Acetaminophen (acetaminophen 650 mg) Dailymed
Generic: acetaminophen is used for the treatment of Fever Glucosephosphate Dehydrogenase Deficiency Liver Diseases Pain
IMPRINT: I 90
SHAPE: oval
COLOR: white
All Imprints
acetaminophen 650 mg - i 06 oval white
acetaminophen 650 mg - i 90 oval white
Go PRO for all pill images
Drug Facts
Active ingredient (in each extended-release tablet) Acetaminophen USP 650 mg
Purpose
Pain reliever/fever reducer
Uses
- temporarily relieves minor aches and pains due to:
- minor pain of arthritis
- muscular aches
- backache
- premenstrual and menstrual cramps
- the common cold
- headache
- toothache
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 6 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- bulers
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do Not Use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product.
Ask a doctor before use if you have liver disease. Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
Stop Use And Ask A Doctor If
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition. If pregnant or breast-feeding, ask a health professional before use.
Keep Out Of Reach Of Children.
Overdose warning:Â In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- do not take more than directed (see overdose warning).
Adults:
- take 2 tablets every 8 hours with water
- swallow whole; do not crush, chew, split or dissolve
- do not take more than 6 tablets in 24 hours
- do not use for more than 10 days unless directed by a doctor.
Under 18 years of age:
- ask a doctor
Other Information
- store at 20o to 25oC (68o to 77oF). Avoid excessive heat 40oC (104oF).Â
- do not use if carton is opened or foil inner seal is broken
- Meets USP dissolution test 3
Inactive Ingredients
colloidal silicon dioxide, hydroxyethyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch (maize), sodium starch glycolate, titanium dioxide, triacetin Questions or comments?  call 1-855-274-4122 Distributed by: AUROHEALTH LLC 279 Princeton-Hightstown Road, East Windsor, NJ 08520 Made in India Code: TS/DRUGS/22/2009
Package Label-principal Display Panel - 650 Mg (225 Tablets Bottle)
AUROHEALTH TO OPEN: 1. PUSH DOWN        NDC 58602-730-28                   2. TURN CAP DO NOT USE WITH OTHER MEDICINES CONTAINING ACETAMINOPHEN 8 HOUR   ARTHRITIS PAIN Acetaminophen Extended-Release Tablets USP 650 mg Pain Reliever/Fever Reducer For the Temporary Relief of Minor Arthritis Pain 225 Extended-release tablets         Â
Package Label-principal Display Panel - 650 Mg (225 Tablets Container Carton)
AUROHEALTH NDC 58602-730-28 *Compare to the Active Ingredient in Tylenol® 8 HR Arthritis Pain DO NOT USE WITH OTHER MEDICINES CONTAINING ACETAMINOPHEN 8 HOUR ARTHRITIS PAIN Acetaminophen Extended-Release Tablets USP 650 mg Pain Reliever/Fever Reducer For the Temporary Relief of Minor Arthritis Pain 225 Extended-release tablets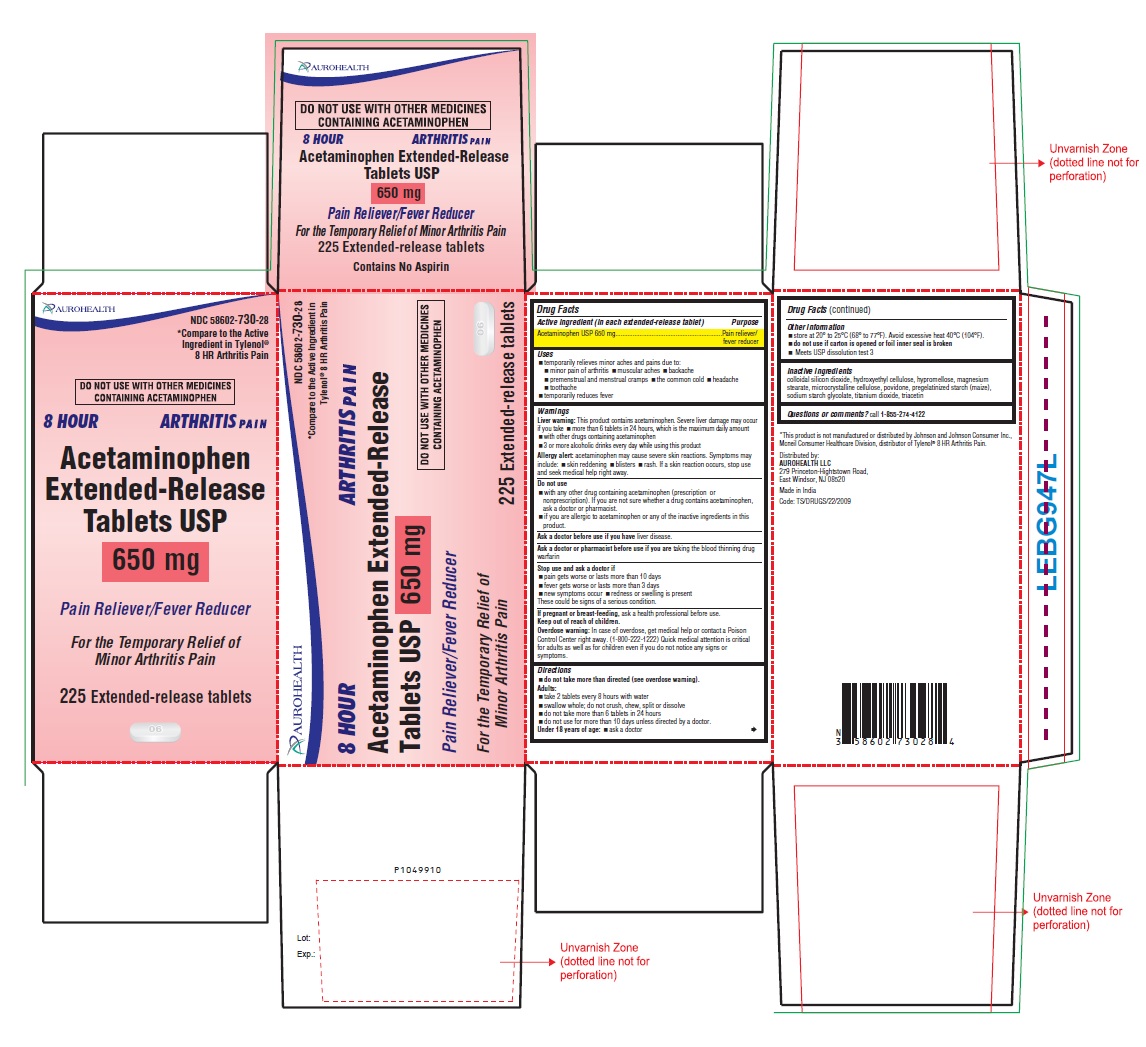
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


