Thyro-Tabs Canine (levothyroxine sodium 0.5 mg) Dailymed
Generic: levothyroxine sodium is used for the treatment of Adrenal Insufficiency Goiter Hypothyroidism Myocardial Infarction Myxedema Thyroid Diseases Thyroid Neoplasms Thyroiditis, Autoimmune Thyrotoxicosis
Go PRO for all pill images
Synthetic thyroxine hormoneFor oral use in dogs only.
Warnings Section
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Description Section
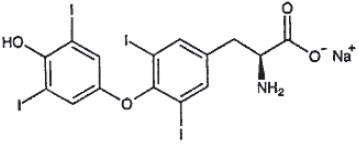
DESCRIPTION: Thyro-Tabs® Canine (levothyroxine sodium tablets), USP contains synthetic crystalline L-3,3',5,5'-tetraiodothyronine sodium salt [levothyroxine (T4) sodium]. Synthetic T4 is identical to that produced in the canine thyroid gland. Levothyroxine sodium has an empirical formula of C15H10I4N NaO4 ∙ H2O, molecular weight of 798.85 g/mol (anhydrous), and structural formula as shown:
Veterinary Indications Section
INDICATION: For replacement therapy for diminished thyroid function in dogs.
Dosage & Administration Section
DOSAGE AND ADMINISTRATION:
The initial total daily dose is 0.1 mg/10 pounds (0.01 mg/lb; 0.022 mg/kg) body weight as a single dose every 24 hours or as a divided dose every 12 hours.
The dose may then be adjusted by monitoring the serum total thyroxine (TT4) concentrations 4 to 6 hours post-tablet administration, along with clinical response, of the dog every 4 to 8 weeks until an adequate maintenance dose is established.
To minimize day-to-day variations in serum TT4 concentrations (see CLINICAL PHARMACOLOGY), owners should consistently administer Thyro-Tabs Canine either with or without food.
When switching from another levothyroxine sodium formulation to Thyro-Tabs Canine, monitor serum TT4 concentrations and clinical response due to potential differences in recommended starting doses and potential differences in bioavailability.
Contraindications Section
CONTRAINDICATIONS: Do not use in dogs with thyrotoxicosis or uncorrected adrenal insufficiency.
Warnings Section
WARNINGS:
SAFE HANDLING WARNING SECTION
USER SAFETY WARNINGS: Not for use in humans. Keep out of reach of children. In the event of accidental ingestion, seek medical advice immediately and show the product label to the physician. Wash hands after handling.
ANIMAL SAFETY WARNINGS: Keep Thyro-Tabs Canine in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
In humans and rodents, excess in utero exposure to thyroid hormones is associated with hypothalamic-pituitary-thyroid axis dysfunction and morphological thyroid gland defects in the offspring. The safety of Thyro-Tabs Canine has not been evaluated in breeding, pregnant, or lactating dogs.
Precautions Section
PRECAUTIONS: Dogs with underlying cardiac disease that are diagnosed with hypothyroidism should be closely monitored during the dose establishment phase. Adjustment of cardiac medication or levothyroxine sodium dosage may be needed depending on clinical response.1-4
Adverse Reactions Section
ADVERSE REACTIONS:
PRE-APPROVAL EXPERIENCE: In a 6-month US field study with 92 dogs, the most commonly reported adverse reactions by percentage of dogs experiencing the reaction included: anorexia (17%), dermatitis (15%), vomiting (15%), otitis externa (14%), lethargy (14%), polydipsia (13%), diarrhea (11%), leukocytosis (9%), pruritus (8%), tachypnea (8%), polyuria (5%), hyperactivity (4%), and seborrhea (1%).
One dog was withdrawn from the study at the owner's request because of increased water consumption and urination, which was possibly related to levothyroxine sodium.
Hematocrit and red blood cell counts exceeded the upper limit of the reference range in seven dogs by the end of the study. Liver enzyme (ALP, ALT, or AST) elevations related to levothyroxine administration were reported in three dogs. In two of the dogs with elevated ALT and AST, the elevations resolved by Day 70 and Day 126, respectively.
POST-APPROVAL EXPERIENCE (2022):
The following adverse events are based on post-approval adverse drug experience reporting for Thyro-Tabs Canine. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported in dogs, are uled in decreasing order of reporting frequency:
Pruritus, high or low serum thyroxine (T4) concentrations, tachypnea, weight loss, lethargy, anorexia, vomiting, polydipsia, alopecia, dermatitis, hyperactivity, diarrhea, and polyuria.
Allergic-type hypersensitivity reactions (including pruritus, hives, facial swelling, and dermatitis) have also been reported.
CONTACT INFORMATION: To report suspected adverse drug experiences, contact LLOYD, Inc. at 1-800-831-0004 or www.lloydinc.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888 FDA-VETS or www.fda.gov/reportanimalae.
