Zebeta (bisoprolol fumarate 10 mg) Dailymed
Generic: bisoprolol fumarate
All Imprints
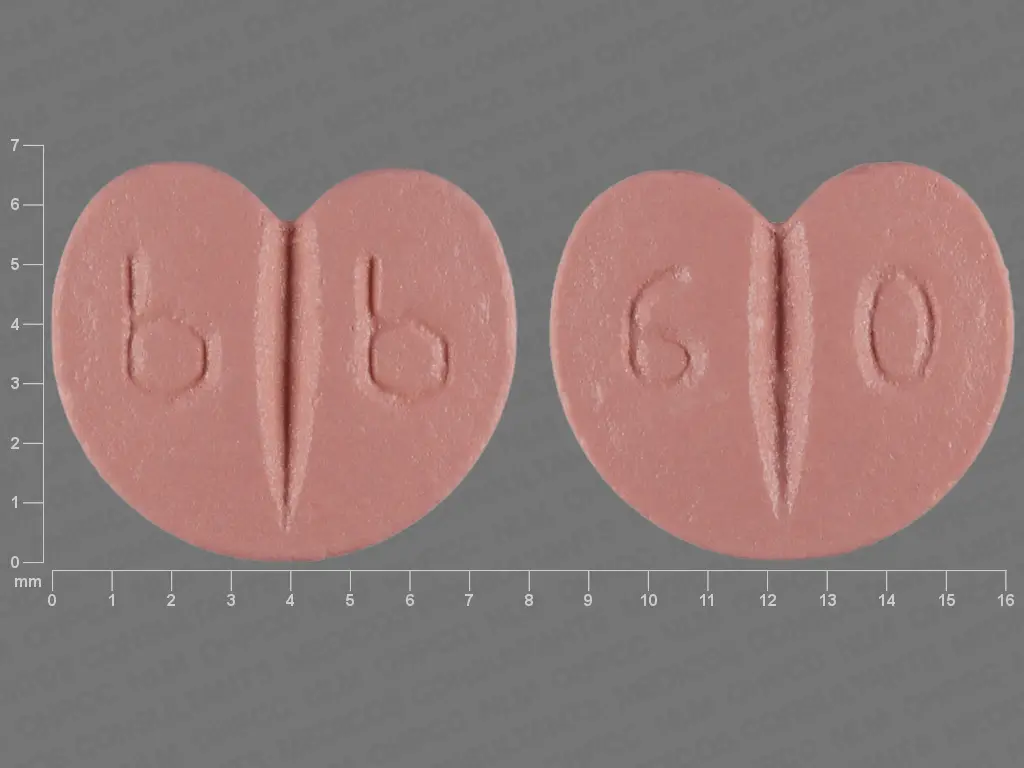
bisoprolol fumarate 5 mg - b b 6 0 freeform pink
bisoprolol fumarate 10 mg - b 61 freeform white
Go PRO for all pill images
Rx only
Rev. 10/2015
Description
ZEBETA (bisoprolol fumarate) is a synthetic, beta1-selective (cardioselective) adrenoceptor blocking agent. The chemical name for bisoprolol fumarate is (¬Ī)-1-[4-[[2-(1-Methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino]-2-propanol(E)-2-butenedioate (2:1) (salt). It possesses an asymmetric carbon atom in its structure and is provided as a racemic mixture. The S(-) enantiomer is responsible for most of the beta-blocking activity. Its empirical formula is (C18H31NO4)2‚ÄĘC4H4O4 and its structure is:
![]()
Bisoprolol fumarate has a molecular weight of 766.97. It is a white crystalline powder which is approximately equally hydrophilic and lipophilic, and is readily soluble in water, methanol, ethanol, and chloroform.
ZEBETA is available as 5 and 10 mg tablets for oral administration.
Inactive ingredients include Colloidal Silicon Dioxide, Corn Starch, Crospovidone, Dibasic Calcium Phosphate, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Polysorbate 80, and Titanium Dioxide. The 5 mg tablets also contain Red and Yellow Iron Oxide.
