VESIcare (solifenacin succinate 10 mg) Dailymed
Generic: solifenacin succinate is used for the treatment of Stomach Diseases Glaucoma, Angle-Closure Urinary Retention Urinary Bladder, Overactive Urinary Incontinence, Urge
IMPRINT: 150
SHAPE: round
COLOR: yellow
All Imprints
solifenacin succinate 5 mg - 150 round yellow
solifenacin succinate 10 mg - logo 151 151 round white
solifenacin succinate 5 mg [vesicare] - logo 150 round yellow
solifenacin succinate 10 mg - 151 round white
solifenacin succinate 10 mg - logo 151 round pink
vesicare (solifenacin succinate) 5 mg - 150 round yellow
solifenacin succinate 10 mg - logo 151 round white
solifenacin succinate 10 mg - 151 round pink
Go PRO for all pill images
1 Indications And Usage
VESIcare¬ģ is indicated for the treatment of adults with overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency.
VESIcare is a muscarinic antagonist indicated for the treatment of adults with overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. (1 )
2 Dosage And Administration
‚ÄĘ 5¬†mg tablet taken orally once daily, and if well tolerated may be increased to 10¬†mg once daily. (2.1 )‚ÄĘ Do not exceed the 5¬†mg dose of VESIcare in patients with:
o Severe renal impairment creatinine clearance < 30 mL/min/1.73 m2. (2.2 ,8.6 )o Moderate hepatic impairment (Child-Pugh B). VESIcare is not recommended in patients with severe hepatic impairment (Child-Pugh C). (2.3 ,8.7 )o Concomitant use of strong CYP3A4 inhibitors. (2.4 ,7.1 )2.1 Dosing Information
The recommended oral dose of VESIcare is 5 mg once daily. If the 5 mg dose is well tolerated, the dose may be increased to 10 mg once daily.
VESIcare should be taken with water and swallowed whole. VESIcare can be administered with or without food.
2.2 Dosing Recommendations in Patients with Renal Impairment
Do not exceed 5 mg once daily in patients with severe renal impairment (CLcr < 30 mL/min/1.73 m2) [see Use in Specific Populations (8.6)].
2.3 Dosing Recommendations in Patients with Hepatic Impairment
Do not exceed 5 mg once daily in patients with moderate hepatic impairment (Child-Pugh B). Do not use VESIcare in patients with severe hepatic impairment (Child-Pugh C) [see Use in Specific Populations (8.7)].
2.4 Dosing Recommendations in Patients Taking CYP3A4 Inhibitors
Do not exceed 5 mg once daily when VESIcare is administered with strong CYP3A4 inhibitors such as ketoconazole [see Drug Interactions (7.1)].
3 Dosage Forms And Strengths
Tablets:
‚ÄĘ 5¬†mg: round, light yellow, debossed with 150‚ÄĘ 10¬†mg: round, light pink, debossed with 151
Tablets: 5 mg and 10 mg. (3 )
4 Contraindications
VESIcare is contraindicated in patients:
‚ÄĘ With urinary retention [see Warnings and Precautions (5.2)],‚ÄĘ With gastric retention [see Warnings and Precautions (5.3)],‚ÄĘ With uncontrolled narrow-angle glaucoma [see Warnings and Precautions (5.5)], and‚ÄĘ Who have demonstrated hypersensitivity to solifenacin succinate or the inactive ingredients in VESIcare. Reported adverse reactions have included anaphylaxis and angioedema [see Adverse Reactions (6.2)].
‚ÄĘ Urinary retention. (4 ,5.2 )‚ÄĘ Gastric retention. (4 ,5.3 )‚ÄĘ Uncontrolled narrow-angle glaucoma. (4 ,5.5 )‚ÄĘ Hypersensitivity to this product or any of its components. (4 ,5.1 ,6.2 )
5 Warnings And Precautions
‚ÄĘ Angioedema and Anaphylactic Reactions: Promptly discontinue VESIcare and provide appropriate therapy. (5.1 )‚ÄĘ Urinary Retention: VESIcare is not recommended for use in patients with clinically significant bladder outlet obstruction. (5.2 )‚ÄĘ Gastrointestinal Disorders: VESIcare is not recommended for use in patients with decreased gastrointestinal motility. (5.3 )‚ÄĘ Central Nervous System Effects: Somnolence has been reported with VESIcare. Advise patients not to drive or operate heavy machinery until they know how VESIcare affects them. (5.4 )‚ÄĘ Controlled Narrow-Angle Glaucoma: Use VESIcare with caution in patients being treated for narrow-angle glaucoma. (5.5 )‚ÄĘ QT Prolongation in Patients at High Risk of QT Prolongation: VESIcare is not recommended for use in patients at high risk of QT prolongation, including patients with a known history of QT prolongation and patients taking medications known to prolong the QT interval. (5.6 )5.1 Angioedema and Anaphylactic Reactions
Angioedema of the face, lips, tongue, and/or larynx have been reported with solifenacin succinate. In some cases, angioedema occurred after the first dose, however, cases have been reported to occur hours after the first dose or after multiple doses. Anaphylactic reactions have also been reported in patients treated with solifenacin succinate. Angioedema associated with upper airway swelling and anaphylactic reactions may be life-threatening.
VESIcare is contraindicated in patients with a known or suspected hypersensitivity to solifenacin succinate [see Contraindications (4)]. If involvement of the tongue, hypopharynx, or larynx occurs, promptly discontinue VESIcare and provide appropriate therapy and/or measures necessary to ensure a patent airway.
5.2 Urinary Retention
The use of VESIcare, like other antimuscarinic drugs, in patients with clinically significant bladder outlet obstruction including patients with urinary retention, may result in further urinary retention and kidney injury. The use of VESIcare is not recommended in patients with clinically significant bladder outlet obstruction and is contraindicated in patients with urinary retention [see Contraindications (4)].
5.3 Gastrointestinal Disorders
The use of VESIcare, like other antimuscarinic drugs, in patients with conditions associated with decreased gastrointestinal motility may result in further decreased gastrointestinal motility. VESIcare is contraindicated in patients with gastric retention [see Contraindications (4)]. The use of VESIcare is not recommended in patients with conditions associated with decreased gastrointestinal motility.
