Ranitidine (ranitidine hydrochloride 300 mg) Dailymed
Generic: ranitidine is used for the treatment of Duodenal Ulcer Esophagitis, Peptic Gastroesophageal Reflux Gastrointestinal Hemorrhage Stomach Ulcer Zollinger-Ellison Syndrome
IMPRINT: V 02
SHAPE: round
COLOR: orange
All Imprints
ranitidine 150 mg oral tablet - v 02 round orange
ranitidine 300 mg oral tablet - v 03 oval yellow
Go PRO for all pill images
Spl Product Data Elements Section
Description
The active ingredient in Ranitidine Tablet USP, 150 mg / Ranitidine Tablet USP, 300 mg is Ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine, HCl. It has the following structure:
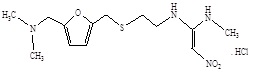
The empirical formula is C13H22N4O3S.HCl, representing molecular weight of 350.87.
Ranitidine HCl is white to pale yellow, crystalline, practically odorless powder, sensitive to light and moisture. Melts at about 140°C with decomposition.
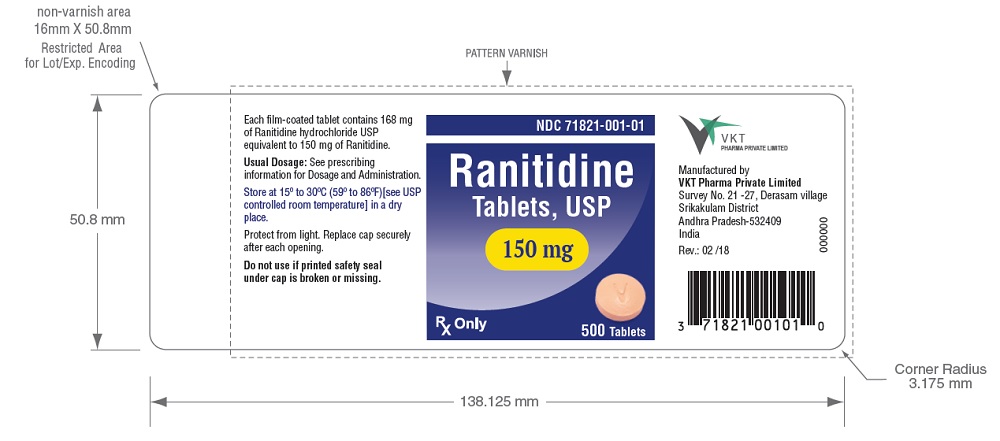
Each Ranitidine Tablet USP, 150 mg for oral administration contains 168 mg of Ranitidine HCl equivalent to 150 mg of ranitidine. Each tablet also contains the inactive ingredients microcrystalline cellulose, croscarmellose sodium, magnesium stearate, Opadry® 200 Orange 203A530006 (Polyvinyl alcohol, talc, titanium dioxide, glycerol monostearate, sodium lauryl sulphate, FD&C yellow 6 and iron oxide yellow), purified water.
Each Ranitidine Tablet USP, 300 mg for oral administration contains 336 mg of Ranitidine HCl equivalent to 300 mg of Ranitidine. Each tablet also contains the inactive ingredients microcrystalline cellulose, croscarmellose sodium, magnesium stearate, Opadry® 200 Yellow 203A520014 (Polyvinyl alcohol, talc, titanium dioxide, glycerol monostearate, sodium lauryl sulphate, FD&C yellow 5 and FD&C Blue 1), purified water.
Clinical Pharmacology
Ranitidine Tablets is a competitive, reversible inhibitor of the action of histamine at the histamine H2-receptors, including receptors on the gastric cells. Ranitidine Tablets does not lower serum Ca++ in hypercalcemic states. Ranitidine Tablets is not an anticholinergic agent.
