Alprazolam (alprazolam 2 mg) Dailymed
Generic: alprazolam is used for the treatment of Agoraphobia Depressive Disorder Glaucoma, Angle-Closure Panic Disorder
IMPRINT: X 74
SHAPE: round
COLOR: white
All Imprints
alprazolam 0.5 mg - x 70 round white
alprazolam 2 mg - x 74 round white
alprazolam tablet, extended release - x 70 round white
alprazolam tablet, extended release - x 74 round white
Boxed Warning
Warning: Risks From Concomitant Use With Opioids; Abuse, Misuse, And Addiction; And Dependence And Withdrawal Reactions
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
- The use of benzodiazepines, including alprazolam extended-release tablets, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing alprazolam extended-release tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction [see Warnings and Precautions (5.2)].
- The continued use of benzodiazepines, including alprazolam extended-release tablets, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of alprazolam extended-release tablets after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam extended-release tablets or reduce the dosage [see Dosage and Administration (2.2), Warnings and Precautions (5.3)].
-
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation. (
5.1 ,7.1 ) -
The use of benzodiazepines, including alprazolam extended-release tablets, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Before prescribing alprazolam extended-release tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction. (
5.2 ) -
Abrupt discontinuation or rapid dosage reduction of alprazolam extended-release tablets after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam extended-release tablets or reduce the dosage. (
2.2 ,5.3 )
Go PRO for all pill images
Recent Major Changes Section
Warnings and Precautions (5.8 )                                                       1/2023
Warning: Risks From Concomitant Use With Opioids; Abuse, Misuse, And Addiction; And Dependence And Withdrawal Reactions
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
- The use of benzodiazepines, including alprazolam extended-release tablets, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing alprazolam extended-release tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction [see Warnings and Precautions (5.2)].
- The continued use of benzodiazepines, including alprazolam extended-release tablets, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of alprazolam extended-release tablets after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam extended-release tablets or reduce the dosage [see Dosage and Administration (2.2), Warnings and Precautions (5.3)].
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS;
ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS See full prescribing information for complete boxed warning.
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation. (
5.1 ,7.1 )- The use of benzodiazepines, including alprazolam extended-release tablets, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Before prescribing alprazolam extended-release tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction. (
5.2 )- Abrupt discontinuation or rapid dosage reduction of alprazolam extended-release tablets after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam extended-release tablets or reduce the dosage. (
2.2 ,5.3 )
1 Indications And Usage
Alprazolam extended-release tablets are indicated for the treatment of panic disorder with or without agoraphobia, in adults.
Alprazolam extended-release tablets are a benzodiazepine indicated for the treatment of panic disorder with or without agoraphobia, in adults. (1 )
2 Dosage And Administration
- Recommended starting oral dosage is 0.5 mg to 1 mg once daily (preferably in the morning). Depending on the response, the dose may be increased at intervals of 3 to 4 days in increments of no more than 1 mg daily. (
2.1 )- Recommended total daily dosage is 3 mg to 6 mg daily. (
2.1 )- Swallow tablets whole; do not divide, crush, or chew. (
2.1 )- When tapering, decrease dosage by no more than 0.5 mg every 3 days. Some patients may require an even slower dosage reduction. (
2.2 ,5.2 )- See the Full Prescribing Information for the recommended dosage in geriatric patients, patients with hepatic impairment, and with use with ritonavir. (
2.3 ,2.4 ,2.5 )2.1 Recommended Dosage
Administer alprazolam extended-release tablets orally once daily, preferably in the morning. Swallow tablets whole; do not divide, crush, or chew.
The recommended starting oral dosage for alprazolam extended-release tablets are 0.5 mg to 1 mg once daily. Depending on the response, the dosage may be adjusted at intervals of every 3 to 4 days in increments of no more than 1 mg daily. The recommended dosage range is 3 mg to 6 mg once daily.
Controlled trials of alprazolam extended-release tablets for the treatment of panic disorder included dosages in the range of 1 mg to 10 mg per day. Most patients showed a response in the dosage range of 3 mg to 6 mg per day. Occasional patients required as much as 10 mg per day.
The longer-term efficacy of alprazolam extended-release tablets has not been systematically evaluated. If alprazolam extended-release tablets are used for periods longer than 8 weeks, the healthcare provider should periodically reassess the usefulness of the drug for the individual patient.
After a period of extended freedom from panic attacks, a carefully supervised tapered discontinuation may be attempted, but there is evidence that this may often be difficult to accomplish without recurrence of symptoms and/or the manifestation of withdrawal phenomena [see Dosage and Administration (2.2), Warnings and Precautions (5.2)].
2.2 Discontinuation or Dosage Reduction of Alprazolam Extended-Release Tablets
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam extended-release tablets or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly [see Warnings and Precautions (5.3), Drug Abuse and Dependence (9.3)].
Reduce the dosage by no more than 0.5 mg every three days. Some patients may benefit from an even more gradual discontinuation. Some patients may prove resistant to all discontinuation regimens.
In a controlled postmarketing discontinuation study of panic disorder patients which compared the recommended taper schedule with a slower taper schedule, no difference was observed between the groups in the proportion of patients who tapered to zero dose; however, the slower schedule was associated with a reduction in symptoms associated with a withdrawal syndrome.