5.4 Central Nervous System Effects
VESIcare is associated with antimuscarinic central nervous system (CNS) adverse reactions [see Adverse Reactions (6.2)]. A variety of CNS antimuscarinic adverse reactions have been reported, including headache, confusion, hallucinations, and somnolence. Monitor patients for signs of antimuscarinic CNS adverse reactions, particularly after beginning treatment or increasing the dose. Advise patients not to drive or operate heavy machinery until they know how VESIcare affects them. If a patient experiences antimuscarinic CNS adverse reactions, consider dose reduction or drug discontinuation.
5.5 Controlled Narrow-Angle Glaucoma
VESIcare should be used with caution in patients being treated for narrow-angle glaucoma [see Contraindications (4)].
5.6 QT Prolongation in Patients at High Risk of QT Prolongation
In a study of the effect of solifenacin succinate on the QT interval conducted in 76 healthy women [see Clinical Pharmacology (12.2)], solifenacin succinate 30 mg (three times the largest maximum recommended dose in adult patients) was associated with a mean increase in the Fridericia-corrected QT interval of 8 msec (90% CI, 4, 13). The QT prolonging effect appeared less with solifenacin succinate 10 mg than with solifenacin succinate 30 mg, and the effect of solifenacin succinate 30 mg did not appear as large as that of the positive control moxifloxacin at its therapeutic dose.
The use of VESIcare is not recommended in patients at high risk of QT prolongation, including patients with a known history of QT prolongation and patients who are taking medications known to prolong the QT interval.
6 Adverse Reactions
The most common adverse reactions (> 4% in VESIcare-treated patients and > placebo-treated patients) were dry mouth and constipation at both 5 mg and 10 mg doses; and urinary tract infection and blurred vision at the 10 mg dose. (6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Astellas Pharma US, Inc. at 1-800-727-7003 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
VESIcare has been evaluated for safety in 1811 adult patients in four randomized, placebo-controlled trials (Studies 1-4) [see Clinical Studies (14)]. Expected adverse reactions of antimuscarinic agents are dry mouth, constipation, blurred vision (accommodation abnormalities), urinary retention, and dry eyes. The incidence of dry mouth and constipation in patients treated with VESIcare was higher in the 10 mg dose group compared to the 5 mg dose group.
In the four 12-week double-blind clinical trials, severe fecal impaction, colonic obstruction, and intestinal obstruction were reported in one patient each, all in the VESIcare 10 mg group. Angioneurotic edema was reported in one patient taking VESIcare 5 mg. Compared to 12 weeks of treatment with VESIcare, the incidence and severity of adverse reactions were similar in patients who remained on drug for up to 12 months in Study 5 [see Clinical Studies (14)].
The most frequent adverse reaction leading to study discontinuation was dry mouth (1.5%). Table 1 uls the rates of identified adverse reactions, in the four randomized, placebo-controlled trials at an incidence greater than placebo and in 1% or more of patients treated with VESIcare 5 or 10 mg once daily for up to 12 weeks.
Table 1: Adverse Reactions Reported by ‚Č• 1% of Patients and Exceeding Placebo in Studies 1, 2, 3 and 4 Placebo (%) VESIcare 5 mg (%) VESIcare 10 mg (%)
Number of Patients
1216
578
1233
GASTROINTESTINAL DISORDERS
     Dry Mouth
4.2
10.9
27.6
     Constipation
2.9
5.4
13.4
     Nausea
2.0
1.7
3.3
     Dyspepsia
1.0
1.4
3.9
     Abdominal Pain Upper
1.0
1.9
1.2
     Vomiting NOS
0.9
0.2
1.1
INFECTIONS AND INFESTATIONS
     Urinary Tract Infection NOS
2.8
2.8
4.8
     Influenza
1.3
2.2
0.9
     Pharyngitis NOS
1.0
0.3
1.1
NERVOUS SYSTEM DISORDERS
     Dizziness
1.8
1.9
1.8
EYE DISORDERS
     Vision Blurred
1.8
3.8
4.8
     Dry Eyes NOS
0.6
0.3
1.6
RENAL AND URINARY DISORDERS
     Urinary Retention
0.6
0
1.4
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS
     Edema Lower Limb
0.7
0.3
1.1
     Fatigue
1.1
1.0
2.1
PSYCHIATRIC DISORDERS
     Depression NOS
0.8
1.2
0.8
RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS
    Cough
0.2
0.2
1.1
VASCULAR DISORDERS
    Hypertension NOS
0.6
1.4
0.5
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of solifenacin succinate in the U.S. and/or outside of the U.S. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General disorders and administration site conditions: peripheral edema, hypersensitivity reactions (including angioedema with airway obstruction, rash, pruritus, urticaria, anaphylactic reaction);
Nervous system disorders: dizziness, headache, confusion, hallucinations, delirium, somnolence;
Cardiac disorders: QT prolongation, Torsade de Pointes, atrial fibrillation, tachycardia, palpitations;
Hepatobiliary disorders: liver disorders mostly characterized by abnormal liver function tests, AST (aspartate aminotransferase), ALT (alanine aminotransferase), GGT (gamma-glutamyl transferase);
Renal and urinary disorders: renal impairment, urinary retention;
Metabolism and nutrition disorders: decreased appetite, hyperkalemia;
Skin and subcutaneous tissue disorders: exfoliative dermatitis, erythema multiforme, dry skin;
Eye disorders: glaucoma;
Gastrointestinal disorders: gastroesophageal reflux disease, ileus, vomiting, abdominal pain, dysgeusia, sialadenitis;
Respiratory, thoracic and mediastinal disorders: dysphonia, nasal dryness;
Musculoskeletal and connective tissue disorders: muscular weakness.