Pharmacokinetics:
Absorption:Â Ranitidine Tablets is 50% absorbed after oral administration, compared to an intravenous (IV) injection with mean peak levels of 440 to 545 ng/mL occurring 2 to 3 hours after a 150 mg dose. Absorption is not significantly impaired by the administration of food or antacids. Propantheline slightly delays and increases peak blood levels of Ranitidine, probably by delaying gastric emptying and transit time. In one study, simultaneous administration of high-potency antacid (150 mmol) in fasting subjects has been reported to decrease the absorption of Ranitidine Tablets.
Distribution: The volume of distribution is about 1.4 L/kg. Serum protein binding averages 15%.
Metabolism: In humans, the N-oxide is the principal metabolite in the urine; however, this amounts to < 4% of the dose. Other metabolites are the S-oxide (1%) and the desmethyl ranitidine (1%). The remainder of the administered dose is found in the stool. Studies in patients with hepatic dysfunction (compensated cirrhosis) indicate that there are minor, but clinically insignificant, alterations in ranitidine half-life, distribution, clearance, and bioavailability.
Excretion: The principal route of excretion is the urine, with approximately 30% of the orally administered dose collected in the urine as unchanged drug in 24 hours. Renal clearance is about 410 mL/min, indicating active tubular excretion. The elimination half-life is 2.5 to 3 hours. Four patients with clinically significant renal function impairment (creatinine clearance 25 to 35 mL/min) administered 50 mg of ranitidine intravenously had an average plasma half-life of 4.8 hours, a Ranitidine clearance of 29 mL/min, and a volume of distribution of 1.76 L/kg. In general, these parameters appear to be altered in proportion to creatinine clearance (see DOSAGE AND ADMINISTRATION).
Geriatrics: The plasma half-life is prolonged and total clearance is reduced in the elderly population due to a decrease in renal function. The elimination half-life is 3 to 4 hours. Peak levels average 526 ng/mL following a 150-mg twice-daily dose occur in about 3 hours (see PRECAUTIONS: Geriatric Use and DOSAGE AND ADMINISTRATION: Dosage adjustment for Patients with impaired renal function).Â
Pediatrics: There are no significant differences in the pharmacokinetic parameter values for ranitidine in pediatric patients (from one month upto 16 yrs of age) and healthy adults when correction is made for body weight. The average bioavailability of Ranitidine is given orally to pediatric patients is 48%, which is comparable to the bioavailability of Ranitidine in the adult population. All other pharmacokinetic parameter values (t1/2, Vd, and CL) are similar to those observed with intravenous Ranitidine use in pediatric patients. Estimates of Cmax and Tmax are displayed in Table 1.
Table 1. Ranitidine Pharmacokinetics in Pediatric Patients Following Oral Dosing Population (age) n Dosage Form (dose) Cmax (ng/mL) Tmax (hours) Gastric or duodenal ulcer (3.5 to 16 years) 12 Tablets (1 to 2 mg/ kg) 54 to 492 2.0 Plasma clearance measured in 2 neonatal patients (less than 1 month of age) was considerably lower (3 mL/min/kg) than children or adults and is likely due to reduced renal function observed in this population (see PRECAUTIONS: Pediatric Use and DOSAGE AND ADMINISTRATION: Pediatric use).
Pharmacodynamics: Serum concentrations necessary to inhibit 50% of stimulated gastric acid secretion are estimated to be 36 to 94 ng/mL. Following a single oral dose of 150 mg, serum concentrations of ranitidine are in this range up to 12 hours. However, blood levels bear no consistent relationship to dose or degree of acid inhibition.
Antisecretory Activity: 1. Effects on Acid Secretion:Â Ranitidine Tablets inhibits both daytime and nocturnal basal gastric acid secretions as well as gastric acid secretion stimulated by food, betazole, and pentagastrin, as shown in below table 2.