2.3 Dosage Recommendations in Geriatric Patients
In geriatric patients, the recommended starting dosage of alprazolam extended-release tablets is 0.5 mg once daily. This may be gradually increased if needed and tolerated. Geriatric patients may be sensitive to the effects of benzodiazepines [see Use in Specific Populations (8.5), Clinical Pharmacology (12.3)].
2.4 Dosage Recommendations in Patients with Hepatic Impairment
In patients with hepatic impairment, the recommended starting dosage of alprazolam extended-release tablets is 0.5 mg once daily. This may be gradually increased if needed and tolerated [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
2.5 Dosage Modifications for Drug Interactions
Alprazolam extended-release tablets should be reduced to half of the recommended dosage when a patient is started on ritonavir and alprazolam extended-release tablets together, or when ritonavir is added to a patient treated with alprazolam extended-release tablets. Increase alprazolam extended-release tablets dosage to the target dose after 10 to 14 days of dosing ritonavir and alprazolam extended-release tablets together. It is not necessary to reduce alprazolam extended-release tablets dosage in patients who have been taking ritonavir for more than 10 to 14 days.
Alprazolam extended-release tablets are contraindicated with concomitant use of all strong CYP3A inhibitors, except ritonavir [see Contraindications (4), Warnings and Precautions (5.5), Drug Interactions (7.1)].
2.6 Switching Patients from Alprazolam Tablets to Alprazolam Extended-Release Tablets
Patients who are currently being treated with divided doses of alprazolam may be switched to alprazolam extended-release tablets at the same total daily dose taken once daily. If the clinical response after switching is inadequate, titrate the dosage as outlined above.
3 Dosage Forms And Strengths
Alprazolam extended-release tablets are available as:
- 0.5 mg:¬†white to off-white, round, biconvex tablets with beveled edge debossed with ‚ÄėX‚Äô on one side and ‚Äė70‚Äô on the other side.
- 1¬†mg:¬†yellow colored, round, biconvex tablets with beveled edge debossed with ‚ÄėX‚Äô on one side and ‚Äė73‚Äô on the other side. The tablets may be mottled.
- 2 mg:¬†blue colored, round, biconvex tablets with beveled edge debossed with ‚ÄėX‚Äô on one side and ‚Äė74‚Äô on the other side. The tablets may be mottled.
- 3 mg:¬†green colored, round, biconvex tablets with beveled edge debossed with ‚ÄėX‚Äô on one side and ‚Äė75‚Äô on the other side. The tablets may be mottled.
Extended Release Tablets: 0.5 mg, 1 mg, 2 mg, and 3 mg (3 )
4 Contraindications
Alprazolam extended-release tablets are contraindicated in patients:
- with known hypersensitivity to alprazolam or other benzodiazepines. Angioedema has been reported [see Adverse Reactions (6.2)].
- taking strong cytochrome P450 3A (CYP3A) inhibitors (e.g., ketoconazole, itraconazole), except ritonavir [see Dosage and Administration (2.5), Warnings and Precautions (5.5), Drug Interactions (7.1)].
- Known hypersensitivity to alprazolam or other benzodiazepines. (
4 )- Concomitant use with strong cytochrome P450 3A (CYP3A) inhibitors, except ritonavir. (
4 ,5.5 ,7.1 )
5 Warnings And Precautions
- Effects on Driving and Operating Machinery: Patients receiving alprazolam extended-release tablets should be cautioned against operating machinery or driving a motor vehicle, as well as avoiding concomitant use of alcohol and other central nervous system (CNS) depressant drugs. (
5.4 )- Patients with Depression: Exercise caution in patients with signs or symptoms of depression. Prescribe the least number of tablets feasible to avoid intentional overdosage. (
5.6 )- Neonatal Sedation and Withdrawal Syndrome: Alprazolam extended-release tablets use during pregnancy can result in neonatal sedation and/or neonatal withdrawal. (
5.8 ,8.1 )5.1 Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including alprazolam extended-release tablets, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe alprazolam extended-release tablets concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of alprazolam extended-release tablets than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking alprazolam extended-release tablets, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when alprazolam extended-release tablets is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined [see Drug Interactions (7.1)].
5.2 Abuse, Misuse, and Addiction
The use of benzodiazepines, including alprazolam extended-release tablets, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death [see Drug Abuse and Dependence (9.2)].
Before prescribing alprazolam extended-release tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of alprazolam extended-release tablets, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of alprazolam extended-release tablets along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
5.3 Dependence and Withdrawal Reactions
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam extended-release tablets or reduce the dosage (a patient-specific plan should be used to taper the dose) [see Dosage and Administration (2.3)].
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including alprazolam extended-release tablets, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of alprazolam extended-release tablets after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) [see Drug Abuse and Dependence (9.3)].
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months [see Drug Abuse and Dependence (9.3)].
Certain adverse clinical events, some life-threatening, are a direct consequence of physical dependence to alprazolam extended-release tablets. These include a spectrum of withdrawal symptoms; the most important is seizure [see Drug Abuse and Dependence (9.3)]. Even after relatively short-term use at doses of < 4 mg/day, there is some risk of dependence. Spontaneous reporting system data suggest that the risk of dependence and its severity appear to be greater in patients treated with doses greater than 4 mg/day and for long periods (more than 12 weeks). However, in a controlled postmarketing discontinuation study of panic disorder patients who received alprazolam, the duration of treatment (3 months compared to 6 months) had no effect on the ability of patients to taper to zero dose. In contrast, patients treated with doses of alprazolam greater than 4 mg/day had more difficulty tapering to zero dose than those treated with less than 4 mg/day.