Clinical Pharmacology
ZEBETA is a beta1-selective (cardioselective) adrenoceptor blocking agent without significant membrane stabilizing activity or intrinsic sympathomimetic activity in its therapeutic dosage range. Cardioselectivity is not absolute, however, and at higher doses (‚Č• 20 mg) bisoprolol fumarate also inhibits beta2-adrenoceptors, chiefly located in the bronchial and vascular musculature; to retain selectivity it is therefore important to use the lowest effective dose.
Pharmacokinetics and Metabolism
The absolute bioavailability after a 10 mg oral dose of bisoprolol fumarate is about 80%. Absorption is not affected by the presence of food. The first pass metabolism of bisoprolol fumarate is about 20%.
Binding to serum proteins is approximately 30%. Peak plasma concentrations occur within 2-4 hours of dosing with 5 to 20 mg, and mean peak values range from 16 ng/mL at 5 mg to 70 ng/mL at 20 mg. Once daily dosing with bisoprolol fumarate results in less than twofold intersubject variation in peak plasma levels. The plasma elimination half-life is 9-12 hours and is slightly longer in elderly patients, in part because of decreased renal function in that population. Steady state is attained within 5 days of once daily dosing. In both young and elderly populations, plasma accumulation is low; the accumulation factor ranges from 1.1 to 1.3, and is what would be expected from the first order kinetics and once daily dosing. Plasma concentrations are proportional to the administered dose in the range of 5 to 20 mg. Pharmacokinetic characteristics of the two enantiomers are similar.
Bisoprolol fumarate is eliminated equally by renal and non-renal pathways with about 50% of the dose appearing unchanged in the urine and the remainder appearing in the form of inactive metabolites. In humans, the known metabolites are labile or have no known pharmacologic activity. Less than 2% of the dose is excreted in the feces. Bisoprolol fumarate is not metabolized by cytochrome P450 II D6 (debrisoquin hydroxylase).
In subjects with creatinine clearance less than 40 mL/min, the plasma half-life is increased approximately threefold compared to healthy subjects.
In patients with cirrhosis of the liver, the elimination of ZEBETA (bisoprolol fumarate) is more variable in rate and significantly slower than that in healthy subjects, with plasma half-life ranging from 8.3 to 21.7 hours.
Pharmacodynamics
The most prominent effect of ZEBETA is the negative chronotropic effect, resulting in a reduction in resting and exercise heart rate. There is a fall in resting and exercise cardiac output with little observed change in stroke volume, and only a small increase in right atrial pressure, or pulmonary capillary wedge pressure at rest or during exercise.
Findings in short-term clinical hemodynamics studies with ZEBETA are similar to those observed with other beta-blocking agents.
The mechanism of action of its antihypertensive effects has not been completely established. Factors which may be involved include:
1. Decreased cardiac output,2. Inhibition of renin release by the kidneys,3. Diminution of tonic sympathetic outflow from the vasomotor centers in the brain.
In normal volunteers, ZEBETA therapy resulted in a reduction of exercise- and isoproterenol-induced tachycardia. The maximal effect occurred within 1-4 hours post-dosing. Effects persisted for 24 hours at doses equal to or greater than 5 mg.
Electrophysiology studies in man have demonstrated that ZEBETA significantly decreases heart rate, increases sinus node recovery time, prolongs AV node refractory periods, and, with rapid atrial stimulation, prolongs AV nodal conduction.
Beta1-selectivity of ZEBETA has been demonstrated in both animal and human studies. No effects at therapeutic doses on beta2-adrenoceptor density have been observed. Pulmonary function studies have been conducted in healthy volunteers, asthmatics, and patients with chronic obstructive pulmonary disease (COPD). Doses of ZEBETA ranged from 5 to 60 mg, atenolol from 50 to 200 mg, metoprolol from 100 to 200 mg, and propranolol from 40 to 80 mg. In some studies, slight, asymptomatic increases in airways resistance (AWR) and decreases in forced expiratory volume (FEV1) were observed with doses of bisoprolol fumarate 20 mg and higher, similar to the small increases in AWR also noted with the other cardioselective beta-blockers. The changes induced by beta-blockade with all agents were reversed by bronchodilator therapy.
