riluzole (riluzole 50 mg) Dailymed
Generic: riluzole is used for the treatment of Amyotrophic Lateral Sclerosis Hypersensitivity
Go PRO for all pill images
1 Indications And Usage
Riluzole tablets are indicated for the treatment of amyotrophic lateral sclerosis (ALS).
Riluzole tablets are indicated for the treatment of amyotrophic lateral sclerosis (ALS) (1 )
2 Dosage And Administration
The recommended dosage for riluzole is 50 mg taken orally twice daily. Riluzole should be taken at least 1 hour before or 2 hours after a meal [see Clinical Pharmacology (12.3)].
Measure serum aminotransferases before and during treatment with riluzole [see Warnings and Precautions (5.1)].
• Recommended dosage: 50 mg twice daily, taken at least 1 hour before or 2 hours after a meal (2 )• Measure serum aminotransferases before and during treatment (2 ,5.1 )
3 Dosage Forms And Strengths
Tablets: 50 mg white to off-white, capsule shaped, beveled edged, film-coated tablets, engraved with ‘381’ on one side and ‘G’ on the other side.
Tablets: 50 mg (3 )
4 Contraindications
Riluzole tablets are contraindicated in patients with a history of severe hypersensitivity reactions to riluzole or to any of its components (anaphylaxis has occurred) [see Adverse Reactions (6.1)].
Patients with a history of severe hypersensitivity reactions to riluzole or to any of its components (4 )
5 Warnings And Precautions
• Hepatic injury: Use of riluzole is not recommended in patients with baseline elevations of serum aminotransferases greater than 5 times upper limit of normal; discontinue riluzole if there is evidence of liver dysfunction (5.1 )• Neutropenia: Advise patients to report any febrile illness (5.2 )• Interstitial lung disease: Discontinue riluzole if interstitial lung disease develops (5.3 )5.1 Hepatic Injury
Cases of drug-induced liver injury, some of which were fatal, have been reported in patients taking riluzole. Asymptomatic elevations of hepatic transaminases have also been reported, and in some patients have recurred upon rechallenge with riluzole.
In clinical studies, the incidence of elevations in hepatic transaminases was greater in riluzole-treated patients than placebo-treated patients. The incidence of elevations of ALT above 5 times the upper limit of normal (ULN) was 2% in riluzole-treated patients. Maximum increases in ALT occurred within 3 months after starting riluzole. About 50% and 8% of riluzole-treated patients in pooled Studies 1 and 2, had at least one elevated ALT level above ULN and above 3 times ULN, respectively [see Clinical Studies (14)].
Monitor patients for signs and symptoms of hepatic injury, every month for the first 3 months of treatment, and periodically thereafter. The use of riluzole is not recommended if patients develop hepatic transaminase levels greater than 5 times the ULN. Discontinue riluzole if there is evidence of liver dysfunction (e.g., elevated bilirubin).
5.2 Neutropenia
Cases of severe neutropenia (absolute neutrophil count less than 500 per mm3) within the first 2 months of riluzole treatment have been reported. Advise patients to report febrile illnesses.
5.3 Interstitial Lung Disease
Interstitial lung disease, including hypersensitivity pneumonitis, has occurred in patients taking riluzole. Discontinue riluzole immediately if interstitial lung disease develops.
6 Adverse Reactions
The following adverse reactions are described below and elsewhere in the labeling:
Most common adverse reactions (incidence greater than or equal to 5% and greater than placebo) were asthenia, nausea, dizziness, decreased lung function, and abdominal pain (6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Glenmark Pharmaceuticals Inc., USA at 1 (888) 721-7115 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Controlled Clinical Trials
In the placebo-controlled clinical trials in patients with ALS (Study 1 and 2), a total of 313 patients received riluzole 50 mg twice daily [see Clinical Studies (14)]. The most common adverse reactions in the riluzole group (in at least 5% of patients and more frequently than in the placebo group) were asthenia, nausea, dizziness, decreased lung function, and abdominal pain. The most common adverse reactions leading to discontinuation in the riluzole group were nausea, abdominal pain, constipation, and elevated ALT.
There was no difference in rates of adverse reactions leading to discontinuation in females and males. However, the incidence of dizziness was higher in females (11%) than in males (4%). The adverse reaction profile was similar in older and younger patients. There were insufficient data to determine if there were differences in the adverse reaction profile in different races.
Table 1 uls adverse reactions that occurred in at least 2% of riluzole-treated patients (50 mg twice daily) in pooled Study 1 and 2, and at a higher rate than placebo.