 
In a controlled clinical trial in which 63 patients were randomized to alprazolam and where withdrawal symptoms were specifically sought, the following were identified as symptoms of withdrawal: heightened sensory perception, impaired concentration, dysosmia, clouded sensorium, paresthesias, muscle cramps, muscle twitch, diarrhea, blurred vision, appetite decrease, and weight loss. Other symptoms, such as anxiety and insomnia, were frequently seen during discontinuation, but it could not be determined if they were due to return of illness, rebound, or withdrawal.
 
Interdose Symptoms
Early morning anxiety and emergence of anxiety symptoms between doses of alprazolam have been reported in patients with panic disorder taking prescribed maintenance doses. These symptoms may reflect the development of tolerance or a time interval between doses which is longer than the duration of clinical action of the administered dose. In either case, it is presumed that the prescribed dose is not sufficient to maintain plasma levels above those needed to prevent relapse, rebound, or withdrawal symptoms over the entire course of the interdosing interval.
5.4 Effects on Driving and Operating Machinery
Because of its CNS depressant effects, patients receiving alprazolam extended-release tablets should be cautioned against engaging in hazardous occupations or activities requiring complete mental alertness such as operating machinery or driving a motor vehicle. For the same reason, patients should be cautioned about the concomitant use of alcohol and other CNS depressant drugs during treatment with alprazolam extended-release tablets [see Drug Interactions (7.1)].
5.5 Interaction with Drugs that Inhibit Metabolism via Cytochrome P450 3A
The initial step in alprazolam metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP3A). Drugs that inhibit this metabolic pathway may have a profound effect on the clearance of alprazolam.
Strong CYP3A Inhibitors Alprazolam extended-release tablets are contraindicated in patients receiving strong inhibitors of CYP3A such as azole antifungal agents [see Contraindications (4)]. Ketoconazole and itraconazole have been shown in vivo to increase plasma alprazolam concentrations 3.98 fold and 2.70 fold, respectively. 
Dosage adjustment is necessary when alprazolam extended-release tablets and ritonavir are initiated concomitantly or when ritonavir is added to a stable dosage of alprazolam extended-release tablets [see Dosage and Administration (2.5), Drug Interactions (7.1)].  
Drugs demonstrated to be CYP3A inhibitors on the basis of clinical studies involving alprazolam: nefazodone, fluvoxamine, and cimetidine [see Drug Interaction (7.1), Clinical Pharmacology (12.3)]. Use caution and consider dose reduction of alprazolam extended-release tablets, as appropriate, during co-administration with these drugs.
5.6 Patients with Depression
Benzodiazepines may worsen depression. Panic disorder has been associated with primary and secondary major depressive disorders and increased reports of suicide among untreated patients. Consequently, appropriate precautions (e.g., limiting the total prescription size and increased monitoring for suicidal ideation) should be considered in patients with depression.
5.7 Mania
Episodes of hypomania and mania have been reported in association with the use of alprazolam extended-release tablets in patients with depression [see Adverse Reactions (6.1)].
5.8 Neonatal Sedation and Withdrawal Syndrome
Use of alprazolam extended-release tablets late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate [see Use in Specific Populations (8.1)]. Monitor neonates exposed to alprazolam extended-release tablets during pregnancy or labor for signs of sedation and monitor neonates exposed to alprazolam extended-release tablets during pregnancy for signs of withdrawal; manage these neonates accordingly.
5.9 Risks in Patients with Impaired Respiratory Function
There have been reports of death in patients with severe pulmonary disease shortly after the initiation of treatment with alprazolam. Closely monitor patients with impaired respiratory function. If signs and symptoms of respiratory depression, hypoventilation, or apnea occur, discontinue alprazolam extended-release tablets.
6 Adverse Reactions
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
- Abuse, Misuse, and Addiction [see Warnings and Precautions (5.2)]
- Dependence and Withdrawal Reactions [see Warnings and Precautions (5.3)]
- Effects on Driving and Operating Machinery [see Warnings and Precautions (5.4)]
- Patients with Depression [see Warnings and Precautions (5.7)]
- Neonatal Sedation and Withdrawal Syndrome [see Warnings and Precautions (5.8)]
- Risks in Patients with Impaired Respiratory Function [see Warnings and Precautions (5.9)]
The most common adverse reactions in panic disorder patients treated with alprazolam extended-release tablets (incidence of > 5% and at least twice that of placebo) include: somnolence, memory impairment, dysarthria, coordination abnormal, ataxia, libido decreased, constipation, and nausea. (6.1 ) To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The information included in the section on Adverse Reactions Observed in Short-Term, Placebo-Controlled Trials with alprazolam extended-release tablets are based on pooled data of five 6- and 8-week placebo-controlled clinical studies in panic disorder.