ZEBETA had minimal effect on serum lipids during antihypertensive studies. In U.S. placebo-controlled trials, changes in total cholesterol averaged +0.8% for bisoprolol fumarate-treated patients, and +0.7% for placebo. Changes in triglycerides averaged +19% for bisoprolol fumarate-treated patients, and +17% for placebo.
ZEBETA (bisoprolol fumarate) has also been given concomitantly with thiazide diuretics. Even very low doses of hydrochlorothiazide (6.25 mg) were found to be additive with bisoprolol fumarate in lowering blood pressure in patients with mild-to-moderate hypertension.
Clinical Studies
In two randomized double-blind placebo-controlled trials conducted in the U.S., reductions in systolic and diastolic blood pressure and heart rate 24 hours after dosing in patients with mild-to-moderate hypertension are shown below. In both studies, mean systolic/diastolic blood pressures at baseline were approximately 150/100 mm Hg, and mean heart rate was 76 bpm. Drug effect is calculated by subtracting the placebo effect from the overall change in blood pressure and heart rate.
Sitting Systolic/Diastolic Pressure (BP) and Heart Rate (HR) Mean Decrease (D) After 3 to 4 Weeks
Study A
Bisoprolol Fumarate
Placebo
5 mg
10 mg
20 mg
n=
61
61
61
61
Total őĒBP (mm Hg)
5.4/3.2
10.4/8.0
11.2/10.9
12.8/11.9
Drug EffectObserved total change from baseline minus placebo.  
-
5.0/4.8
5.8/7.7
7.4/8.7
Total őĒHR (bpm)
0.5
7.2
8.7
11.3
Drug Effect
-
6.7
8.2
10.8
Study B
Bisoprolol Fumarate
Placebo
2.5 mg
10 mg
n=
56
59
62
Total őĒBP (mm Hg)
3.0/3.7
7.6/8.1
13.5/11.2
Drug Effect
-
4.6/4.4
10.5/7.5
Total őĒHR (bpm)
1.6
3.8
10.7
Drug Effect
-
2.2
9.1
Blood pressure responses were seen within one week of treatment and changed little thereafter. They were sustained for 12 weeks and for over a year in studies of longer duration. Blood pressure returned to baseline when bisoprolol fumarate was tapered over two weeks in a long-term study.
Overall, significantly greater blood pressure reductions were observed on bisoprolol fumarate than on placebo regardless of race, age, or gender. There were no significant differences in response between black and nonblack patients.
Indications And Usage
ZEBETA is indicated in the management of hypertension. It may be used alone or in combination with other antihypertensive agents.
Contraindications
ZEBETA is contraindicated in patients with cardiogenic shock, overt cardiac failure, second or third degree AV block, and marked sinus bradycardia.
Warnings
Cardiac Failure
Sympathetic stimulation is a vital component supporting circulatory function in the setting of congestive heart failure, and beta-blockade may result in further depression of myocardial contractility and precipitate more severe failure. In general, beta-blocking agents should be avoided in patients with overt congestive failure. However, in some patients with compensated cardiac failure it may be necessary to utilize them. In such a situation, they must be used cautiously.
In Patients Without a History of Cardiac Failure
Continued depression of the myocardium with beta-blockers can, in some patients, precipitate cardiac failure. At the first signs or symptoms of heart failure, discontinuation of ZEBETA should be considered. In some cases, beta-blocker therapy can be continued while heart failure is treated with other drugs.
Abrupt Cessation of Therapy
Exacerbation of angina pectoris, and, in some instances, myocardial infarction or ventricular arrhythmia, have been observed in patients with coronary artery disease following abrupt cessation of therapy with beta-blockers. Such patients should, therefore, be cautioned against interruption or discontinuation of therapy without the physician’s advice. Even in patients without overt coronary artery disease, it may be advisable to taper therapy with ZEBETA over approximately one week with the patient under careful observation. If withdrawal symptoms occur, ZEBETA therapy should be reinstituted, at least temporarily.
