Phenazopyridine Hydrochloride (phenazopyridine hydrochloride 100 mg) Dailymed
Generic: phenazopyridine hydrochloride is used for the treatment of Kidney Diseases Liver Diseases Pain Pyelonephritis Urological Manifestations
IMPRINT: 115
SHAPE: round
COLOR: brown
All Imprints
phenazopyridine hydrochloride 200 mg oral tablet - 115 round brown
Go PRO for all pill images
Description
Phenazopyridine Hydrochloride is dark brown fine granular powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain.
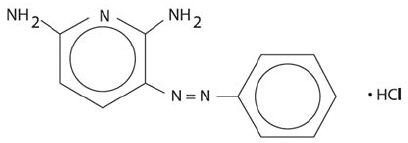
Phenazopyridine HCl tablets contain the following inactive ingredients: hypromellose, polyethylene glycol, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, pregelatinized starch, maize(corn) starch, silicone dioxide, polyvinylpyrrolidone.
Clinical Pharmacology
Phenazopyridine HCl is excreted in the urine where it exerts a topical analgesic effect on the mucosa of the urinary tract. This action helps to relieve pain, burning, urgency and frequency. The precise mechanism of action is not known. The pharmacokinetic properties of Phenazopyridine HCl have not been determined. Phenazopyridine HCl is rapidly excreted by the kidneys, with as much as 66% of an oral dose being excreted unchanged in the urine.
Indications And Usage
Phenazopyridine HCl is indicated for the symptomatic relief of pain, burning, urgency, frequency, and other discomforts arising from irritation of the lower urinary tract mucosa caused by infection, trauma, surgery, endoscopic procedures, or the passage of sounds or catheters. The use of Phenazopyridine HCl for relief of symptoms should not delay definitive diagnosis and treatment of causative conditions. Because it provides only symptomatic relief, prompt appropriate treatment of the cause of pain must be instituted and Phenazopyridine HCl should be discontinued when symptoms are controlled. The analgesic action may reduce or eliminate the need for systemic analgesics or narcotics. It is, however, compatible with antibacterial therapy and can help to relieve pain and discomfort during the interval before antibacterial therapy controls the infection. Treatment of a urinary tract infection with Phenazopyridine HCl should not exceed 2 days because there is a lack of evidence that the combined administration of Phenazopyridine HCl and an antibacterial provides greater benefit than administration of the antibacterial alone after 2 days. (See  DOSAGE AND ADMINISTRATION section.)
Contraindications
Phenazopyridine HCl should not be used in patients who have previously exhibited hypersensitivity to it. The use of Phenazopyridine HCl is contraindicated in patients with renal insufficiency.
Adverse Reactions
Headache, rash, pruritus and occasional gastrointestinal disturbance. An anaphylactoid-like reaction has been described. Methemoglobinemia, hemolytic anemia, renal and hepatic toxicity have been reported, usually at overdosage levels (see OVERDOSAGE Section).
Precautions
General: A yellowish tinge of the skin or sclera may indicate accumulation due to impaired renal excretion and the need to discontinue therapy. The decline in renal function associated with advanced age should be kept in mind. While our product does not contain Sulfur, it has been indicated to exhibit in rare circumstances a sulfur-type reaction. Use of this product in patients sensitive to sulfur should be done so under the supervision of a Physician.
NOTE: Patients should be informed that Phenazopyridine HCl produces a reddish-orange discoloration of the urine and may stain fabric. Staining of contact lenses has been reported.
Laboratory Test Interaction: Due to its properties as an azo dye, Phenazopyridine HCl may interfere with urinalysis based on spectrometry or color reactions.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term administration of Phenazopyridine HCl has induced neoplasia in rats (large intestine) and mice (liver).  Although no association between Phenazopyridine HCl and human neoplasia has been reported, adequate epidemiological studies along these lines have not been conducted.
Pregnancy Category B: Reproduction studies have been performed in rats at doses up to 50 mg/kg/day and have revealed no evidence of impaired fertility or harm to the fetus due to Phenazopyridine HCl. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing mothers: No information is available on the appearance of Phenazopyridine HCl, or its metabolites in human milk.
Dosage And Administration
100 mg Tablets: Average adult dosage is two tablets 3 times a day after meals. 200 mg Tablets: Average adult dosage is one tablet 3 times a day after meals.
When used concomitantly with an antibacterial agent for the treatment of a urinary tract infection, the administration of Phenazopyridine HCl should not exceed 2 days.
To report SUSPECTED ADVERSE REACTIONS, contact Winder Laboratories, LLC at 1-770-307-0703, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Overdosage
Exceeding the recommended dose in patients with good renal function or administering the usual dose to patients with impaired renal function (common in elderly patients) may lead to increased serum levels and toxic reactions. Methemoglobinemia generally follows a massive, acute overdose. Methylene blue, 1 to 2 mg/kg/body weight intravenously or ascorbic acid 100 to 200 mg given orally should cause prompt reduction of the methemoglobinemia and disappearance of the cyanosis which is an aid in diagnosis. Oxidative Heinz body hemolytic anemia may also occur, and "bite cells" (degmacytes) may be present in a chronic overdosage situation. Red blood cell G-6-PD deficiency may predispose to hemolysis. Renal and hepatic impairment and occasional failure, usually due to hypersensitivity, may also occur.
How Supplied
100 mg Tablets: Supplied in bottles of 100 (NDC 75826-114-10) counts. Appearance: Dark brown to maroon colored, round, film coated tablets debossed "114" on one side and plain on the other side.
200 mg Tablets: Supplied in bottles of 100 (NDC 75826-115-10) counts. Appearance: Dark brown to maroon colored, round, film coated tablets debossed "115" on one side and plain on the other side.
DISPENSE contents with a child-resistant closure (as¬†required) and in a tight container as defined in the USP. STORE at 20¬į to 25¬įC (68¬į to 77¬įF); excursions permitted¬†to 15¬į to 30¬įC (59¬į to 86¬įF) [See USP Controlled Room¬†Temperature].
Disclaimer: This is not an Orange Book Product and has not been subjected to FDA therapeutic equivalency or other equivalency testing.
Manufactured by: Winder Laboratories, LLC.716 Patrick Industrial Lane Winder, GA 30680
RLS.115.99-2.0 Rev. Dec 2021
Package Label.principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site