7 Drug Interactions
CYP3A4 Inhibitors: Do not exceed the 5 mg dose of VESIcare with concomitant use of strong CYP3A4 inhibitors. (7.1 )
 
7.1 Strong CYP3A4 Inhibitors
Solifenacin is a substrate of CYP3A4. Concomitant use of ketoconazole, a strong CYP3A4 inhibitor, significantly increased the exposure of solifenacin [see Clinical Pharmacology (12.3)]. The dosage of VESIcare greater than 5 mg once daily is not recommended when concomitantly used with strong CYP3A4 inhibitors [see Dosage and Administration (2.4)].
8 Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no studies with the use of solifenacin succinate in pregnant women to inform a drug-associated risk of major birth defects, miscarriages, or adverse maternal or fetal outcomes. No adverse developmental outcomes were observed in animal reproduction studies with oral administration of solifenacin succinate to pregnant mice during the period of organogenesis at a dose resulting in 1.2 times the systemic exposure at the maximum recommended human dose (MRHD) of 10 mg/day. However, administration of doses 3.6 times and greater than the MRHD during organogenesis produced maternal toxicity in the pregnant mice and resulted in developmental toxicity and reduced fetal body weights in offspring [see Data].
In the U.S. general population, the estimated background risk of major birth defects or miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Animal Data
Oral administration of 14C-solifenacin succinate to pregnant mice resulted in the recovery of radiolabel in the fetus indicating that solifenacin-related product can cross the placental barrier. In pregnant mice, administration of solifenacin succinate at a dose of 250 mg/kg/day (7.9 times the systemic exposure at the MRHD of 10 mg), resulted in an increased incidence of cleft palate and increased maternal lethality. Administration of solifenacin succinate to pregnant mice during organogenesis at greater than or equal to 3.6 times (100 mg/kg/day and greater) the systemic exposure at the MRHD, resulted in reduced fetal body weights and reduced maternal body weight gain. No embryo-fetal toxicity or teratogenicity was observed in fetuses from pregnant mice treated with solifenacin succinate at a dose of 30 mg/kg/day (1.2 times the systemic exposure at the MRHD). Administration of solifenacin succinate to pregnant rats and rabbits at a dose of 50 mg/kg/day (< 1 times and 1.8 times the systemic exposure at the MRHD, respectively), resulted in no findings of embryo-fetal toxicity. Oral pre- and post-natal administration of solifenacin succinate at 100 mg/kg/day (3.6 times the systemic exposure at the MRHD) during the period of organogenesis through weaning, resulted in reduced peripartum and postnatal survival, reduced body weight gain by the pups, and delayed physical development (eye opening and vaginal patency). An increase in the percentage of male offspring was also observed in litters from offspring (F2 generation) exposed to maternal doses of 250 mg/kg/day. There were no effects on natural delivery in mice treated with 1.2 times (30 mg/kg/day) the expected systemic exposure at the MRHD.
8.2 Lactation
Risk Summary
There is no information on the presence of solifenacin in human milk, the effects on the breastfed child, or the effects on milk production. Solifenacin is present in mouse milk [see Data]. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VESIcare and any potential adverse effects on the breastfed child from VESIcare or from the underlying maternal condition.
Animal Data
Oral administration of 14C-solifenacin succinate to lactating mice resulted in the recovery of radioactivity in maternal milk. Lactating female mice orally administered solifenacin succinate at a maternally toxic dose of 100 mg/kg/day (3.6 times the systemic exposure at the MRHD) had increased postpartum pup mortality, pups with reduced body weights, or delays in the onset of reflex and physical development. Pups from lactating dams orally administered solifenacin succinate at a dose of 30 mg/kg/day (1.2 times the systemic exposure at the MRHD) had no discernible adverse findings. The concentrations of solifenacin in animal milk does not necessarily predict the concentration of drug in human milk.
8.4 Pediatric Use
The safety and effectiveness of VESIcare Tablets have not been established in pediatric patients.
8.5 Geriatric Use
In placebo-controlled clinical studies, similar safety and effectiveness were observed between geriatric patients (623¬†patients ‚Č•¬†65¬†years and 189¬†patients ‚Č•¬†75¬†years) and younger adult patients (1188¬†patients <¬†65¬†years) treated with VESIcare [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Solifenacin plasma concentrations are greater in patients with severe renal impairment compared to subjects with normal renal function [see Clinical Pharmacology (12.3)]. Because increased solifenacin plasma concentrations increase the risk of antimuscarinic adverse reactions, the maximum recommended dose of VESIcare in patients with severe renal impairment (CLcr < 30 mL/min/1.73 m2) is 5 mg once daily [see Dosage and Administration (2.2)]. The recommended dose in patients with mild or moderate renal impairment is the same as in patients with normal renal function.
8.7 Hepatic Impairment
Solifenacin plasma concentrations are greater in patients with moderate hepatic impairment compared to subjects with normal hepatic function [see Clinical Pharmacology (12.3)]. Because increased solifenacin plasma concentrations increase the risk of antimuscarinic adverse reactions, the maximum recommended dose of VESIcare in patients with moderate hepatic impairment (Child-Pugh B) is 5 mg once daily [see Dosage and Administration (2.3)] and VESIcare is not recommended for use in patients with severe hepatic impairment (Child-Pugh C).
8.8 Gender
The pharmacokinetics of solifenacin is not significantly influenced by gender.
10 Overdosage
Overdosage with VESIcare can potentially result in severe antimuscarinic effects and should be treated accordingly. The highest dose ingested in an accidental overdose of solifenacin succinate was 280 mg (28 times the maximum dosage) in a 5-hour period. This case was associated with mental status changes. Some cases reported a decrease in the level of consciousness.
Intolerable antimuscarinic adverse reactions (fixed and dilated pupils, blurred vision, failure of heel-to-toe exam, tremors, and dry skin) occurred on day 3 in normal volunteers taking 50 mg daily (5 times the maximum recommended therapeutic dose) and resolved within 7 days following discontinuation of drug.
In the event of overdose with VESIcare, treat with gastric lavage and appropriate supportive measures. ECG monitoring is also recommended.