Table 1. Adverse Reactions in Pooled Placebo-Controlled Trials (Studies 1 and 2) in Patients with ALS
Riluzole
50 mg twice daily
(N=313)
Placebo
(N=320)
Asthenia
19%
12%
Nausea
16%
11%
Decreased lung function
10%
9%
Hypertension
5%
4%
Abdominal pain
5%
4%
Vomiting
4%
2%
Arthralgia
4%
3%
Dizziness
4%
3%
Dry mouth
4%
3%
Insomnia
4%
3%
Pruritus
4%
3%
Tachycardia
3%
1%
Flatulence
3%
2%
Increased cough
3%
2%
Peripheral edema
3%
2%
Urinary Tract Infection
3%
2%
Circumoral paresthesia
2%
0%
Somnolence
2%
1%
Vertigo
2%
1%
Eczema
2%
1%
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of riluzole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
• Acute hepatitis and icteric toxic hepatitis [see Warnings and Precautions (5.1)]• Renal tubular impairment• Pancreatitis
7 Drug Interactions
• Strong to moderate CYP1A2 inhibitors: Coadministration may increase riluzole-associated adverse reactions (7.1 )• Strong to moderate CYP1A2 inducers: Coadministration may result in decreased efficacy (7.2 )• Hepatotoxic drugs: Riluzole-treated patients that take other hepatotoxic drugs may be at increased risk for hepatotoxicity (7.3 )7.1 Agents that may Increase Riluzole Blood Concentrations
CYP1A2 inhibitors
Co-administration of riluzole (a CYP1A substrate) with CYP1A2 inhibitors was not evaluated in a clinical trial; however, in vitro findings suggest an increase in riluzole exposure is likely. The concomitant use of strong or moderate CYP1A2 inhibitors (e.g., ciprofloxacin, enoxacin, fluvoxamine, methoxsalen, mexiletine, oral contraceptives, thiabendazole, vemurafenib, zileuton) with riluzole may increase the risk of riluzole-associated adverse reactions [see Clinical Pharmacology (12.3)].
7.2 Agents that may Decrease Riluzole Plasma Concentrations
CYP1A2 inducers
Co-administration of riluzole (a CYP1A substrate) with CYP1A2 inducers was not evaluated in a clinical trial; however, in vitro findings suggest a decrease in riluzole exposure is likely. Lower exposures may result in decreased efficacy [see Clinical Pharmacology (12.3)].
7.3 Hepatotoxic Drugs
Clinical trials in ALS patients excluded patients on concomitant medications which were potentially hepatotoxic (e.g., allopurinol, methyldopa, sulfasalazine). Riluzole-treated patients who take other hepatotoxic drugs may be at an increased risk for hepatotoxicity [see Warnings and Precautions (5.1)].
8 Use In Specific Populations
• Pregnancy: Based on animal data, may cause fetal harm (8.1 )8.1 Pregnancy
Risk Summary
There are no studies of riluzole in pregnant women, and case reports have been inadequate to inform the drug-associated risk. The background risk for major birth defects and miscarriage in patients with amyotrophic lateral sclerosis is unknown. In the U.S. general population, the background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
In studies in which riluzole was administered orally to pregnant animals, developmental toxicity (decreased embryofetal/offspring viability, growth, and functional development) was observed at clinically relevant doses [see Data]. Based on these results, women should be advised of a possible risk to the fetus associated with use of riluzole during pregnancy.
Data
 Animal Data Oral administration of riluzole (3, 9, or 27 mg/kg/day) to pregnant rats during the period of organogenesis resulted in decreases in fetal growth (body weight and length) at the high dose. The mid dose, a no-effect dose for embryofetal developmental toxicity, is approximately equal to the recommended human daily dose (RHDD, 100 mg) on a mg/m2 basis. When riluzole was administered orally (3, 10, or 60 mg/kg/day) to pregnant rabbits during the period of organogenesis, embryofetal mortality was increased at the high dose and fetal body weight was decreased and morphological variations increased at all but the lowest dose tested. The no-effect dose (3 mg/kg/day) for embryofetal developmental toxicity is less than the RHDD on a mg/m2 basis. Maternal toxicity was observed at the highest dose tested in rat and rabbit. When riluzole was orally administered (3, 8, or 15 mg/kg/day) to male and female rats prior to and during mating and to female rats throughout gestation and lactation, increased embryofetal mortality and decreased postnatal offspring viability, growth, and functional development were observed at the high dose. The mid dose, a no-effect dose for pre-and postnatal developmental toxicity, is approximately equal to the RHDD on a mg/m2 basis.8.2 Lactation
Risk Summary
It is not known if riluzole is excreted in human milk. Riluzole or its metabolites have been detected in milk of lactating rats. Women should be advised that many drugs are excreted in human milk and that the potential for serious adverse reactions in nursing infants from riluzole is unknown.