Peripheral Vascular Disease
Beta-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease. Caution should be exercised in such individuals.
Bronchospastic Disease
PATIENTS WITH BRONCHOSPASTIC DISEASE SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS. Because of its relative beta1-selectivity, however, ZEBETA may be used with caution in patients with bronchospastic disease who do not respond to, or who cannot tolerate other antihypertensive treatment. Since beta1-selectivity is not absolute, the lowest possible dose of ZEBETA should be used, with therapy starting at 2.5 mg. A beta2 agonist (bronchodilator) should be made available.
Major Surgery
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes and Hypoglycemia
Beta-blockers may mask some of the manifestations of hypoglycemia, particularly tachycardia. Nonselective beta-blockers may potentiate insulin-induced hypoglycemia and delay recovery of serum glucose levels. Because of its beta1-selectivity, this is less likely with ZEBETA. However, patients subject to spontaneous hypoglycemia, or diabetic patients receiving insulin or oral hypoglycemic agents, should be cautioned about these possibilities and bisoprolol fumarate should be used with caution.
Thyrotoxicosis
Beta-adrenergic blockade may mask clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of beta-blockade may be followed by an exacerbation of the symptoms of hyperthyroidism or may precipitate thyroid storm.
Precautions
Impaired Renal or Hepatic Function
Use caution in adjusting the dose of ZEBETA in patients with renal or hepatic impairment (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Drug Interactions
ZEBETA should not be combined with other beta-blocking agents. Patients receiving catecholamine-depleting drugs, such as reserpine or guanethidine, should be closely monitored, because the added beta-adrenergic blocking action of ZEBETA may produce excessive reduction of sympathetic activity. In patients receiving concurrent therapy with clonidine, if therapy is to be discontinued, it is suggested that ZEBETA be discontinued for several days before the withdrawal of clonidine.
ZEBETA should be used with care when myocardial depressants or inhibitors of AV conduction, such as certain calcium antagonists (particularly of the phenylalkylamine [verapamil] and benzothiazepine [diltiazem] classes), or antiarrhythmic agents, such as disopyramide, are used concurrently.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Concurrent use of rifampin increases the metabolic clearance of ZEBETA, resulting in a shortened elimination half-life of ZEBETA. However, initial dose modification is generally not necessary. Pharmacokinetic studies document no clinically relevant interactions with other agents given concomitantly, including thiazide diuretics and cimetidine. There was no effect of ZEBETA on prothrombin time in patients on stable doses of warfarin.
Risk of Anaphylactic Reaction: While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reactions.
Information for Patients
Patients, especially those with coronary artery disease, should be warned about discontinuing use of ZEBETA without a physician’s supervision. Patients should also be advised to consult a physician if any difficulty in breathing occurs, or if they develop signs or symptoms of congestive heart failure or excessive bradycardia.
Patients subject to spontaneous hypoglycemia, or diabetic patients receiving insulin or oral hypoglycemic agents, should be cautioned that beta-blockers may mask some of the manifestations of hypoglycemia, particularly tachycardia, and bisoprolol fumarate should be used with caution.
Patients should know how they react to this medicine before they operate automobiles and machinery or engage in other tasks requiring alertness.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies were conducted with oral bisoprolol fumarate administered in the feed of mice (20 and 24 months) and rats (26 months). No evidence of carcinogenic potential was seen in mice dosed up to 250 mg/kg/day or rats dosed up to 125 mg/kg/day. On a body weight basis, these doses are 625 and 312 times, respectively, the maximum recommended human dose (MRHD) of 20 mg, (or 0.4 mg/kg/day based on a 50 kg individual); on a body surface area basis, these doses are 59 times (mice) and 64 times (rats) the MRHD. The mutagenic potential of bisoprolol fumarate was evaluated in the microbial mutagenicity (Ames) test, the point mutation and chromosome aberration assays in Chinese hamster V79 cells, the unscheduled DNA synthesis test, the micronucleus test in mice, and the cytogenetics assay in rats. There was no evidence of mutagenic potential in these in vitro and in vivo assays.