Table 2. Effect of Oral Ranitidine Tablets on Gastric Acid Secretion Time After Dose, hours % Inhibition of Gastric Acid Output by Dose, mg 75 – 80 100 150 200 Basal Up to 4 99 95 Nocturnal Up to 13 95 96 92 Betazole Up to 3 97 99 Pentagastrin Up to 5 58 72 72 80 Meal Up to 3 73 79 95 It appears basal-, nocturnal-, and betazole- stimulated secretions are most sensitive to inhibition by Ranitidine Tablets responding almost completely to doses of 100 mg or less, while pentagastrin- and food-stimulated secretions are more difficult to suppress.
2. Effects on other gastrointestinal secretions:
Pepsin:Â Oral Ranitidine Tablets does not affect pepsin secretion. Total pepsin output is reduced in proportion to the decrease in volume of gastric juice.
Intrinsic Factor: Oral Ranitidine Tablets has no significant effect on pentagastrin-stimulated intrinsic factor secretion.
Serum Gastrin:Â Ranitidine Tablets has little or no effect on fasting or postprandial serum gastrin.
Other Pharmacologic Actions:
- Gastric bacterial flora-increase in nitrate-reducing organisms, significance not known.
- Prolactin levels-no effect in recommended oral or IV dosage, but small, transient, dose-related increases in serum prolactin have been reported after IV bolus injections of 100 mg or more.
- Other pituitary hormones-no effect on serum gonadotropins, TSH or GH. Possible impairment of vasopressin release.
- No change in cortisol, aldosterone, androgen or estrogen levels.
- No anti androgenic action.
- No effect on count, motility or morphology of sperm.
Pediatrics:Â Oral doses of 6 to 10 mg/kg/day in 2 or 3 divided doses maintain gastric pH >4 throughout most of the dosing interval.
Clinical Trials: Â Active Duodenal Ulcer: In a multicenter, double-blind, controlled, US study of endoscopically diagnosed duodenal ulcers, earlier healing was seen in patients treated with Ranitidine Tablets as shown in Table 3.
Table 3. Duodenal Ulcer Patient Healing Rates Ranitidine Tablets All patients were permitted antacids as needed for relief of pain Placebo Number Entered Healed/ Evaluable Number Entered Healed/ Evaluable Outpatients 195 69/182 (38%) P < 0.0001 188 31/164 (19%) Week 2 Week 4 137/187 (73%) 76/168 (45%) In these studies, patients treated with Ranitidine Tablets reported a reduction in both daytime and nocturnal pain, and they also consumed less antacid than the placebo-treated patients.
Table 4. Mean Daily Doses of Antacid Ulcer Healed Ulcer Not Healed Ranitidine Tablets 0.06 0.71 Placebo 0.71 1.43 Foreign studies have shown that patients heal equally well with 150 mg twice daily and 300 mg at bedtime (85% versus 84%, respectively) during a usual 4-week course of therapy. If patients require extended therapy of 8 weeks, the healing rate may be higher for 150 mg twice daily as compared to 300 mg at bedtime (92% versus 87%, respectively).
Studies have been limited to short-term treatment of acute duodenal ulcer. Patients whose ulcers healed during therapy had recurrences of ulcers at the usual rates.
Maintenance therapy in duodenal ulcer:Â Ranitidine has been found to be effective as maintenance therapy for patients following healing of acute duodenal ulcers. In 2 independent, double-blind, multi-center, controlled trials, the number of duodenal ulcers observed was significantly less in patients treated with Ranitidine Tablets (150 mg at bedtime) than in patients treated with placebo over a 12-month period.
Table 5. Duodenal Ulcer Prevalence % = Life table estimate.
RAN = ranitidine
PLC = Placebo.
Double-Blind, Multicenter, Placebo-Controlled Trials Duodenal Ulcer Prevalence Multicenter Trial Drug 0-4 Months 0-8 Months 0-12 Months No. of Patients USA RAN 20% = P < 0.05 (Ranitidine Tablets versus comparator). 24% 35% 138 PLC 44% 54% 59% 139 Foreign RAN 12% 21% 28% 174 PLC 56% 64% 68% 165 As with other H2-antagonists, the factors responsible for the significant reduction in the prevalence of duodenal ulcers include prevention of recurrence of ulcers, more rapid healing of ulcers that may occur during maintenance therapy, or both.