8.3 Females and Males of Reproductive Potential
In rats, oral administration of riluzole resulted in decreased fertility indices and increases in embryolethality [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of riluzole in pediatric patients have not been established.
8.5 Geriatric Use
In clinical studies of riluzole, 30% of patients were 65 years and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
Patients with mild [Child-Pugh’s (CP) score A] or moderate (CP score B) hepatic impairment had increases in AUC compared to patients with normal hepatic function. Thus, patients with mild or moderate hepatic impairment may be at increased risk of adverse reactions. The impact of severe hepatic impairment on riluzole exposure is unknown.
Use of riluzole is not recommended in patients with baseline elevations of serum aminotransferases greater than 5 times upper limit of normal or evidence of liver dysfunction (e.g., elevated bilirubin) [Clinical Pharmacology (12.3)].
8.7 Japanese Patients
Japanese patients are more likely to have higher riluzole concentrations. Consequently, the risk of adverse reactions may be greater in Japanese patients [see Clinical Pharmacology (12.3)].
10 Overdosage
Reported symptoms of overdose following ingestion of riluzole ranging from 1.5 to 3 grams (30 to 60 times the recommended dose) included acute toxic encephalopathy, coma, drowsiness, memory loss, and methemoglobinemia.
No specific antidote for the treatment of riluzole overdose is available. For current information on the management of poisoning or overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
11 Description
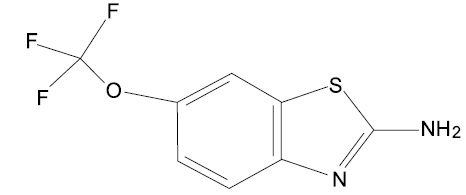
Riluzole, USP is a member of the benzothiazole class. The chemical designation for riluzole is 2-amino-6-(trifluoromethoxy)benzothiazole. Its molecular formula is C8H5F3N2OS, and its molecular weight is 234.2 g/mol. The chemical structure is:
Riluzole USP is a white to slightly yellow powder or crystalline powder that is freely soluble in acetonitrile, in alcohol and in methylene chloride; slightly soluble in hexane, very slightly soluble in water.
Each film-coated tablet for oral use contains 50 mg of riluzole, USP and the following inactive ingredients: anhydrous dibasic calcium phosphate, colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400 and titanium dioxide.
12 Clinical Pharmacology
12.1 Mechanism of Action
The mechanism by which riluzole exerts its therapeutic effects in patients with ALS is unknown.
12.2 Pharmacodynamics
The clinical pharmacodynamics of riluzole has not been determined in humans.
12.3 Pharmacokinetics
Table 2 displays the pharmacokinetic parameters of riluzole.
Table 2. Pharmacokinetic Parameters of Riluzole
Absorption
 Bioavailability (oral)
Approximately 60%
 Dose Proportionality
Linear over a dose range of 25 mg to 100 mg every 12 hours (1/2 to 2 times the recommended dosage)
 Food effect
AUC ↓ 20% and Cmax ↓ 45% (high fat meal)
Distribution
 Plasma Protein Binding
96% (Mainly to albumin and lipoproteins)
Elimination
 Elimination half-life
• 12 hours (CV=35%)• The high interindividual variability in the clearance of riluzole is potentially attributable to variability of CYP1A2. The clinical implications are not known.
 Accumulation
Approximately 2-fold
Metabolism
 Fraction metabolized (% dose)
At least 88%
 Primary metabolic pathway(s) [in vitro]
• Oxidation: CYP1A2• Direct and sequential glucoronidation: UGT-HP4
 Active Metabolites
Some metabolites appear pharmacologically active in vitro but the clinical implications are not known.