Reproduction studies in rats did not show any impairment of fertility at doses up to 150 mg/kg/day of bisoprolol fumarate, or 375 and 77 times the MRHD on the basis of body weight and body surface area, respectively.
Pregnancy Category C
In rats, bisoprolol fumarate was not teratogenic at doses up to 150 mg/kg/day which is 375 and 77 times the MRHD on the basis of body weight and body surface area, respectively. Bisoprolol fumarate was fetotoxic (increased late resorptions) at 50 mg/kg/day and maternotoxic (decreased food intake and body weight gain) at 150 mg/kg/day. The fetotoxicity in rats occurred at 125 times the MRHD on a body weight basis and 26 times the MRHD on the basis of body surface area. The maternotoxicity occurred at 375 times the MRHD on a body weight basis and 77 times the MRHD on the basis of body surface area. In rabbits, bisoprolol fumarate was not teratogenic at doses up to 12.5 mg/kg/day, which is 31 and 12 times the MRHD based on body weight and body surface area, respectively, but was embryolethal (increased early resorptions) at 12.5 mg/kg/day.
There are no adequate and well-controlled studies in pregnant women. ZEBETA (bisoprolol fumarate) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Small amounts of bisoprolol fumarate (< 2% of the dose) have been detected in the milk of lactating rats. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk caution should be exercised when bisoprolol fumarate is administered to nursing women.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
ZEBETA has been used in elderly patients with hypertension. Response rates and mean decreases in systolic and diastolic blood pressure were similar to the decreases in younger patients in the U.S. clinical studies. Although no dose response study was conducted in elderly patients, there was a tendency for older patients to be maintained on higher doses of bisoprolol fumarate.
Observed reductions in heart rate were slightly greater in the elderly than in the young and tended to increase with increasing dose. In general, no disparity in adverse experience reports or dropouts for safety reasons was observed between older and younger patients. Dose adjustment based on age is not necessary.
Adverse Reactions
Safety data are available in more than 30,000 patients or volunteers. Frequency estimates and rates of withdrawal of therapy for adverse events were derived from two U.S. placebo-controlled studies.
In Study A, doses of 5, 10, and 20 mg bisoprolol fumarate were administered for 4 weeks. In Study B, doses of 2.5, 10, and 40 mg of bisoprolol fumarate were administered for 12 weeks. A total of 273 patients were treated with 5-20 mg of bisoprolol fumarate; 132 received placebo.
Withdrawal of therapy for adverse events was 3.3% for patients receiving bisoprolol fumarate and 6.8% for patients on placebo. Withdrawals were less than 1% for either bradycardia or fatigue/lack of energy.
The following table presents adverse experiences, whether or not considered drug related, reported in at least 1% of patients in these studies, for all patients studied in placebo-controlled clinical trials (2.5-40 mg), as well as for a subgroup that was treated with doses within the recommended dosage range (5-20 mg). Of the adverse events uled in the table, bradycardia, diarrhea, asthenia, fatigue, and sinusitis appear to be dose related.
Body System/Adverse Experience
All Adverse Experiences (%percentage of patients with event ) Bisoprolol Fumarate
Placebo (n=132)
5-20 mg (n=273)
2.5-40 mg (n=404)
%
%
%
Skin
     increased sweating
1.5
0.7
1.0
Musculoskeletal
     arthralgia
2.3
2.2
2.7
Central Nervous System
     dizziness
3.8
2.9
3.5
      headache
11.4
8.8
10.9
     hypoaesthesia
0.8
1.1
1.5
Autonomic Nervous System
     dry mouth
1.5
0.7
1.3
Heart Rate/Rhythm
     bradycardia
0
0.4
0.5
Psychiatric
     vivid dreams
0
0
0
     insomnia
2.3
1.5
2.5
     depression
0.8
0
0.2
Gastrointestinal
     diarrhea
1.5
2.6
3.5
     nausea
1.5
1.5
2.2
     vomiting
0
1.1
1.5
Respiratory
     bronchospasm
0
0
0
     cough
4.5
2.6
2.5
     dyspnea
0.8
1.1
1.5
     pharyngitis
2.3
2.2
2.2
     rhinitis
3.0
2.9
4.0
     sinusitis
1.5
2.2
2.2
     URI
3.8
4.8
5.0
Body as a Whole
     asthenia
0
0.4
1.5
     chest pain
0.8
1.1
1.5
     fatigue
1.5
6.6
8.2
     edema (peripheral)
3.8
3.7
3.0
The following is a comprehensive ul of adverse experiences reported with bisoprolol fumarate in worldwide studies, or in postmarketing experience (in italics):
Central Nervous System
Dizziness, unsteadiness, vertigo, syncope, headache, paresthesia, hypoesthesia, hyperesthesia, somnolence, sleep disturbances, anxiety/restlessness, decreased concentration/memory.
