. Protect from moisture.
Dispense in a tight container as defined in the USP/NF.
17 Patient Counseling Information
17.1 Counseling Information For All Users of NIRAVAM
To assure safe and effective use of benzodiazepines, all patients prescribed NIRAVAM should be provided with the following guidance.
1. Do not remove NIRAVAM tablets from the bottle until just prior to dosing. With dry hands, open the bottle, remove the tablet, and immediately place on the tongue to dissolve and be swallowed with the saliva. The tablet may also be taken with water.2. Discard any cotton that was included in the bottle and reseal the bottle tightly to prevent introducing moisture that might cause the tablets to disintegrate.3. Store at room temperature in a dry place. Protect from moisture.4. Inform your physician about any alcohol consumption and medicine you are taking now, including medication you may buy without a prescription. Alcohol should generally not be used during treatment with benzodiazepines.5. NIRAVAM is not recommended for use in pregnancy. Therefore, inform your physician if you are pregnant, if you are planning to have a child, or if you become pregnant while you are taking this medication.6. Inform your physician if you are nursing.7. Until you experience how this medication affects you, do not drive a car or operate potentially dangerous machinery, etc.8. Do not increase the dose even if you think the medication “does not work anymore” without consulting your physician. Benzodiazepines, even after relatively short-term use at the doses recommended, may produce emotional and/or physical dependence.9. Do not stop taking this medication abruptly or decrease the dose without consulting your physician, since withdrawal symptoms can occur even after relatively short-term use at the doses recommended. You should follow a gradual dosage tapering schedule.17.2 Additional Counseling Information for Panic Disorder Patients
The use of NIRAVAM at doses greater than 4 mg per day, often necessary to treat panic disorder, is accompanied by risks that you need to carefully consider. When used at doses greater than 4 mg per day, which may or may not be required for your treatment, NIRAVAM has the potential to cause severe psychological and physical dependence in some patients and these patients may find it exceedingly difficult to terminate treatment. In two controlled trials of 6 to 8 weeks duration where the ability of patients to discontinue medication was measured, 7 to 29% of patients treated with NIRAVAM did not completely taper off therapy. In a controlled postmarketing discontinuation study of panic disorder patients, the patients treated with doses of NIRAVAM greater than 4 mg per day had more difficulty tapering to zero dose than patients treated with less than 4 mg per day. In all cases, it is important that your physician help you discontinue this medication in a careful and safe manner to avoid overly extended use of NIRAVAM.
In addition, the extended use at doses greater than 4 mg per day appears to increase the incidence and severity of withdrawal reactions when NIRAVAM is discontinued. These are generally minor but seizure can occur, especially if you reduce the dose too rapidly or discontinue the medication abruptly. Seizure can be life-threatening.
Manufactured for: Jazz Pharmaceuticals Commercial Corp . Philadelphia, PA 19103
U.S. Patent Nos. 6,024,981 and 6,221,392.
NIRAVAM® is a registered trademark of UCB Manufacturing, Inc.
Principal Display Panel - 0.25 Mg Tablets
NDC 18860-321-01
100 tablets
NIRAVAM® 0.25 mg (alprazolam orally disintegrating tablets)
Rx only   CIV
Jazz Pharmaceuticals
orally disintegrating tablets
Each orange-flavored, orally disintegrating tablet contains 0.25 mg alprazolam.
USUAL DOSAGE: See package insert for further information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86ºF) [See USP Controlled Room Temperature]. Protect from moisture.
Dispense in a tight container as defined in the USP/NF.
KEEP OUT OF REACH OF CHILDREN.
Distributed by:Jazz Pharmaceuticals Commercial Corp.Philadelphia, PA 19103
U.S. Patent Nos. 6,024,981 and 6,221,392
NIRAt-10-12Rev. 05/12 3E
NIRAVAM® is a registered trademark of UCB Manufacturing, Inc.
00017961
LOT:EXP:
![]()
.25 mg Label
Principal Display Panel - 0.5 Mg Tablets
NDC 18860-322-01
100 tablets
NIRAVAM® 0.5 mg (alprazolam orally disintegrating tablets)
Rx only   CIV
Jazz Pharmaceuticals
orally disintegrating tablets
Each orange-flavored, orally disintegrating tablet contains 0.5 mg alprazolam.
USUAL DOSAGE: See package insert for further information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86ºF) [See USP Controlled Room Temperature]. Protect from moisture.
Dispense in a tight container as defined in the USP/NF.
KEEP OUT OF REACH OF CHILDREN.
Distributed by:Jazz Pharmaceuticals Commercial Corp.Philadelphia, PA 19103
U.S. Patent Nos. 6,024,981 and 6,221,392
NIRAt-10-12Rev. 05/12 3E
NIRAVAM® is a registered trademark of UCB Manufacturing, Inc.
00017960
LOT:EXP:
![]()
.50 mg Label
Principal Display Panel - 1 Mg Tablets
NDC 18860-323-01
100 tablets
NIRAVAM® 1 mg (alprazolam orally disintegrating tablets)
Rx only   CIV
Jazz Pharmaceuticals
orally disintegrating tablets
Each orange-flavored, orally disintegrating tablet contains 1 mg alprazolam.
USUAL DOSAGE: See package insert for further information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86ºF) [See USP Controlled Room Temperature]. Protect from moisture.
Dispense in a tight container as defined in the USP/NF.
KEEP OUT OF REACH OF CHILDREN.
Distributed by:Jazz Pharmaceuticals Commercial Corp.Philadelphia, PA 19103
U.S. Patent Nos. 6,024,981 and 6,221,392
NIRAt-10-12Rev. 05/12 3E
NIRAVAM® is a registered trademark of UCB Manufacturing, Inc.
00017959
LOT:EXP:
![]()
1 mg Label
Principal Display Panel - 2 Mg Tablets
NDC 18860-324-01
100 tablets
NIRAVAM® 2 mg (alprazolam orally disintegrating tablets)
Rx only   CIV
Jazz Pharmaceuticals
orally disintegrating tablets
Each orange-flavored, orally disintegrating tablet contains 2 mg alprazolam.
USUAL DOSAGE: See package insert for further information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86ºF) [See USP Controlled Room Temperature]. Protect from moisture.
Dispense in a tight container as defined in the USP/NF.
KEEP OUT OF REACH OF CHILDREN.
Distributed by:Jazz Pharmaceuticals Commercial Corp.Philadelphia, PA 19103
U.S. Patent Nos. 6,024,981 and 6,221,392
NIRAt-10-12Rev. 05/12 3E
NIRAVAM® is a registered trademark of UCB Manufacturing, Inc.
00017958
LOT:EXP:
![]()
2 mg Label
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site