Excretion
 Primary elimination pathways (% dose)
• Feces: 5%• Urine: 90% (2% unchanged riluzole)
Specific Populations
 Hepatic Impairment Compared with healthy volunteers, the AUC of riluzole was approximately 1.7-fold greater in patients with mild chronic hepatic impairment (CP score A) and approximately 3-fold greater in patients with moderate chronic hepatic impairment (CP score B). The pharmacokinetics of riluzole have not been studied in patients with severe hepatic impairment (CP score C) [see Use in Specific Populations (8.6)]. Race The clearance of riluzole was 50% lower in male Japanese subjects than in Caucasian subjects, after normalizing for body weight [see Use in Specific Populations (8.7)]. Gender The mean AUC of riluzole was approximately 45% higher in female patients than male patients. Smokers The clearance of riluzole in tobacco smokers was 20% greater than in nonsmokers. Geriatric Patients and Patients with Moderate to Severe Renal Impairment Age 65 years or older, and moderate to severe renal impairment do not have a meaningful effect on the pharmacokinetics of riluzole. The pharmacokinetics of riluzole in patients undergoing hemodialysis are unknown.
Drug Interaction Studies
 Drugs Highly Bound To Plasma Proteins Riluzole and warfarin are highly bound to plasma proteins. In vitro, riluzole did not show any displacement of warfarin from plasma proteins. Riluzole binding to plasma proteins was unaffected by warfarin, digoxin, imipramine and quinine at high therapeutic concentrations in vitro.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Riluzole was not carcinogenic in mice or rats when administered for 2 years at daily oral doses up to 20 and 10 mg/kg/day, respectively, which are approximately equal to the recommended human daily dose (RHDD, 100 mg) on a mg/m2 basis.
Mutagenesis
Riluzole was negative in in vitro (bacterial reverse mutation (Ames), mouse lymphoma tk, chromosomal aberration assay in human lymphocytes), and in vivo (rat cytogenetic and mouse micronucleus) assays.
N-hydroxyriluzole, the major active metabolite of riluzole, was positive for clastogenicity in the in vitro mouse lymphoma tk assay and in the in vitro micronucleus assay using the same mouse lymphoma cell line. N-hydroxyriluzole was negative in the HPRT gene mutation assay, the Ames assay (with and without rat or hamster S9), the in vitro chromosomal aberration assay in human lymphocytes, and the in vivo mouse micronucleus assay.
Impairment of Fertility
When riluzole (3, 8, or 15 mg/kg) was administered orally to male and female rats prior to and during mating and continuing in females throughout gestation and lactation, fertility indices were decreased and embryolethality was increased at the high dose. This dose was also associated with maternal toxicity. The mid dose, a no-effect dose for effects on fertility and early embryonic development, is approximately equal to the RHDD on a mg/m2 basis.
14 Clinical Studies
The efficacy of riluzole was demonstrated in two studies (Study 1 and 2) that evaluated riluzole 50 mg twice daily in patients with amyotrophic lateral sclerosis (ALS). Both studies included patients with either familial or sporadic ALS, a disease duration of less than 5 years, and a baseline forced vital capacity greater than or equal to 60% of normal.
Study 1 was a randomized, double-blind, placebo-controlled clinical study that enrolled 155 patients with ALS. Patients were randomized to receive riluzole 50 mg twice daily (n=77) or placebo (n=78) and were followed for at least 13 months (up to a maximum duration of 18 months). The clinical outcome measure was time to tracheostomy or death.
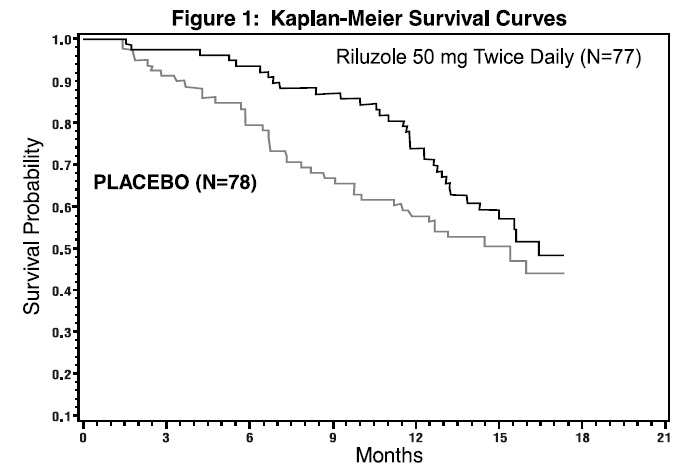
The time to tracheostomy or death was longer for patients receiving riluzole compared to placebo. There was an early increase in survival in patients receiving riluzole compared to placebo. Figure 1 displays the survival curves for time to death or tracheostomy. The vertical axis represents the proportion of individuals alive without tracheostomy at various times following treatment initiation (horizontal axis). Although these survival curves were not statistically significantly different when evaluated by the analysis specified in the study protocol (Logrank test p=0.12), the difference was found to be significant by another appropriate analysis (Wilcoxon test p=0.05). As seen in Figure 1, the study showed an early increase in survival in patients given riluzole. Among the patients in whom the endpoint of tracheostomy or death was reached during the study, the difference in median survival between the riluzole 50 mg twice daily and placebo groups was approximately 90 days.