Gastric Ulcer:Â In a multicentre, double-blind, controlled, US study of endoscopically diagnosed gastric ulcers, earlier healing was seen in the patients treated with Ranitidine Tablets as shown in Table 6.
Table 6. Gastric Ulcer Patient Healing Rates Ranitidine Tablets All patients were permitted antacids as needed for relief of pain. Placebo Number entered Healed / Evaluable Number entered Healed / Evaluable Outpatients 92 16/83 (19%) 94 10/83 (12%) Week 2 Week 6 50/73 (68%) P= 0.009 35/69 (51%) In this multicenter trial, significantly, more patients treated with Ranitidine Tablets became pain free during therapy.
Maintenance of healing Gastric Ulcers: In 2 multicentre, double-blind, randomized, placebo-controlled, 12-month trials conducted in patients whose gastric ulcers had been previously healed, Ranitidine Tablets 150 mg at bed time was significantly more effective than placebo in maintaining healing of gastric ulcers.
Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome):Â Ranitidine Tablets inhibits gastric acid secretion and reduces occurrence of diarrhea, anorexia, and pain in patients with pathological hypersecretion associated with Zollinger- Ellison syndrome, systemic mastocytosis, and other pathological hypersecretory conditions. (eg. Postoperative, "short-gut" syndrome, idiopathic). Use of Ranitidine Tablets was followed by healing of ulcers in 8 of 19 (42%) patients who were intractable to previous therapy.
Gastroesophageal Reflux Disease (GERD):Â In 2 multicenter, double-blind, placebo-controlled, 6-week trials performed in the United States and Europe, ranitidine tablets USP, 150 mg twice daily was more effective than placebo for the relief of heartburn and other symptoms associated with GERD. Ranitidine- treated patients consumed significantly less antacid than did placebo- treated patients.
The US trial indicated that Ranitidine Tablets USP, 150 mg twice daily significantly reduced the frequency of heartburn attacks and severity of heartburn pain within 1 to 2 weeks after starting therapy. The improvement was maintained throughout the 6-week trial period. Moreover, patient response rates demonstrated that the effect of heartburn extends through both the day and night time periods.
In 2 additional US multicenter, double-blind, placebo- controlled, 2- week trials, Ranitidine Tablets USP, 150 mg twice daily was shown to provide relief of heartburn pain within 24 hours of initiating therapy and a reduction in the frequency of severity of heartburn.
Erosive Esophagitis:Â In 2 multicenter, double- blind, randomized, placebo- controlled, 12- week trials performed in the United States, Ranitidine Tablets USP, 150 mg 4 times daily was significantly more effective than placebo in healing endoscopically diagnosed erosive esophagitis and in relieving associated heartburn. The erosive esophagitis healing rates were as follows:
Table 7. Erosive Esophagitis Patient Healing Rates  Healed/Evaluable Placebo All patients were permitted antacids as needed for relief of pain. n = 229Ranitidine Tablets USP, 150 mg 4 times daily n = 215 Week 4 43/198 (22%) 96/206 (47%) P < 0.001 versus placebo. Week 8 63/176 (36%) 142/200 (71%) Week 12 92/159 (58%) 162/192 (84%) No additional benefit in healing of esophagitis or in relief of heartburn was seen with a ranitidine dose of 300 mg 4 times daily.
Maintenance of Healing of Erosive Esophagitis:Â In 2 multicenter, double-blind, randomized, placebo-controlled, 48-week trials conducted in patients whose erosive esophagitis had been previously healed, Ranitidine Tablets USP, 150 mg twice daily was significantly more effective than placebo in maintaining healing of erosive esophagitis.