11 Description
VESIcare (solifenacin succinate) is a muscarinic receptor antagonist. Chemically, solifenacin succinate is a butanedioic acid compound with (1S)-(3R)-1-azabicyclo[2.2.2]oct-3-yl 3,4-dihydro-1-phenyl-2(1H)-iso-quinolinecarboxylate (1:1) having an empirical formula of C23H26N2O2‚ÄĘC4H6O4, and a molecular weight of 480.55. The structural formula of solifenacin succinate is:
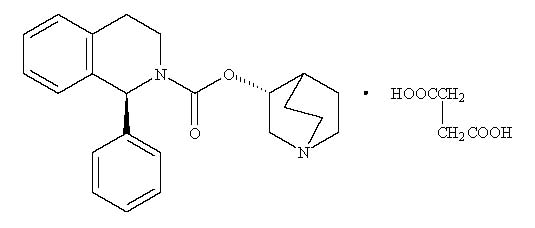
Solifenacin succinate is a white to pale-yellowish-white crystal or crystalline powder. It is freely soluble at room temperature in water, glacial acetic acid, dimethyl sulfoxide, and methanol.
Each VESIcare tablet contains 5 or 10 mg of solifenacin succinate and is for oral administration. In addition to the active ingredient solifenacin succinate, each VESIcare tablet also contains the following inactive ingredients: lactose monohydrate, corn starch, hypromellose 2910, magnesium stearate, talc, polyethylene glycol 8000 and titanium dioxide with yellow ferric oxide (5 mg VESIcare tablet) or red ferric oxide (10 mg VESIcare tablet).
12 Clinical Pharmacology
12.1 Mechanism of Action
Solifenacin is a competitive muscarinic receptor antagonist. Muscarinic receptors play an important role in several major cholinergically mediated functions, including contractions of urinary bladder smooth muscle.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of 10 mg and 30 mg solifenacin succinate (three times the maximum recommended dose) on the QT interval was evaluated at the time of peak plasma concentration of solifenacin in a multi-dose, randomized, double-blind, placebo and positive-controlled (moxifloxacin 400 mg) trial [see Warnings and Precautions (5.6)]. After receiving placebo and moxifloxacin sequentially, subjects were randomized to one of two treatment groups. One group (n=51) completed 3 additional sequential periods of dosing with solifenacin succinate 10, 20, and 30 mg while the second group (n=25) in parallel completed a sequence of placebo and moxifloxacin. Study subjects were female volunteers aged 19 to 79 years. The 30 mg dose of solifenacin succinate (three times the highest recommended dose) was chosen for use in this study because this dose results in a solifenacin exposure that covers those observed upon coadministration of 10 mg VESIcare with strong CYP3A4 inhibitors (e.g., ketoconazole, 400 mg). Due to the sequential dose escalating nature of the study, baseline ECG measurements were separated from the final QT assessment (of the 30 mg dose level) by 33 days.
The median difference from baseline in heart rate associated with the 10 and 30 mg doses of solifenacin succinate compared to placebo was -2 and 0 beats/minute, respectively. Because a significant period effect on QTc was observed, the QTc effects were analyzed utilizing the parallel placebo control arm rather than the pre-specified intra-patient analysis. Representative results are shown in Table 2.
Table 2: QTc changes in msec (90% CI) from baseline at Tmax (relative to placebo) Results displayed are those derived from the parallel design portion of the study and represent the comparison of Group 1 to time-matched placebo effects in Group 2. Drug/Dose Fridericia method (using mean difference)
Solifenacin succinate 10 mg
2 (-3, 6)
Solifenacin succinate 30 mg
8 (4, 13)
Moxifloxacin was included as a positive control in this study and, given the length of the study, its effect on the QT interval was evaluated in 3 different sessions. The placebo-subtracted mean changes (90% CI) in QTcF for moxifloxacin in the three sessions were 11 (7, 14), 12 (8, 17), and 16 (12, 21), respectively.
The QT interval prolonging effect of the highest solifenacin succinate dose (three times the maximum therapeutic dose) studied was not as large as that of the positive control moxifloxacin at its recommended dose. However, the confidence intervals overlapped, and this study was not designed to draw direct statistical conclusions between the drugs or the dose levels.
12.3 Pharmacokinetics
Absorption
After oral administration of VESIcare in healthy volunteers, peak plasma concentrations (Cmax) of solifenacin were reached within 3 to 8 hours after administration and, at steady-state, ranged from 32.3 to 62.9 ng/mL for the 5 and 10 mg VESIcare tablets, respectively. The absolute bioavailability of solifenacin is approximately 90%, with plasma concentrations of solifenacin proportional to the dose administered.
  Effect of Food  VESIcare may be administered without regard to meals. A single 10 mg dose administration of VESIcare with food increased Cmax and AUC of solifenacin by 4% and 3%, respectively.
Distribution
Solifenacin is approximately 98% (in vivo) bound to human plasma proteins, principally to őĪ1-acid glycoprotein. Solifenacin is highly distributed to non-CNS tissues, having a mean steady-state volume of distribution of 600¬†L.
Elimination
The elimination half-life (t1/2) of solifenacin following chronic dosing is approximately 45-68 hours.
  Metabolism  Solifenacin is extensively metabolized in the liver. The primary pathway for elimination is by way of CYP3A4; however, alternate metabolic pathways exist. The primary metabolic routes of solifenacin are through N-oxidation of the quinuclidin ring and 4R-hydroxylation of the tetrahydroisoquinoline ring. One pharmacologically active metabolite (4R-hydroxy solifenacin), occurring at low concentrations and unlikely to contribute significantly to clinical activity, and three pharmacologically inactive metabolites (N-glucuronide and the N-oxide and 4R-hydroxy-N-oxide of solifenacin) have been found in human plasma after oral dosing. Excretion  Following the administration of 10 mg of 14C-solifenacin succinate to healthy volunteers, 69% of the radioactivity was recovered in the urine and 23% in the feces over 26 days. Less than 15% (as mean value) of the dose was recovered in the urine as intact solifenacin. The major metabolites identified in urine were N-oxide of solifenacin, 4R-hydroxy solifenacin, and 4R-hydroxy-N-oxide of solifenacin and, in feces, 4R-hydroxy solifenacin.