Clinical Pharmacology Section
CLINICAL PHARMACOLOGY: Levothyroxine sodium has poor oral bioavailability in dogs (10-20%) with peak serum TT4 concentrations within 4 to 6 hours (fasted state). Administration of levothyroxine sodium with food reduces oral bioavailability.5 In most dogs, the estimated half-life is approximately 10-14 hours. Levothyroxine sodium is excreted in the feces.
EFFECTIVENESS: In a US field study with 92 dogs, dogs were administered a starting daily dose of 0.1 mg/10 lb (0.022 mg/kg) body weight. The dose was administered either once every 24 hours or as 0.05 mg/10 lb (0.011 mg/kg) body weight every 12 hours. The dose could be increased or decreased (without a change in frequency) after 6, 10, and 18 weeks, based on clinical findings and serum thyroid hormone concentrations. The majority of the dogs (80%) were dosed at 0.08-0.12 mg/10 lb (0.008-0.012 mg/lb; 0.018-0.026 mg/kg) by the end of the study, with the majority of dogs (69.7%) requiring one dose change.
The product was considered effective if the serum TT4, free thyroxine (fT4), and thyroid stimulating hormone (TSH) concentrations were all within the desired treatment ranges when collected 4 to 6 hours post-tablet administration after 182 ± 5 days of treatment (TT4: 15-94 nmol/L; fT4: 8-36 pmol/L; TSH: ≤ 37 mU/L). Of the 78 evaluable cases, 59 (75.6%) were considered treatment successes. There was no statistical difference in the success rate between the once daily or divided dose treatment groups.
Clinical signs of hypothyroidism (weight gain, lethargy, bradycardia, seborrhea, alopecia, hyperpigmentation, scaling, and hypercholesterolemia) generally improved by the end of the study (Day 182 ± 5). Respiratory rate, excluding panting dogs, increased during the study along with activity level.
TARGET ANIMAL SAFETY: In a laboratory study, levothyroxine sodium was administered to 32 healthy, 7-10 month old, euthyroid Beagle dogs (4 males and 4 females per group) at 0×, 2×, 6×, and 10× the initial dose of 0.10 mg/10 lb (0.01 mg/lb) once daily for 26 weeks. Increased serum TT4 and fT4 concentrations were directly proportional to an increasing dose of levothyroxine sodium. Decreased serum TSH concentrations were inversely proportional to an increasing dose of levothyroxine sodium. Dogs treated with levothyroxine sodium had elevated red blood cell indices (hemoglobin, hematocrit, and red blood cell count) and ALT but these did not exceed the normal reference ranges. Dogs treated with levothyroxine sodium had lower albumin, calcium, globulins, and total protein values but these did not fall below the normal reference ranges. Vomiting, diarrhea, excitation, rapid respiration, tachycardia, and feces with blood were observed in all treatment groups, but were seen with greater frequency in dogs treated with levothyroxine sodium. Decreased pituitary gland and thyroid/parathyroid gland organ weights were also observed in euthyroid dogs treated with levothyroxine sodium.
Storage And Handling Section
STORAGE CONDITIONS: Store at controlled room temperature 20°-25°C (68°-77°F) with excursions allowed between 15° and 30°C (59° and 86°F). Protect from light and moisture.
How Supplied Section
HOW SUPPLIED: Thyro-Tabs Canine (levothyroxine sodium tablets), USP is available as scored, color-coded ovoid tablets in 9 strengths: 0.1 mg–yellow; 0.2 mg–pink; 0.3 mg–green; 0.4 mg–maroon; 0.5 mg–white; 0.6 mg–purple; 0.7 mg–orange; 0.8 mg–blue; and 1.0 mg–tan, in bottles of 120 and 1,000 tablets.
Approved by FDA under NADA # 141-448
Manufactured by: LLOYD, Inc.Shenandoah, IA 51601
Rev. 0523
References Section
REFERENCES:
- Phillips DE, Harkin KR. Hypothyroidism and myocardial failure in two Great Danes. J Am Anim Hosp Assoc 2003;39:133-137.
- Flood JA, Hoover JP. Improvement in myocardial dysfunction in a hypothyroid dog. Can Vet J 2009;50:828-834.
- Chow B, French A. Conversion of atrial fibrillation after levothyroxine in a dog with hypothyroidism and arterial thromboembolism. J Small Anim Pract 2014;55:278-282.
- Sangster JK, Panciera DL, Abbott JA. Cardiovascular effects of thyroid disease. Compend Contin Educ Vet 2013;35:E5.
- Le Traon G, Burgaud S, Horspool LJ. Pharmacokinetics of total thyroxine in dogs after administration of an oral solution of levothyroxine sodium. J Vet Pharmacol Ther 2008;31:95-101.
LLOYD P.O. Box 130Shenandoah, IA 51601 U.S.A.712-246-4000
Principal Display Panel - 0.1 Mg Tablet Bottle Label
NDC 11789-251-20
Thyro-Tabs® Canine(levothyroxine sodium tablets), USP
0.1 mg
Approved by FDA under NADA # 141-448
1,000 Tablets
Caution: Federal law restricts this drug to use by or onthe order of a licensed veterinarian.
LLOYD®