Adverse Reactions Observed in Short-Term, Placebo-Controlled Trials of Alprazolam Extended-Release Tablets
Adverse Reactions Reported as Reasons for Discontinuation of Treatment in Placebo-Controlled Trials
Approximately 17% of the 531 patients who received alprazolam extended-release tablets in placebo-controlled clinical trials for panic disorder had at least 1 adverse event that led to discontinuation compared to 8% of 349 placebo-treated patients. The most common events leading to discontinuation and considered to be drug-related (i.e., leading to discontinuation in at least 1% of the patients treated with alprazolam extended-release tablets at a rate at least twice that of placebo) are shown in Table 1.
Table 1: Adverse Reactions Leading to Discontinuation in ‚Č•1% of alprazolam extended-release tablets-treated Patients and at least twice the Rate of Placebo-treated Patients in Placebo-Controlled Trials n=number of patients ¬† Percentage of Patients Discontinuing Due to Adverse Reactions ¬† Alprazolam extended-release tablets (n=531) Placebo (n=349) Nervous system disorders ¬†¬†¬†¬†Sedation¬†¬†¬†¬†Somnolence¬†¬†¬†¬†Dysarthria¬†¬†¬†¬†Coordination abnormal¬†¬†¬†¬†Memory impairment 7.53.22.11.91.5 ¬†0.60.300.30.3 General disorders/administration site conditions ¬†¬†¬†¬†Fatigue ¬†1.7 ¬†0.6 Psychiatric disorders ¬†¬†¬†¬†Depression ¬†2.5 ¬†1.2
Adverse Reactions Occurring at an Incidence of 1% or More Among Patients Treated with Alprazolam Extended-Release Tablets
Table 2 shows the incidence of adverse reactions that occurred during 6- and 8-week placebo-controlled trials in 1% or more of patients treated with alprazolam extended-release tablets where the incidence in patients treated with alprazolam extended-release tablets was greater than the incidence in placebo-treated patients. The most commonly observed adverse reactions in panic disorder patients treated with alprazolam extended-release tablets (incidence of 5% or greater and at least twice the incidence in placebo patients) were: sedation, somnolence, memory impairment, dysarthria, coordination abnormal, ataxia, libido decreased.
Table 2: Adverse Reactions Occuring in¬†‚Č•1% in alprazolam-treated Patients and Greater than Placebo-treated Patients in 6 and 8 week Placebo-Controlled Trials Panic Disorder ¬† Alprazolam extended-release tablets (n=531) Placebo (n=349) Nervous system disorders ¬†¬†¬†¬†Sedation¬†¬†¬†¬†Somnolence¬†¬†¬†¬†Memory Impairment¬†¬†¬†¬†Dysartharia¬†¬†¬†¬†Coordination abnormal¬†¬†¬†¬†Mental impairment¬†¬†¬†¬†Ataxia¬†¬†¬†¬†Disturbance in attention¬†¬†¬†¬†Balance impaired¬†¬†¬†¬†Dyskinesia¬†¬†¬†¬†Hypoesthesia¬†¬†¬†¬†Hypersomnia ¬†45%23%15%11%9%7%7%3%3%2%1%1% ¬†23%6%7%3%1%6%3%1%1%1%<1%0% General disorders/administration site conditions ¬†¬†¬†¬†Fatigue¬†¬†¬†¬†Lethargy ¬†14%2% ¬†9%1% Psychiatric disorders¬† ¬†¬†¬†¬†Depression¬†¬†¬†¬†Libido decreased¬†¬†¬†¬†Disorientation¬†¬†¬†¬†Confusion¬†¬†¬†¬†Depressed mood ¬†12%6%2%2%1% ¬†9%2%0%1%<1% Metabolism and nutrition disorders ¬†¬†¬†¬†Appetite increased¬†¬†¬†¬†Anorexia ¬†7%2% ¬†6%0% Gastrointestinal disorders ¬†¬†¬†¬†Constipation¬†¬†¬†¬†Nausea ¬†8%6% ¬†4%3% Investigations ¬†¬†¬†¬†Weight increased ¬†5 ¬†4 Injury, poisoning, and procedural complications ¬†¬†¬†¬†Road traffic accident ¬†2% ¬†0% Reproductive system and breast disorders ¬†¬†¬†¬†Dysmenorrhea¬†¬†¬†¬†Sexual dysfunction ¬†4%2% ¬†3%1% Musculoskeletal and connective tissue disorder ¬†¬†¬†¬†Arthralgia¬†¬†¬†¬†Myalgia¬†¬†¬†¬†Pain in limb ¬†2%2%1% ¬†1%1%0% Respiratory, thoracic, and mediatinal disorders ¬†¬†¬†¬†Dyspnea ¬†2% ¬†0%
Other Adverse Reactions Observed During the Premarketing Evaluation of Alprazolam Extended-Release Tablets
Following is a ul of other adverse reaction reported by 531 patients with panic disorder treated with alprazolam extended-release tablets. Adverse reactions are further categorized by body system and uled in order of decreasing frequency according to the following definitions: those occurring in at least l/l00 patients (frequent); those occurring in less than l/100 patients but at least l/1000 patients (infrequent); those occurring in fewer than l/1000 patients (rare).