Specific Populations
  Geriatric Patients  Multiple dose studies of VESIcare in geriatric volunteers (65 to 80 years) showed that Cmax, AUC and t1/2 values of solifenacin were 20-25% higher compared to the younger adult volunteers (18 to 55 years). [See Use in Specific Populations (8.5)]. Patients with Renal Impairment  In studies with solifenacin succinate 10 mg, there was a 2.1-fold increase in AUC and a 1.6-fold increase in t1/2 of solifenacin in patients with severe renal impairment compared to subjects with normal renal function [see Use in Specific Populations (8.6)]. Patients with Hepatic Impairment  In studies with solifenacin succinate 10 mg, there was a 2-fold increase in the t1/2 and a 35% increase in AUC of solifenacin in patients with moderate hepatic impairment compared to subjects with normal hepatic function [see Use in Specific Populations (8.7)]. VESIcare has not been studied in patients with severe hepatic impairment.
Drug Interaction Studies
  Strong CYP3A4 Inhibitors  In a crossover study, following blockade of CYP3A4 by coadministration of the strong CYP3A4 inhibitor, ketoconazole 400 mg once daily for 21 days, the mean Cmax and AUC of solifenacin increased by 1.5 and 2.7-fold, respectively [see Dosage and Administration (2.4) and Drug Interactions (7.1)]. CYP3A4 Inducers  Because solifenacin is a substrate of CYP3A4, inducers of CYP3A4 may decrease the concentration of solifenacin. Warfarin  In a crossover study, subjects received a single oral dose of warfarin 25 mg on the 10th day of dosing with either solifenacin succinate 10 mg or matching placebo once daily for 16 days. For R-warfarin, when it was coadministered with solifenacin succinate, the mean Cmax increased by 3% and AUC decreased by 2%. For S-warfarin, when it was coadministered with solifenacin succinate, the mean Cmax and AUC increased by 5% and 1%, respectively. Oral Contraceptives  In a crossover study, subjects received 2 cycles of 21 days of oral contraceptives containing 30 ug ethinyl estradiol and 150 ug levonorgestrel. During the second cycle, subjects received additional solifenacin succinate 10 mg or matching placebo once daily for 10 days starting from the 12th day of receipt of oral contraceptives. For ethinyl estradiol, when it was administered with solifenacin succinate, the mean Cmax and AUC increased by 2% and 3%, respectively. For levonorgestrel, when it was administered with solifenacin succinate, the mean Cmax and AUC decreased by 1%. Digoxin  In a crossover study, subjects received digoxin (loading dose of 0.25 mg on day 1, followed by 0.125 mg from days 2 to 8) for 8 days. Consecutively, they received solifenacin succinate 10 mg or matching placebo with digoxin 0.125 mg for an additional 10 days. When digoxin was coadministered with solifenacin succinate, the mean Cmax and AUC increased by 13% and 4%, respectively. Drugs Metabolized by Cytochrome P450 Enzymes  In vitro studies demonstrated that, at therapeutic concentrations, solifenacin does not inhibit CYP1A1/2, 2C9, 2C19, 2D6, or 3A4 derived from human liver microsomes.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No increase in tumors was found following the administration of solifenacin succinate to male and female mice for 104 weeks at doses up to 200 mg/kg/day (5 and 9 times, respectively, of the exposure at the maximum recommended human dose [MRHD] of 10 mg), and male and female rats for 104 weeks at doses up to 20 and 15 mg/kg/day, respectively (< 1 times the exposure at the MRHD).
Solifenacin succinate was not mutagenic in the in vitro Salmonella typhimurium or Escherichia coli microbial mutagenicity test or chromosomal aberration test in human peripheral blood lymphocytes with or without metabolic activation or in the in vivo micronucleus test in rats.
Solifenacin succinate had no effect on reproductive function, fertility, or early embryonic development of the fetus in male and female mice treated with 250 mg/kg/day (13 times the exposure at the MRHD) of solifenacin succinate, and in male rats treated with 50 mg/kg/day (< 1 times the exposure at the MRHD) and female rats treated with 100 mg/kg/day (1.7 times the exposure at the MRHD) of solifenacin succinate.
13.2 Animal Toxicology and/or Pharmacology
Juvenile Animal Toxicology Data
Dose-related increased mortality without preceding clinical signs occurred in juvenile mice treated before weaning for a duration of 12 weeks, from day 10 after birth, with doses that achieved a pharmacological effect. Animals dosed from postnatal day 10 onwards had higher mortality compared to the mortality in adult mice. No increased frequency in mortality was observed in juvenile mice that were treated after weaning for a duration of 4 weeks, from day 21 after birth onwards. Plasma exposure at postnatal day 10 was higher than in adult mice; the systemic exposure at postnatal day 21 was comparable to the systemic exposure in adult mice.
14 Clinical Studies
VESIcare was evaluated in four twelve-week, double-blind, randomized, placebo-controlled, parallel group, multicenter clinical trials for the treatment of overactive bladder in adult patients having symptoms of urinary frequency, urgency, and/or urge or mixed incontinence (with a predominance of urge). Entry criteria required that patients have symptoms of overactive bladder for ‚Č•¬†3¬†months duration. These studies involved 3027¬†patients (1811 on VESIcare and 1216 on placebo), and approximately 90% of these patients completed the 12-week studies. Two of the four studies evaluated the 5 and 10¬†mg VESIcare doses (Studies 1 and 2) and the other two evaluated only the 10¬†mg dose (Studies 3 and 4). All patients completing the 12-week studies were eligible to enter an open-label, long-term extension study (Study 5) and 81% of patients enrolling completed the additional 40-week treatment period. The majority of patients were Caucasian (93%) and female (80%) with a mean age of 58¬†years.
The primary endpoint in all four trials was the mean change from baseline to 12 weeks in number of micturitions/24 hours. Secondary endpoints included mean change from baseline to 12 weeks in number of incontinence episodes/24 hours, and mean volume voided per micturition.