Indications And Usage
Ranitidine Tablets is indicated in
- Short- term treatment of active duodenal ulcer. Most patients heal within 4 weeks. Studies available to date have not assessed the safety of Ranitidine in uncomplicated duodenal ulcer for periods of more than 8 weeks.
- Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of acute ulcers. No placebo – controlled comparative studies have been carried out for periods longer than 1 year.
- The treatment of pathological hypersecretory conditions. (e.g., Zollinger-Ellison syndrome and systemic mastocytosis).
- Short-term treatment of active, benign gastric ulcer. Most patients heal within 6 weeks and the usefulness of further treatment has not been demonstrated. Studies available to date have not assessed the safety of ranitidine in uncomplicated, benign gastric ulcer for periods of more than 6 weeks.
- Maintenance therapy for gastric ulcer patients at reduced dosage after healing of acute ulcers. Placebo-controlled studies have been carried out for 1 year.
- Treatment of GERD. Symptomatic relief commonly occurs within 24 hours after starting therapy with Ranitidine Tablets USP, 150 mg twice daily.
- Treatment of endoscopically diagnosed erosive esophagitis. Symptomatic relief of heartburn commonly occurs within 24 hours of therapy initiation with Ranitidine Tablets USP, 150 mg 4 times daily.
- Maintenance of healing of erosive esophagitis. Placebo-controlled trials have been carried out for 48 weeks.
Concomitant antacids should be given as needed for pain relief to patients with active duodenal ulcer; active, benign gastric ulcer; hypersecretory states; GERD; and erosive esophagitis.
Contraindications
Ranitidine Tablets is contraindicated for patients known to have hypersensitivity to the drug or any of the ingredients (see PRECAUTIONS)
Precautions
General:
- Symptomatic response to therapy with Ranitidine Tablets does not preclude the presence of gastric malignancy.
- Since Ranitidine Tablets is excreted primarily by the kidney, dosage should be adjusted in patients with impaired renal function. (see DOSAGE AND ADMINISTRATION). Caution should be observed in patients with hepatic dysfunction since Ranitidine Tablets is metabolized in the liver.
- Rare reports suggest that Ranitidine Tablets may precipitate acute porphyric attacks in patients with acute porphyria. Ranitidine Tablets should therefore be avoided in patients with a history of acute porphyria.
Laboratory Tests: False-positive tests for urine protein with MULTISTIX® may occur during therapy with Ranitidine Tablets, and therefore testing with sulfosalicylic acid is recommended.
Drug Interactions: Ranitidine has been reported to affect the bioavailability of other drugs through several different mechanisms such as competition for renal tubular secretion, alteration of gastric pH and inhibition of cytochrome P450 enzymes.
Procainamide:Â Ranitidine, a substrate of the renal organic cation transport system, may affect the clearance of other drugs eliminated by this route. High doses of ranitidine (e.g., such as those used in the treatment of Zollinger-Ellison syndrome) have been shown to reduce the renal excretion of procainamide and N-acetylprocainamide resulting in increased plasma levels of these drugs. Although this interaction is unlikely to be clinically relevant at usual ranitidine doses, it may prudent to monitor for procainamide toxicity when administered with oral ranitidine at a dose exceeding 300 mg per day.
Warfarin:Â There have been reports of altered prothrombin time among patients on concomitant warfarin and ranitidine therapy. Due to the narrow therapeutic index, close monitoring of increased or decreased prothrombin time is recommended during concurrent treatment with ranitidine.
Ranitidine may alter the absorption of drugs in which gastric pH is an important determinant of bio-availability. This can result in either an increase in absorption (e.g., triazolam, midazolam, glipizide) or a decrease in absorption (e.g., ketoconazole, Atazanavir, delavirdine, gefitinib). Appropriate clinical monitoring is recommended.
Atazanavir: Atazanavir absorption may be impaired based on known interactions with other agents that increase gastric pH. Use with caution. see atazanavir label for specific recommendations.
Delavirdine: Delavirdine absorption may be impaired based on known interactions with other agents that increase gastric pH. Chronic use of H2-receptor antagonists with delavirdine is not recommended.