Figure 1. Time to Tracheostomy or Death in ALS Patients in Study 1 (Kaplan-Meier Curves)
Study 2 was a randomized, double-blind, placebo-controlled clinical study that enrolled 959 patients with ALS. Patients were randomized to riluzole 50 mg twice daily (n=236) or placebo (n=242) and were followed for at least 12 months (up to a maximum duration of 18 months). The clinical outcome measure was time to tracheostomy or death.
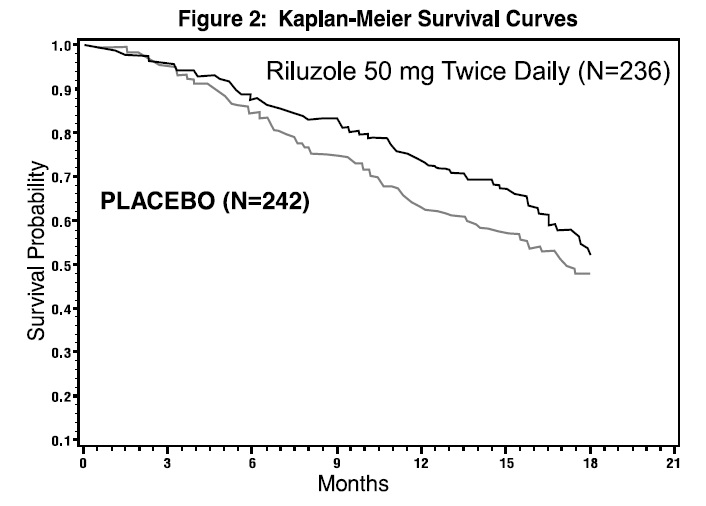
The time to tracheostomy or death was longer for patients receiving riluzole compared to placebo. Figure 2 displays the survival curves for time to death or tracheostomy for patients randomized to either riluzole 100 mg per day or placebo. Although these survival curves were not statistically significantly different when evaluated by the analysis specified in the study protocol (Logrank test p=0.076), the difference was found to be significant by another appropriate analysis (Wilcoxon test p=0.05). Not displayed in Figure 2 are the results of riluzole 50 mg per day (one-half of the recommended daily dose), which could not be statistically distinguished from placebo, or the results of riluzole 200 mg per day (two times the recommended daily dose), which were not distinguishable from the 100 mg per day results. Among the patients in whom the endpoint of tracheostomy or death was reached during the study, the difference in median survival between riluzole and placebo was approximately 60 days.
Although riluzole improved survival in both studies, measures of muscle strength and neurological function did not show a benefit.
Figure 2. Time to Tracheostomy or Death in ALS Patients in Study 2 (Kaplan-Meier Curves)
16 How Supplied/storage And Handling
Riluzole tablets USP, 50 mg are white to off-white, capsule shaped, beveled edged, film-coated tablets, engraved with ‘381’ on one side and ‘G’ on the other side. Riluzole tablets USP, 50 mg are supplied in:
 Bottles of 60 tablets NDC 68462-381-60 Bottles of 1000 tablets NDC 68462-381-10
Store at 20°C to 25°C (68°F to77°F) [see USP Controlled Room Temperature] and protect from bright light. Keep out of the reach of children.
17 Patient Counseling Information
Advise patients to inform their healthcare provider if they experience:
• Yellowing of the whites of the eyes [see Warnings and Precautions (5.1)]• Fever [see Warnings and Precautions (5.2)]• Respiratory symptoms—for example, dry cough and difficult or labored breathing [see Warnings and Precautions (5.3)]
Manufactured by:
Glenmark Pharmaceuticals Limited Colvale-Bardez, Goa 403513, India
Manufactured for:

Glenmark Pharmaceuticals Inc., USA Mahwah, NJ 07430
Questions? 1 (888) 721-7115www.glenmarkpharma-us.com
Product of India
July 2020
Package/label Principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site