The efficacy of VESIcare was similar across patient age groups and gender. The mean reduction in the number of micturitions per 24 hours was significantly greater with VESIcare 5 mg (2.3; p < 0.001) and VESIcare 10 mg (2.7; p < 0.001) compared to placebo (1.4). The mean reduction in the number of incontinence episodes per 24 hours was significantly greater with VESIcare 5 mg (1.5; p < 0.001) and VESIcare 10 mg (1.8; p < 0.001) treatment groups compared to the placebo treatment group (1.1). The mean increase in the volume voided per micturition was significantly greater with VESIcare 5 mg (32.3 mL; p < 0.001) and VESIcare 10 mg (42.5 mL; p < 0.001) compared with placebo (8.5 mL).
The results for the primary and secondary endpoints in the four individual 12-week clinical studies of VESIcare are reported in Tables 3 through 6.
Table 3: Mean Changes from Baseline to Week 12 in Efficacy Endpoints in Study 1 Parameter Placebo (N=253) Mean (SE) VESIcare 5 mg (N=266) Mean (SE) VESIcare 10 mg (N=264) Mean (SE)
Urinary Frequency (Number of Micturitions/24 hours)Primary endpoint
     Baseline
     Reduction
     P value vs. placebo
12.2 (0.26)
1.2 (0.21)
12.1 (0.24)
2.2 (0.18)
< 0.001
12.3 (0.24)
2.6 (0.20)
< 0.001
Number of Incontinence Episodes/24 hoursSecondary endpoint
     Baseline
     Reduction
     P value vs. placebo
2.7 (0.23)
0.8 (0.18)
2.6 (0.22)
1.4 (0.15)
< 0.01
2.6 (0.23)
1.5 (0.18)
< 0.01
Volume Voided per Micturition [mL]
     Baseline
     Increase
     P value vs. placebo
143.8 (3.37)
7.4 (2.28)
149.6 (3.35)
32.9 (2.92)
< 0.001
147.2 (3.15)
39.2 (3.11)
< 0.001
Table 4: Mean Changes from Baseline to Week 12 in Efficacy Endpoints in Study 2 Parameter Placebo (N=281) Mean (SE) VESIcare 5 mg (N=286) Mean (SE) VESIcare 10 mg (N=290) Mean (SE)
Urinary Frequency (Number of Micturitions/24 hours)Primary endpoint
     Baseline
     Reduction
     P value vs. placebo
12.3 (0.23)
1.7 (0.19)
12.1 (0.23)
2.4 (0.17)
< 0.001
12.1 (0.21)
2.9 (0.18)
< 0.001
Number of Incontinence Episodes/24 hoursSecondary endpoint
     Baseline
     Reduction
     P value vs. placebo
3.2 (0.24)
1.3 (0.19)
2.6 (0.18)
1.6 (0.16)
< 0.01
2.8 (0.20)
1.6 (0.18)
0.016
Volume Voided per Micturition [mL]
     Baseline
     Increase
     P value vs. placebo
147.2 (3.18)
11.3 (2.52)
148.5 (3.16)
31.8 (2.94)
< 0.001
145.9 (3.42)
36.6 (3.04)
< 0.001
Table 5: Mean Changes from Baseline to Week 12 in Efficacy Endpoints in Study 3 Parameter Placebo (N=309) Mean (SE) VESIcare 10 mg (N=306) Mean (SE)
Urinary Frequency (Number of Micturitions/24 hours)Primary endpoint
     Baseline
     Reduction
     P value vs. placebo
11.5 (0.18)
1.5 (0.15)
11.7 (0.18)
3.0 (0.15)
< 0.001
Number of Incontinence Episodes/24 hoursSecondary endpoint
     Baseline
     Reduction
     P value vs. placebo
3.0 (0.20)
1.1 (0.16)
3.1 (0.22)
2.0 (0.19)
< 0.001
Volume Voided per Micturition [mL]
     Baseline
     Increase
     P value vs. placebo
190.3 (5.48)
2.7 (3.15)
183.5 (4.97)
47.2 (3.79)
< 0.001
Table 6: Mean Changes from Baseline to Week 12 in Efficacy Endpoints in Study 4 Parameter Placebo (N=295) Mean (SE) VESIcare 10 mg (N=298) Mean (SE)
Urinary Frequency (Number of Micturitions/24 hours)Primary endpoint
     Baseline
     Reduction
     P value vs. placebo
11.8 (0.18)
1.3 (0.16)
11.5 (0.18)
2.4 (0.15)
< 0.001
Number of Incontinence Episodes/24 hoursSecondary endpoint
     Baseline
     Reduction
     P value vs. placebo
2.9 (0.18)
1.2 (0.15)
2.9 (0.17)
2.0 (0.15)
< 0.001
Volume Voided per Micturition [mL]
     Baseline
     Increase
     P value vs. placebo
175.7 (4.44)
13.0 (3.45)
174.1 (4.15)
46.4 (3.73)
< 0.001
16 How Supplied/storage And Handling
VESIcare is supplied as round, film-coated tablets, available in bottles as follows:
Each 5¬†mg tablet is light yellow and debossed with a logo and ‚Äú150‚ÄĚ and is available as follows:
  Bottle of 30                                 NDC 51248-150-01
Each 10¬†mg tablet is light pink and debossed with a logo and ‚Äú151‚ÄĚ and is available as follows:
  Bottle of 30                                  NDC 51248-151-01  Bottle of 90                                  NDC 51248-151-03 
Store at 25¬įC (77¬įF) with excursions permitted from 15¬įC to 30¬įC (59¬įF to 86¬įF) [see USP Controlled Room Temperature].
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Angioedema and Anaphylactic Reactions
Inform patients that angioedema and anaphylactic reactions have been reported in patients treated with VESIcare. Angioedema and anaphylactic reactions may be life-threatening. Advise patients to promptly discontinue VESIcare therapy and seek immediate attention if they experience edema of the tongue or laryngopharynx, or difficulty breathing [see Contraindications (4) and Warnings and Precautions (5.1)].