Gefitinib: Gefitinib exposure was reduced by 44% with the co-administration of ranitidine and sodium bicarbonate (dosed to maintain gastric pH above 5.0). Use with caution.
Glipizide: In diabetic patients, glipizide exposure was increased by 34% following a single 150 mg dose of oral ranitidine. Use appropriate clinical monitoring when initiating or discontinuing ranitidine.
Ketoconazole: Oral ketoconazole exposure was reduced by up to 95% when oral ranitidine was co-administered in a regimen to maintain a gastric pH of 6 or above. The degree of interaction with usual dose of ranitidine (150 mg twice daily) is unknown.
Midazolam: Oral midazolam exposure in 5 healthy volunteers was increased by up to 65% when administered with oral ranitidine at a dose of 150 mg twice daily. However, in another interaction trial in 8 volunteers receiving IV midazolam, a 300 mg oral dose of ranitidine increased midazolam exposure by about 9%. Monitor patients for excessive or prolonged sedation when ranitidine is co-administered with oral midazolam.
Triazolam: Triazolam exposure in healthy volunteers was increased by approximately 30% when administered with oral ranitidine at a dose of 150 mg twice daily. Monitor patients for excessive or prolonged sedation.
Carcinogenesis, Mutagenesis, Impairment of Fertility: There was no indication of tumorigenic or carcinogenic effects in life-span studies in mice and rats at dosages up to 2,000 mg/kg/day.
Ranitidine was not mutagenic in standard bacterial tests (Salmonella, Escherichia coli) for mutagenecity at concentrations up to the maximum recommended for these assays.
In a dominant lethal assay, a single oral dose of 1,000 mg/kg to male rats was without effect on the outcome of 2 matings per week for the next 9 weeks.
Pregnancy: Teratogenic Effects: Pregnancy Category B. Reproduction studies have been performed in rats and rabbits at doses upto 160 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to Ranitidine Tablets. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers: Ranitidine is secreted in human milk. Caution should be exercised when Ranitidine Tablets is administered to a nursing mother.
Pediatric Use: The safety and effectiveness of Ranitidine Tablets have been established in the age-group of 1 month to 16 years for the treatment of duodenal and gastric ulcers, gastroesophageal reflux disease and erosive esophagitis, and the maintenance of healed duodenal and gastric ulcer. Use of Ranitidine Tablets in this age group is supported by adequate and well-controlled studies in adults, as well as additional pharmacokinetic data in pediatric patients and an analysis of the published literature (see CLINICAL PHARMACOLOGY: Pediatrics and DOSAGE AND ADMINSTRATION: Pediatric Use).
Safety and effectiveness in pediatric patients for the treatment of pathological hypersecretory conditions or the maintenance of healing of erosive esophagitis have not been establishment.
Safety and effectiveness in neonates (less than 1 month of age) have not been established (see CLINICAL PHARMACOLOGY: Pediatrics).Â
Geriatric Use: Of the total number of subjects enrolled in US and foreign controlled clinical trials of oral formulations of Ranitidine Tablets, for which there were subgroup analyses, 4,197 were 65 and over, while 899 were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, caution should be exercised in dose selection, and it may be useful to monitor renal function. (See CLINICAL PHARMACOLGY: Pharmacokinetics: Geriatrics and DOSAGE AND ADMINISTRATION: Dosage Adjustment for Patients with Impaired Renal Function).
Adverse Reactions
The following have been reported as events in clinical trials or in the routine management of patients treated with Ranitidine Tablets. The relationship to therapy with Ranitidine Tablets has been unclear in many cases. Headache, sometimes severe, seems to be related to administration of Ranitidine Tablets.
Central Nervous System: Rarely, malaise, dizziness, somnolence, insomnia and vertigo. Rare cases of reversible mental confusion, agitation, depression, and hallucinations have been reported, predominantly in severely ill elderly patients. Rare cases of reversible blurred vision suggestive of a change in accommodation have been reported. Rare reports of reversible involuntary motor disturbances have been received.