Cardiac disorders: Frequent: palpitation; Infrequent: sinus tachycardia
Ear and Labyrinth disorders:  Frequent: Vertigo; Infrequent: tinnitus, ear pain
Eye disorders:  Frequent: blurred vision; Infrequent: mydriasis, photophobia
Gastrointestinal disorders : Frequent: diarrhea, vomiting, dyspepsia, abdominal pain; Infrequent: dysphagia, salivary hypersecretion
General disorders and administration site conditions : Frequent: malaise, weakness, chest pains; Infrequent: fall, pyrexia, thirst, feeling hot and cold, edema, feeling jittery, sluggishness, asthenia, feeling drunk, chest tightness, increased energy, feeling of relaxation, hangover, loss of control of legs, rigors
Musculoskeletal and connective tissue disorders : Frequent: back pain, muscle cramps, muscle twitching
Nervous system disorders : Frequent: headache, dizziness, tremor; Infrequent: amnesia, clumsiness, syncope, hypotonia, seizures, depressed level of consciousness, sleep apnea syndrome, sleep talking, stupor
Psychiatric system disorders : Frequent: irritability, insomnia, nervousness, derealization, libido increased, restlessness, agitation, depersonalization, nightmare; Infrequent: abnormal dreams, apathy, aggression, anger, bradyphrenia, euphoric mood, logorrhea, mood swings, dysphonia, hallucination, homicidal ideation, mania, hypomania, impulse control, psychomotor retardation, suicidal ideation
Renal and urinary disorders : Frequent: difficulty in micturition; Infrequent: urinary frequency, urinary incontinence
Respiratory, thoracic, and mediastinal disorders : Frequent: nasal congestion, hyperventilation; Infrequent: choking sensation, epistaxis, rhinorrhea
Skin and subcutaneous tissue disorders : Frequent: sweating increased; Infrequent: clamminess, rash, urticaria
Vascular disorders : Infrequent: hypotension
Discontinuation-Emergent Adverse Reactions Occurring at an Incidence of 5% or More Among Patients Treated with Alprazolam Extended-Release Tablets
Table 3 shows the incidence of discontinuation-emergent adverse reactions that occurred during short-term, placebo-controlled trials in 5% or more of patients treated with alprazolam extended-release tablets where the incidence in patients treated with alprazolam extended-release tablets was 2 times greater than the incidence in placebo-treated patients.
Table 3: Discontinuation-Emergent Symptom Incidence Reported in ‚Č•5% of alprazolam extended-release tablets-treated Patients and at least twice the Rate of Placebo-treated Patients in Short-Term, Placebo-Controlled Trials ¬† Alprazolam extended-release tablets n=422 (%) Placebo n=261(%) Nervous system disorders ¬†¬†¬†¬†Tremor ¬†¬†¬†¬†Headache¬†¬†¬†¬†Hypoesthesia ¬†¬†¬†¬†Paraesthesia ¬†28.226.57.87.1 ¬†10.712.62.32.7 Psychiatric disorders ¬†¬†¬†¬†Insomnia¬†¬†¬†¬†Nervousness¬†¬†¬†¬†Depression¬†¬†¬†¬†Derealization¬†¬†¬†¬†Anxiety¬†¬†¬†¬†Depersonalization ¬†24.221.810.98.07.85.7 ¬†9.68.85.03.82.71.9 Gastrointestinal disorders ¬†¬†¬†¬†Diarrhea ¬†12.1 ¬†3.1 Respiratory, thoracic and mediastinal disorders ¬†¬†¬†¬†Hyperventilation ¬†8.5 ¬†2.7 Metabolism and nutrition disorders ¬†¬†¬†¬†Appetite decreased ¬†9.5 ¬†3.8 Musculosketal and connective tissue disorders ¬†¬†¬†¬†Muscle twitching ¬†7.4 ¬†2.7 Vascular disorders ¬†¬†¬†¬†Hot flushes ¬†5.9 ¬†2.7
There have also been reports of withdrawal seizures upon rapid decrease or abrupt discontinuation of alprazolam [see Warning and Precautions (5.2), Drug Abuse and Dependence (9.3)].
Paradoxical reactions such as stimulation, increased muscle spasticity, sleep disturbances, hallucinations, and other adverse behavioral effects such as agitation, rage, irritability, and aggressive or hostile behavior have been reported rarely. In many of the spontaneous case reports of adverse behavioral effects, patients were receiving other CNS drugs concomitantly and/or were described as having underlying psychiatric conditions. Should any of the above events occur, alprazolam should be discontinued. Isolated published reports involving small numbers of patients have suggested that patients who have borderline personality disorder, a prior history of violent or aggressive behavior, or alcohol or substance abuse may be at risk for such events. Instances of irritability, hostility, and intrusive thoughts have been reported during discontinuation of alprazolam in patients with posttraumatic stress disorder.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of alprazolam and/or alprazolam extended-release tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Endocrine disorders: Hyperprolactinemia
General disorders and administration site conditions: Edema peripheral
Hepatobiliary disorders: Hepatitis, hepatic failure, jaundice
Investigations: Liver enzyme elevations
Psychiatric disorders: Hypomania, mania
Reproductive system and breast disorders: Gynecomastia, galactorrhea, menstruation irregular
Skin and subcutaneous tissue disorders: Photosensitivity reaction, angioedema, Stevens-Johnson syndrome
7 Drug Interactions
- Use with Opioids: Increase the risk of respiratory depression. (
7.1 )- Use with Other CNS Depressants: Produces additive CNS depressant effects. (
7.1 )- Use with Digoxin: Increase the risk of digoxin toxicity. (
7.1 )- Use with CYP3A Inhibitors (except ritonavir): Increase the risk of adverse reactions of alprazolam. (
4 ,5.5 ,7.1 )- Use with CYP3A Inducers: Increase the risk of reduced efficacy of alprazolam. (
7.1 )7.1 Drugs Having Clinically Important Interactions with Alprazolam Extended-Release Tablets
Table 4 includes clinically significant drug interactions with alprazolam extended-release tablets [see Clinical Pharmacology (12.3)].