Autonomic Nervous System
Dry mouth.
Cardiovascular
Bradycardia, palpitations and other rhythm disturbances, cold extremities, claudication, hypotension, orthostatic hypotension, chest pain, congestive heart failure, dyspnea on exertion.
Psychiatric
Vivid dreams, insomnia, depression.
Gastrointestinal
Gastric/epigastric/abdominal pain, gastritis, dyspepsia, nausea, vomiting, diarrhea, constipation, peptic ulcer.
Musculoskeletal
Muscle/joint pain, arthralgia, back/neck pain, muscle cramps, twitching/tremor.
Skin
Rash, acne, eczema, psoriasis, skin irritation, pruritus, flushing, sweating, alopecia, dermatitis, angioedema, exfoliative dermatitis, cutaneous vasculitis.
Special Senses
Visual disturbances, ocular pain/pressure, abnormal lacrimation, tinnitus, decreased hearing, earache, taste abnormalities.
Metabolic
Gout.
Respiratory
Asthma/bronchospasm, bronchitis, coughing, dyspnea, pharyngitis, rhinitis, sinusitis, URI.
Genitourinary
Decreased libido/impotence, Peyronie’s disease, cystitis, renal colic, polyuria.
Hematologic
Purpura.
General
Fatigue, asthenia, chest pain, malaise, edema, weight gain, angioedema.
In addition, a variety of adverse effects have been reported with other beta-adrenergic blocking agents and should be considered potential adverse effects of ZEBETA:
Central Nervous System
Reversible mental depression progressing to catatonia, hallucinations, an acute reversible syndrome characterized by disorientation to time and place, emotional lability, slightly clouded sensorium.
Allergic
Fever, combined with aching and sore throat, laryngospasm, respiratory distress.
Hematologic
Agranulocytosis, thrombocytopenia, thrombocytopenic purpura.
Gastrointestinal
Mesenteric arterial thrombosis, ischemic colitis.
Miscellaneous
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with ZEBETA (bisoprolol fumarate) during investigational use or extensive foreign marketing experience.
LABORATORY ABNORMALITIES
In clinical trials, the most frequently reported laboratory change was an increase in serum triglycerides, but this was not a consistent finding.
Sporadic liver test abnormalities have been reported. In the U.S. controlled trials experience with bisoprolol fumarate treatment for 4-12 weeks, the incidence of concomitant elevations in SGOT and SGPT from 1 to 2 times normal was 3.9%, compared to 2.5% for placebo. No patient had concomitant elevations greater than twice normal.
In the long-term, uncontrolled experience with bisoprolol fumarate treatment for 6-18 months, the incidence of one or more concomitant elevations in SGOT and SGPT from 1 to 2 times normal was 6.2%. The incidence of multiple occurrences was 1.9%. For concomitant elevations in SGOT and SGPT of greater than twice normal, the incidence was 1.5%. The incidence of multiple occurrences was 0.3%. In many cases these elevations were attributed to underlying disorders, or resolved during continued treatment with bisoprolol fumarate.
Other laboratory changes included small increases in uric acid, creatinine, BUN, serum potassium, glucose, and phosphorus and decreases in WBC and platelets. These were generally not of clinical importance and rarely resulted in discontinuation of bisoprolol fumarate.