Cardiovascular: As with other H2-blockers, rare reports of arrhythmias such as tachycardia, bradycardia, atrioventricular block, and premature ventricular beats.
Gastrointestinal: Constipation, diarrhea, nausea/vomiting, abdominal discomfort/pain and rare reports of pancreatitis.
Hepatic: There have been occasional reports of hepatocellular, cholestatic, or mixed hepatitis, with or without jaundice. In such circumstances, ranitidine should be immediately discontinued. These events are usually reversible, but in rare circumstances death has occurred. Rare cases of hepatic failure have also been reported. In normal volunteers, SGPT values were increased to atleast twice the pretreatment levels in 6 of 12 subjects receiving 100 mg intravenously 4 times daily for 7 days, and in 4 of 24 subjects receiving 50 mg intravenously 4 times daily for 5 days.
Musculoskeletal: Rare reports of arthralgias and myalgias.
Hematologic: Blood count changes (leukopenia, granulocytopenia, and thrombocytopenia) have occurred in few patients. These were usually reversible. Rare cases of agranulocytosis, pancytopenia, sometimes with narrow hypoplasia, and aplastic anemia and exceedingly rare cases of acquired immune hemolytic anemia have been reported.
Endocrine: Controlled studies in animals and man have shown no stimulation of any pituitary hormone by Ranitidine Tablets and no antiadrenogenic activity, and cimetidine-induced gynecomastia and impotence in hypersecretory patients have resolved when Ranitidine Tablets has been substituted.
However, occasional cases of impotence and loss of libido have been reported in male patients receiving Ranitidine Tablets, but the incidence did not differ from that in the general population. Rare cases of breast symptoms and conditions, including galactorrhea and gynecomastia, have been reported in both males and females.
Integumentary: Rash, including rare cases of erythema multiforme. Rare cases of alopecia and vasculitis.
Respiratory: A large epidemiological study suggested an increased risk of developing pneumonia in current users of histamine-2-receptor antagonists (H2RAs) compared with patients who had stopped H2RA treatment, with an observed adjusted relative risk of 1.63 (95% CI, 1.07 – 2.48). However, a casual relationship between use of H2RAs and pneumonia has not been established.
Other: Rare cases of hypersensitivity reactions (e.g., bronchospasm, fever, rash, eosinophilia), anaphylaxis, angioneurotic edema, acute interstitial nephritis, and small increases in serum creatinine.
Overdosage
There has been limited experience with overdosage. Reported acute ingestions of up to 18 g orally have been associated with transient adverse effects similar to those encountered in normal clinical experience (see ADVERSE REACTIONS). In addition, abnormalities of gait and hypotension have been reported.
When overdosage occurs, the usual measures to remove unabsorbed material from the gastrointestinal tract, clinical monitoring, and supportive therapy should be employed.
Studies in dogs receiving dosages of Ranitidine Tablets, in excess of 225 mg/kg/day have shown muscular tremors, vomiting, and rapid respiration. Single oral doses of 1,000 mg/kg in mice and rats were not lethal. Intravenous LD50, values in mice and rats were 77 and 83 mg/kg, respectively.
Dosage And Administration
Active Duodenal Ulcer: The current recommended adult oral dosage of Ranitidine Tablets for duodenal ulcer is 150 mg twice daily. An alternative dosage of 300 mg once daily after the evening meal or at bedtime can be used for patients in whom dosing convenience is important. The advantages of one treatment regimen compared with the other in a particular patient population have yet to be demonstrated (see Clinical Trials: Active Duodenal Ulcer). Smaller doses have been shown to be equally effective in inhibiting gastric acid secretion in US studies, and several foreign trials have shown that 100 mg twice daily is as effective as the 150 mg dose.