Principal Display Panel - 0.2 Mg Tablet Bottle Label
NDC 11789-252-20
Thyro-Tabs® Canine(levothyroxine sodium tablets), USP
0.2 mg
Approved by FDA under NADA # 141-448
1,000 Tablets
Caution: Federal law restricts this drug to use by or onthe order of a licensed veterinarian.
LLOYD®

Principal Display Panel - 0.3 Mg Tablet Bottle Label
NDC 11789-253-20
Thyro-Tabs® Canine(levothyroxine sodium tablets), USP
0.3 mg
Approved by FDA under NADA # 141-448
1,000 Tablets
Caution: Federal law restricts this drug to use by or onthe order of a licensed veterinarian.
LLOYD®

Principal Display Panel - 0.4 Mg Tablet Bottle Label
NDC 11789-254-20
Thyro-Tabs® Canine(levothyroxine sodium tablets), USP
0.4 mg
Approved by FDA under NADA # 141-448
1,000 Tablets
Caution: Federal law restricts this drug to use by or onthe order of a licensed veterinarian.
LLOYD®

Principal Display Panel - 0.5 Mg Tablet Bottle Label
NDC 11789-255-20
Thyro-Tabs® Canine(levothyroxine sodium tablets), USP
0.5 mg
Approved by FDA under NADA # 141-448
1,000 Tablets
Caution: Federal law restricts this drug to use by or onthe order of a licensed veterinarian.
LLOYD®

Principal Display Panel - 0.6 Mg Tablet Bottle Label
NDC 11789-256-20
Thyro-Tabs® Canine(levothyroxine sodium tablets), USP
0.6 mg
Approved by FDA under NADA # 141-448
1,000 Tablets
Caution: Federal law restricts this drug to use by or onthe order of a licensed veterinarian.
LLOYD®

Principal Display Panel - 0.7 Mg Tablet Bottle Label
NDC 11789-257-20
Thyro-Tabs® Canine(levothyroxine sodium tablets), USP
0.7 mg
Approved by FDA under NADA # 141-448
1,000 Tablets
Caution: Federal law restricts this drug to use by or onthe order of a licensed veterinarian.
LLOYD®

Principal Display Panel - 0.8 Mg Tablet Bottle Label
NDC 11789-258-20
Thyro-Tabs® Canine(levothyroxine sodium tablets), USP
0.8 mg
Approved by FDA under NADA # 141-448
1,000 Tablets
Caution: Federal law restricts this drug to use by or onthe order of a licensed veterinarian.
LLOYD®

Principal Display Panel - 1.0 Mg Tablet Bottle Label
NDC 11789-268-20
Thyro-Tabs® Canine(levothyroxine sodium tablets), USP
1.0 mg
Approved by FDA under NADA # 141-448
1,000 Tablets
Caution: Federal law restricts this drug to use by or onthe order of a licensed veterinarian.
LLOYD®

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