As with other beta-blockers, ANA conversions have also been reported on bisoprolol fumarate. About 15% of patients in long-term studies converted to a positive titer, although about one-third of these patients subsequently reconverted to a negative titer while on continued therapy.
Overdosage
The most common signs expected with overdosage of a beta-blocker are bradycardia, hypotension, congestive heart failure, bronchospasm, and hypoglycemia. To date, a few cases of overdose (maximum: 2000 mg) with bisoprolol fumarate have been reported. Bradycardia and/or hypotension were noted. Sympathomimetic agents were given in some cases, and all patients recovered.
In general, if overdose occurs, ZEBETA therapy should be stopped and supportive and symptomatic treatment should be provided. Limited data suggest that bisoprolol fumarate is not dialyzable. Based on the expected pharmacologic actions and recommendations for other beta-blockers, the following general measures should be considered when clinically warranted:
Bradycardia
Administer IV atropine. If the response is inadequate, isoproterenol or another agent with positive chronotropic properties may be given cautiously. Under some circumstances, transvenous pacemaker insertion may be necessary.
Hypotension
IV fluids and vasopressors should be administered. Intravenous glucagon may be useful.
Heart Block (second or third degree)
Patients should be carefully monitored and treated with isoproterenol infusion or transvenous cardiac pacemaker insertion, as appropriate.
Congestive Heart Failure
Initiate conventional therapy (ie, digitalis, diuretics, inotropic agents, vasodilating agents).
Bronchospasm
Administer bronchodilator therapy such as isoproterenol and/or aminophylline.
Hypoglycemia
Administer IV glucose.
Dosage And Administration
The dose of ZEBETA must be individualized to the needs of the patient. The usual starting dose is 5 mg once daily. In some patients, 2.5 mg may be an appropriate starting dose (see Bronchospastic Disease in WARNINGS). If the antihypertensive effect of 5 mg is inadequate, the dose may be increased to 10 mg and then, if necessary, to 20 mg once daily.
Patients with Renal or Hepatic Impairment
In patients with hepatic impairment (hepatitis or cirrhosis) or renal dysfunction (creatinine clearance less than 40 mL/min), the initial daily dose should be 2.5 mg and caution should be used in dose-titration. Since limited data suggest that bisoprolol fumarate is not dialyzable, drug replacement is not necessary in patients undergoing dialysis.
Geriatric Patients
It is not necessary to adjust the dose in the elderly, unless there is also significant renal or hepatic dysfunction (see above and Geriatric Use in PRECAUTIONS).
Pediatric Patients
There is no pediatric experience with ZEBETA.
How Supplied
ZEBETA¬ģ (bisoprolol fumarate) is supplied as 5 mg and 10 mg tablets.
The 5 mg tablet is pink, heart-shaped, biconvex, film-coated, vertically scored in half on both sides, with an engraved stylized b/stylized b on one side and 6/0 on the reverse side, supplied as follows:
30
Unit-of-use
NDC 51285-060-01
The 10 mg tablet is white, heart-shaped, biconvex, film-coated, with an engraved stylized b on one side and 61 on the reverse side, supplied as follows:
30
Unit-of-use
NDC 51285-061-01
STORAGE AND HANDLING SECTION
Store at 20o to 25o C (68o to 77oF) [See USP Controlled Room Temperature].
Protect from moisture.
Dispense in tight containers.
Distributed by:
TEVA PHARMACEUTICALS USA, INC.
North Wales, PA 19454
Rev. 10/2015
Package/label Display Panel
![]()
Zebeta (bisoprolol fumarate) Tablets 5 mg, 30s Label Text
NDC 51285-060-01
ZEBETA¬ģ
(bisoprolol fumarate)
Tablets
5 mg
30 Tablets
Unit-of-use
TEVA
Rx only
Package/label Display Panel
![]()
Zebeta (bisoprolol fumarate) Tablets 10 mg, 30s Label Text
NDC 51285-061-01
ZEBETA¬ģ
(bisoprolol fumarate)
Tablets
10 mg
30 Tablets
Unit-of-use
TEVA
Rx only
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site