Table 4: Clinically Significant Drug Interactions with Alprazolam Extended-Release Tablets   Opioids Clinical implication The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at gamma-aminobutyric acid (GABAA) sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Prevention or management Limit dosage and duration of concomitant use of alprazolam extended-release tablets and opioids, and monitor patients closely for respiratory depression and sedation [see Warnings and Precautions (5.1)]. Examples Morphine, buprenorphine, hydromorphone, oxymorphone, oxycodone, fentanyl, methadone, alfentanil, butorpenol, codeine, dihydrocodeine, meperidine, pentazocine, remifentanil, sufentanil, tapentadol, tramadol. CNS Depressants Clinical implication The benzodiazepines, including alprazolam, produce additive CNS depressant effects when coadministered with other CNS depressants. Prevention or management Limit dosage and duration of alprazolam extended-release tablets during concomitant use with CNS depressants [see Warnings and Precautions (5.3)]. Examples Psychotropic medications, anticonvulsants, antihistaminics, ethanol, and other drugs which themselves produce CNS depression. Strong Inhibitors of CYP3A (except ritonavir) Clinical implication Concomitant use of alprazolam extended-release tablets with strong CYP3A inhibitors has a profound effect on the clearance of alprazolam, resulting in increased concentrations of alprazolam and increased risk of adverse reactions [see Clinical Pharmacology (12.3)]. Prevention or management Concomitant use of alprazolam extended-release tablets with a strong CYP3A4 inhibitor (except ritonavir) is contraindicated [see Contraindications (4), Warnings and Precautions (5.5)]. Examples Ketoconazole, itraconazole, clarithromycin Moderate or Weak Inhibitors of CYP3A Clinical implication Concomitant use of alprazolam extended-release tablets with CYP3A inhibitors may increase the concentrations of alprazolam extended-release tablets, resulting in increased risk of adverse reactions [see Clinical Pharmacology (12.3)].   Prevention or management Avoid use and consider appropriate dose reduction when alprazolam extended-release tablets are coadministered with a moderate or weak CYP3A inhibitor [see Warnings and Precautions (5.5)]. Examples Nefazodone, fluvoxamine, cimetidine, erythromycin CYP3A Inducers Clinical implication Concomitant use of CYP3A inducers can increase alprazolam metabolism and therefore can decease plasma levels of alprazolam [see Clinical Pharmacology (12.3)]. Prevention or management Caution is recommended during coadministration with alprazolam. Examples Carbamazepine, phenytoin Ritonavir Clinical implication Interactions involving ritonavir and alprazolam are complex and time dependent. Short term administration of ritonavir increased alprazolam exposure due to CYP3A4 inhibition. Following long term treatment of ritonavir (> 10 to 14 days), CYP3A4 induction offsets this inhibition. Alprazolam exposure was not meaningfully affected in the presence of ritonavir. Prevention or management Reduce alprazolam extended-release tablets dose when a patient is initiated with ritonavir and alprazolam extended-release tablets concomitantly, or when ritonavir is added to a regimen where alprazolam extended-release tablets are stabilized.  Increase alprazolam extended-release tablets dosage to the target dosage after 10 to 14 days of dosing ritonavir and alprazolam extended-release tablets concomitantly. No dosage adjustment of alprazolam extended-release tablets is necessary in patients receiving ritonavir for more than 10 to 14 days [see Dosage and Administration (2.5)].  Concomitant use of alprazolam extended-release tablets with a strong CYP3A inhibitor, except ritonavir, is contraindicated [see Contraindications (4), Warnings and Precautions (5.5)].   Digoxin   Clinical implication Increased digoxin concentrations have been reported when alprazolam was given, especially in geriatric patients (>65 years of age). Prevention or management In patients on digoxin therapy, measure serum digoxin concentrations before initiating alprazolam extended-release tablets. Continue monitoring digoxin serum concentration and toxicity frequently. Reduce the digoxin dose if necessary. 7.2 Drug/Laboratory Test Interactions
Although interactions between benzodiazepines and commonly employed clinical laboratory tests have occasionally been reported, there is no consistent pattern for a specific drug or specific test.
8 Use In Specific Populations
Lactation: Breastfeeding not recommended. (8.2 )
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to psychiatric medications, including alprazolam extended-release tablets during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/pregnancyregistry/.
Risk Summary
Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal [see Warnings and Precautions (5.8), and Clinical Considerations)]. Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects. The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal adverse reactions  
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia, and sedation in neonates. Monitor neonates exposed to alprazolam extended-release tablets during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to alprazolam extended-release tablets during pregnancy for signs of withdrawal. Manage these neonates accordingly [see Warnings and Precautions (5.8)].