Urinary Retention
Inform patients that VESIcare may cause urinary retention in patients with conditions associated with bladder outlet obstruction [see Warnings and Precautions (5.2)].
Gastrointestinal Disorders
Inform patients that VESIcare may cause further decrease in gastrointestinal motility in patients with conditions associated with decreased gastrointestinal motility. VESIcare has been associated with constipation and dry mouth. Advise patients to contact their health care providers if they experience severe abdominal pain or become constipated for 3 or more days [see Warnings and Precautions (5.3)].
Central Nervous System Effects
Because VESIcare, like other antimuscarinic agents, may cause central nervous system effects or blurred vision, advise patients to exercise caution in decisions to engage in potentially dangerous activities until the drug’s effect on the patient has been determined [see Warnings and Precautions (5.4)].
Narrow-Angle Glaucoma
Inform patients that VESIcare, like other antimuscarinics, may cause worsening of the glaucoma condition in patients with narrow-angle glaucoma [see Warnings and Precautions (5.5)].
Dry Skin
Inform patients that VESIcare, like other antimuscarinics, may cause dry skin due to decreased sweating. Heat prostration due to decreased sweating can occur when VESIcare is used in a hot environment [see Adverse Reactions (6.2)].
Marketed and Distributed by: Astellas Pharma US, Inc. Northbrook, IL 60062
VESIcare is a registered trademark of Astellas Pharma Inc.
¬© 2004 ‚Äď 2022 Astellas Pharma US, Inc.
361078-VES
Spl Patient Package Insert Section
Patient Information
VESIcare¬ģ (VES-ih-care)
(solifenacin succinate)
Tablet
Read the Patient Information that comes with VESIcare¬ģ before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or treatment.
What is VESIcare?
VESIcare is a prescription medicine for adults used to treat the following symptoms due to a condition called overactive bladder:
‚ÄĘ Urge urinary incontinence: a strong need to urinate with leaking or wetting accidents‚ÄĘ Urgency: a strong need to urinate right away‚ÄĘ Frequency: urinating often
VESIcare 5 mg and 10 mg tablets are not approved for use in children.
Who should not take VESIcare?
Do not take VESIcare if you:
‚ÄĘ are not able to empty your bladder (urinary retention)‚ÄĘ have delayed or slow emptying of your stomach (gastric retention)‚ÄĘ have an eye problem called ‚Äúuncontrolled narrow-angle glaucoma‚ÄĚ‚ÄĘ are allergic to solifenacin succinate or any of the ingredients in VESIcare. See the end of this leaflet for a complete ul of ingredients.
What should I tell my doctor before taking VESIcare?
Before you take VESIcare, tell your doctor if you:
‚ÄĘ have any stomach or intestinal problems or problems with constipation‚ÄĘ have trouble emptying your bladder or you have a weak urine stream‚ÄĘ have an eye problem called ‚Äúnarrow-angle glaucoma‚ÄĚ‚ÄĘ have liver problems‚ÄĘ have kidney problems‚ÄĘ have a rare heart problem called ‚ÄúQT prolongation‚ÄĚ‚ÄĘ are pregnant or plan to become pregnant. It is not known if VESIcare will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.‚ÄĘ are breastfeeding or plan to breastfeed. It is not known if VESIcare passes into your breast milk. You and your doctor should decide if you will take VESIcare or breastfeed.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. VESIcare may affect the way other medicines work, and other medicines may affect how VESIcare works.
How should I take VESIcare?
‚ÄĘ Take VESIcare exactly as your doctor tells you to take it.‚ÄĘ You should take 1 VESIcare tablet 1¬†time a day.‚ÄĘ You should take VESIcare with water and swallow the tablet whole.‚ÄĘ You can take VESIcare with or without food.‚ÄĘ If you miss a dose of VESIcare, begin taking VESIcare again the next day. Do not take 2¬†doses of VESIcare the same day.‚ÄĘ If you take too much VESIcare, call your doctor or go to the nearest hospital emergency room right away.
What should I avoid while taking VESIcare?
VESIcare can cause blurred vision or drowsiness. Do not drive or operate heavy machinery until you know how VESIcare affects you.
What are the possible side effects of VESIcare?
VESIcare may cause serious side effects including:
‚ÄĘ Serious allergic reaction. Stop taking VESIcare and get medical help right away if you have:
‚ÄĘ hives, skin rash or swelling‚ÄĘ severe itching‚ÄĘ swelling of your face, mouth or tongue‚ÄĘ trouble breathing
The most common side effects of VESIcare include:
‚ÄĘ dry mouth‚ÄĘ constipation. Call your doctor if you get severe stomach area (abdominal) pain or become constipated for 3 or more days.‚ÄĘ urinary tract infection‚ÄĘ blurred vision
Other side effects have been observed with anticholinergic drugs such as VESIcare and may include:
‚ÄĘ dry skin due to decreased sweating. Heat exhaustion or heat stroke can happen due to decreased sweating when VESIcare is used in hot environments. Symptoms may include:
‚ÄĘ decreased sweating‚ÄĘ dizziness‚ÄĘ tiredness‚ÄĘ nausea‚ÄĘ increase in body temperature
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of VESIcare. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store VESIcare?
‚ÄĘ Store VESIcare between 59¬įF to 86¬įF (15¬įC to 30¬įC). Keep the bottle closed.‚ÄĘ Safely throw away medicine that is out of date or no longer needed.
Keep VESIcare and all medicines out of the reach of children.
General information about the safe and effective use of VESIcare
Medicines are sometimes prescribed for purposes other than those uled in Patient Information leaflets. Do not use VESIcare for a condition for which it was not prescribed. Do not give VESIcare to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about VESIcare. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about VESIcare that is written for health professionals.
What are the ingredients in VESIcare?
Active ingredient: solifenacin succinate
Inactive ingredients: lactose monohydrate, corn starch, hypromellose 2910, magnesium stearate, talc, polyethylene glycol 8000 and titanium dioxide with yellow ferric oxide (5 mg VESIcare tablet) or red ferric oxide (10 mg VESIcare tablet)
What is overactive bladder?