Antacid should be given as needed for relief of pain (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
Maintenance of Healing of Duodenal Ulcers: The current recommended adult oral dosage is 150 mg at bedtime.
Pathological Hypersecretory Conditions (such as Zollinger - Ellison syndrome): The current recommended adult oral dosage is 150 mg twice daily. In some patients it may be necessary to administer Ranitidine Tablets USP, 150 mg doses more frequently. Dosages should be adjusted to individual patient needs, and should continue as long as clinically indicated. Dosages up to 6 g/day have been employed in patients with severe disease.
Benign Gastric Ulcer: The current recommended adult oral dosage is 150 mg twice daily.
Maintenance of Healing of Gastric Ulcers: The current recommended adult oral dosage is 150 mg at bed time.
GERD: The current recommended adult oral dosage is 150 mg twice daily.
Erosive Esophagitis: The current recommended adult oral dosage is 150 mg 4 times daily.
Maintenance of Healing of Erosive Esophagitis: The current recommended adult oral dosage is 150 mg twice daily.
Pediatric Use: The safety and effectiveness of Ranitidine Tablets have been established in the age- group of 1 month to 16 years. There is insufficient information about the pharmacokinetics of Ranitidine Tablets in neonatal patients (less than 1 month of age) to make dosing recommendations.
The following 3 subsections provide dosing information for each of the pediatric indications.
Treatment of Duodenal and Gastric Ulcers:Â The recommended oral dose for the treatment of active duodenal and gastric ulcers is 2 to 4 mg/kg twice daily to a maximum of 300 mg/day. This recommendation is derived from adult clinical studies and pharmacokinetic data in pediatric patients.
Maintenance of Healing of Duodenal and Gastric Ulcers:Â The recommended oral dose for the maintenance of healing of duodenal and gastric ulcers is to 2 to 4 mg/kg once daily to a maximum of 150 mg/day. This recommendation is derived from adult clinical studies and pharmacokinetic data in pediatric patients.
Treatment of GERD and Erosive Esophagitis:Â Although limited data exist for these conditions in pediatric patients, published literature supports a dosage of 5 to 10 mg/kg/day, usually given as 2 divided doses.
Dosage Adjustment for Patients with Impaired Renal Function: On the basis of experience with a group of subjects with severely impaired renal function treated with Ranitidine Tablets, the recommended dosage in patients with a creatinine clearance < 50 mL/min is 150 mg every 24 hours. Should the patient's condition require, the frequency of dosing may be increased to every 12 hours or even further with caution. Hemodialysis reduces the level of circulating ranitidine. Ideally, the dosing schedule should be adjusted so that the timing of a scheduled dose coincides with the end of hemodialysis.
Elderly patients are more likely to have decreased renal function, therefore caution should be exercised in dose selection, and it may be useful to monitor renal function. (see CLINICAL PHARMACOLOGY: Pharmacokinetics: Geriatrics and PRECAUTIONS : Geriatric Use).
How Supplied
Ranitidine Tablets USP, 150 mg (Ranitidine HCl equivalent to 150 mg of ranitidine) are orange, film-coated, round shaped tablets debossed with 'V' on one side and '02' on the other side. They are available in following counts
- Bottles of 500 (NDC 71821-001-01) tablets
Ranitidine Tablets USP, 300 mg (Ranitidine HCl equivalent to 300 mg of ranitidine) are yellow, film-coated, oval shaped tablets debossed with 'V' on one side and '03' on the other side. They are available in following counts
- Bottles of 250 (NDC 71821-002-01) tablets
Store between 15º and 30ºC (59º and 86ºF) in a dry place. Protect from light. Replace cap securely after each opening.
Contains color additives including FD&C Yellow No.5 (tartrazine)
MULTISTIX is a registered trademark of Bayer Healthcare LLC.
VKT Pharma Private Limited
Survey No.21-27, Derasam Village
Srikakulam District,
Andhra Pradesh
India-532409
February 2018
Package Label.principal Display Panel
Package Label.principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site