Data 
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco, and other medications, have not confirmed these findings.
8.2 Lactation
Risk Summary
Limited data from published literature reports the presence of alprazolam in human breast milk. There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. The effects of alprazolam on lactation are unknown.
Because of the potential for serious adverse reactions, including sedation and withdrawal symptoms in breastfed infants, advise patients that breastfeeding is not recommended during treatment with alprazolam extended-release tablets. 
8.4 Pediatric Use
Safety and effectiveness of alprazolam extended-release tablets have not been established in pediatric patients.
8.5 Geriatric Use
Alprazolam extended-release tablets-treated geriatric patients had higher plasma concentrations of alprazolam (due to reduced clearance) compared to younger adults receiving the same doses. Therefore, dosage reduction of alprazolam extended-release tablets is recommended in geriatric patients [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
Patients with alcoholic liver disease exhibit a longer elimination half-life (19.7 hours), compared to healthy subjects (11.4 hours). This may be caused by decreased clearance of alprazolam in patients with alcoholic liver disease. Dosage reduction of alprazolam extended-release tablets is recommended in patients with hepatic impairment [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
9 Drug Abuse And Dependence
9.1 Controlled Substance
Alprazolam extended-release tablets contain alprazolam, which is a Schedule IV controlled substance.
9.2 Abuse
Alprazolam extended-release tablets are a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.  Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders [see Warnings and Precautions (5.2)].
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
9.3 Dependence
Physical Dependence
Alprazolam extended-release tablets may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use [see Warnings and Precautions (5.3)].
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam extended-release tablets or reduce the dosage [see Dosage and Administration (2.3), Warnings and Precautions (5.3)].
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used. Tolerance Tolerance to alprazolam extended-release tablets may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of alprazolam extended-release tablets may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
10 Overdosage
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, exclient, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal [see Warnings and Precautions (5.2)]. Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway management. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting the Poison Help Line at (1-800-222-1222), or a medical toxicologist for additional overdosage management recommendations.
11 Description
Alprazolam extended-release tablets USP contain alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.
The chemical name of alprazolam is 8-chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-őĪ] [1,4] benzodiazepine. The molecular formula is C17H13ClN4 which corresponds to a molecular weight of 308.76. ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†
The structural formula is represented below: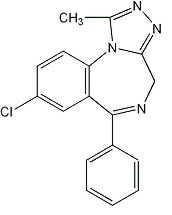
Alprazolam USP is a white to off-white, crystalline powder, which is soluble in methanol or ethanol but which has no appreciable solubility in water at physiological pH.
Each alprazolam extended-release tablet USP, for oral administration, contains 0.5 mg, 1 mg, 2 mg, or 3 mg of alprazolam USP. The inactive ingredients are colloidal silicon dioxide, hypromellose, lactose monohydrate, and magnesium stearate. In addition, the 1 mg and 3 mg tablets contain D&C Yellow No. 10 aluminum lake and the 2 mg and 3 mg tablets contain FD&C Blue No. 2 lake.
Meets USP Dissolution Test 4.
12 Clinical Pharmacology
12.1 Mechanism of Action
Alprazolam is a 1,4 benzodiazepine. Alprazolam exerts its effect for the treatment of panic disorder through binding to the benzodiazepine site of gamma-aminobutyric acid-A (GABAA) receptors in the brain and enhances GABA-mediated synaptic inhibition.
12.3 Pharmacokinetics
The pharmacokinetics of alprazolam and two of its major active metabolites (4-hydroxyalprazolam and őĪ-hydroxyalprazolam) are linear, and concentrations are proportional up to 10 mg alprazolam extended-release tablets given once daily.
 
Absorption   
Following oral administration of alprazolam extended-release tablets in the morning, peak plasma concentration of alprazolam (Cmax) occurs in about 10 hours postdose. Compared to morning dosing, alprazolam Cmax increased by 30% and the Tmax decreased by an hour following dosing at night.
The mean absolute bioavailability of alprazolam following administration of alprazolam extended-release tablets is approximately 90%, and the relative bioavailability compared to alprazolam is about 100%. The bioavailability and pharmacokinetics of alprazolam following administration of alprazolam extended-release tablets are similar to that for alprazolam, with the exception of a slower rate of absorption.
Effect of Food
A high-fat meal given up to 2 hours before dosing with alprazolam extended-release tablets increased the mean Cmax by about 25%. The effect of this meal on Tmax depended on the timing of the meal, with a reduction in Tmax by about 1/3 for subjects eating immediately before dosing and an increase in Tmax by about 1/3 for subjects eating 1 hour or more after dosing. The extent of exposure (AUC) and elimination half-life (t1/2) were not affected by eating.
Distribution  
The apparent volume of distribution of alprazolam is similar for alprazolam extended-release tablets and alprazolam. Alprazolam is 80% bound to human serum protein, and albumin accounts for the majority of the binding.
Elimination  
The mean plasma elimination half-life of alprazolam following administration of alprazolam extended-release tablets ranges from 10.7 to 15.8 hours in healthy adults.