Overactive bladder occurs when you cannot control your bladder contractions. When these muscle contractions happen too often or cannot be controlled you can get symptoms of overactive bladder, which are urinary frequency, urinary urgency, and urinary incontinence (leakage). Marketed and Distributed by:
Astellas Pharma US, Inc.
Northbrook, Illinois 60062 VESIcare is a registered trademark of Astellas Pharma Inc. ¬© 2004 ‚Äď 2022 Astellas Pharma US, Inc. For more information, call 1-800-727-7003. 361078-VES
  This Patient Information has been approved by the U.S. Food and Drug Administration.                Revised: 10/2022
Principal Display Panel - 5 Mg Carton
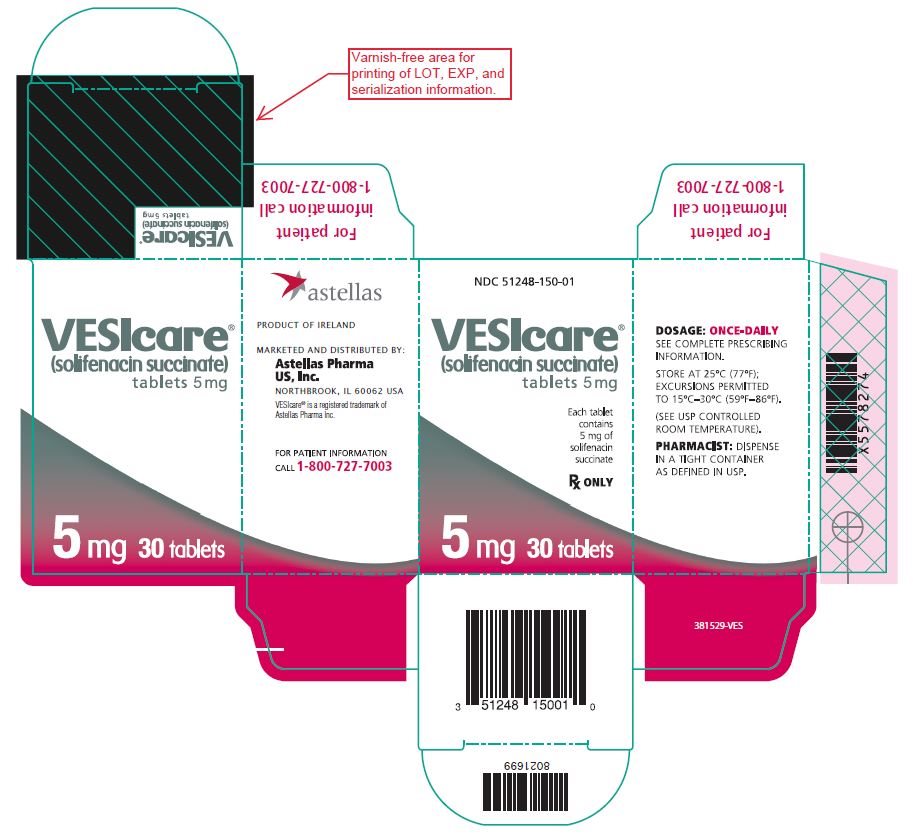
NDC 51248-150-01
VESIcare¬ģ (solifenacin succinate)tablets 5 mg
Each tablet contains 5 mg of solifenacin succinate
Rx ONLY
5 mg 30 tablets
Principal Display Panel 10 Mg Carton
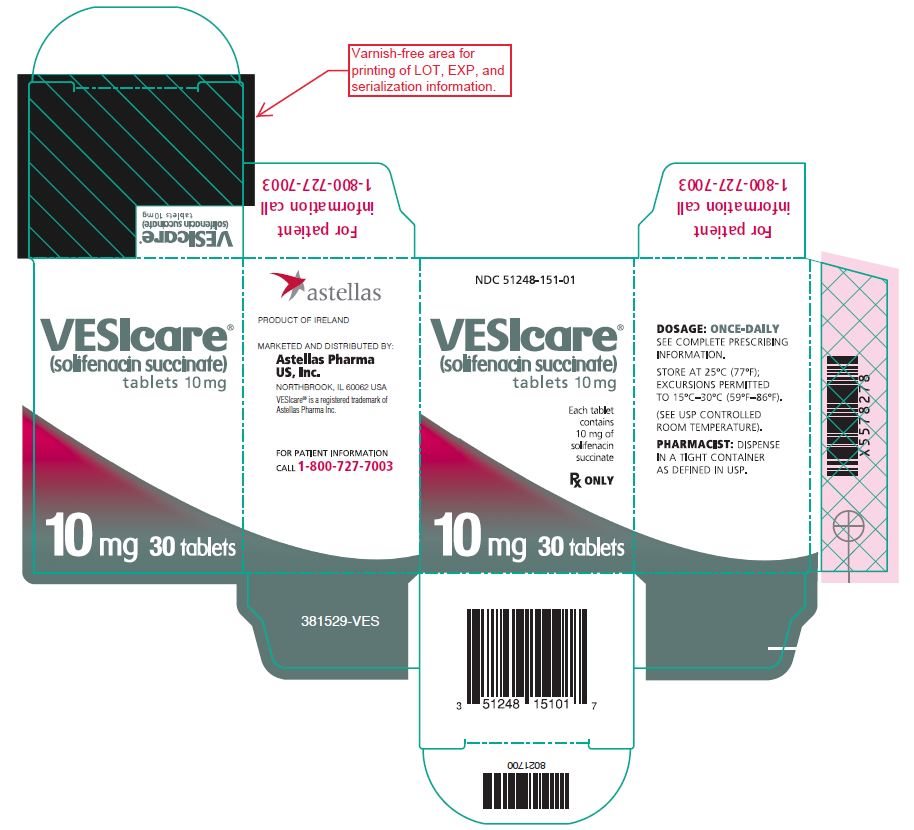
NDC 51248-151-01
VESIcare¬ģ (solifenacin succinate)tablets 10 mg
Each tablet contains 10 mg of solifenacin succinate
Rx ONLY
10 mg 30 tablets
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