Metabolism
Alprazolam is extensively metabolized in humans, primarily by cytochrome P450 3A4 (CYP3A4), to two major active metabolites in the plasma: 4-hydroxyalprazolam and őĪ-hydroxyalprazolam. The plasma circulation levels of the two active metabolites after both alprazolam extended-release tablets and alprazolam are less than 10% and 4% of the parent, respectively. The reported relative potencies in benzodiazepine receptor binding experiments and in animal models of induced seizure inhibition are 0.20 and 0.66, respectively, for 4-hydroxyalprazolam and őĪ-hydroxyalprazolam. The low concentrations and low potencies of 4-hydroxyalprazolam and őĪ-hydroxyalprazolam indicate that they unlikely contribute much to the effects of alprazolam. A benzophenone derived from alprazolam is also found in humans. Their half-lives appear to be similar to that of alprazolam. The pharmacokinetic parameters at steady-state for the two hydroxylated metabolites of alprazolam (4-hydroxyalprazolam and őĪ-hydroxyalprazolam) were similar for alprazolam and alprazolam extended-release tablets, indicating that the metabolism of alprazolam is not affected by absorption rate. Excretion
Alprazolam and its metabolites are excreted primarily in the urine. Specific Populations
Geriatric Patients 
The mean T1/2 of alprazolam was 16.3 hours (range: 9.0 to 26.9 hours) in healthy elderly subjects compared to 11.0 hours (range: 6.3 to 15.8 hours, n=16) in healthy adult subjects. Obese Patients
The mean T1/2 of alprazolam was 21.8 hours (range: 9.9 to 40.4 hours) in a group of obese subjects.   
Patients with Hepatic Impairment
The mean T1/2 of alprazolam was 19.7 hours (range: 5.8 to 65.3 hours) in patients with alcoholic liver disease. 
Racial or Ethnic Groups
Maximal concentrations and T1/2 of alprazolam are approximately 15% and 25% higher in Asians compared to Caucasians.
Smoking
Alprazolam concentrations may be reduced by up to 50% in smokers compared to non-smokers.  
Drug Interaction Studies
In Vivo Studies
Most of the interactions that have been documented with alprazolam are with drugs that modulate CYP3A4 activity.  
Compounds that are inhibitors or inducers of CYP3A would be expected to increase or decrease plasma alprazolam concentrations, respectively. Drug products that have been studied in vivo, along with their effect on increasing alprazolam AUC, are as follows: ketoconazole, 3.98 fold; itraconazole, 2.66 fold; nefazodone, 1.98 fold; fluvoxamine, 1.96 fold; and erythromycin, 1.61 fold [see Contraindications (4), Warnings and Precautions (5.5), Drug Interactions (7.2)]. Other studied drugs include:
Cimetidine : Coadministration of cimetidine increased the maximum plasma concentration of alprazolam by 82%, decreased clearance by 42%, and increased T1/2 by 16%.
Fluoxetine : Coadministration of fluoxetine with alprazolam increased the maximum plasma concentration of alprazolam by 46%, decreased clearance by 21%, increased T1/2 by 17%, and decreased measured psychomotor performance. Oral Contraceptives : Coadministration of oral contraceptives increased the maximum plasma concentration of alprazolam by 18%, decreased clearance by 22%, and increased T1/2 by 29%. Carbamazepine : The oral clearance of alprazolam (given in a 0.8 mg single dose) was increased from 0.90¬Ī0.21 mL/min/kg to 2.13¬Ī0.54 mL/min/kg and the elimination T1/2 was shortened (from 17.1¬Ī4.9 to 7.7¬Ī1.7 hour) following administration of 300 mg per day carbamazepine for 10 days [see Drug Interactions (7.2)]. However, the carbamazepine dose used in this study was fairly low compared to the recommended doses (1000 to 1200 mg per day); the effect at usual carbamazepine doses is unknown.
Ritonavir : Interactions involving HIV protease inhibitors (e.g., ritonavir) and alprazolam are complex and time dependent. Short-term low doses of ritonavir (4 doses of 200 mg) increased mean AUC of alprazolam by about 2.5-fold, and did not significantly affect Cmax of alprazolam. The elimination T1/2 was prolonged (30 hours versus 13 hours). However, upon extended exposure to ritonavir (500 mg, twice daily for 10 days), CYP3A induction offset this inhibition. Alprazolam AUC and Cmax was reduced by 12% and 16%, respectively, in the presence of ritonavir. The elimination T1/2 of alprazolam was not significantly changed [see Warnings and Precautions (5.5)].
Sertraline : A single dose of alprazolam 1 mg and steady state dose of sertraline (50 to 150 mg per day) did not reveal any clinically significant changes in the pharmacokinetics of alprazolam.
Imipramine and Desipramine : The steady state plasma concentrations of imipramine and desipramine have been reported to be increased an average of 31% and 20%, respectively, by the concomitant administration of alprazolam in doses up to 4 mg per day. 
Warfarin : Alprazolam did not affect the prothrombin or plasma warfarin levels in male volunteers administered sodium warfarin orally. In Vitro Studies Data from in vitro studies of alprazolam suggest a possible drug interaction of alprazolam with paroxetine. The ability of alprazolam to induce human hepatic enzyme systems has not been determined.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No evidence of carcinogenic potential was observed in rats or mice administered alprazolam for 2 years at doses up to 30 and 10 mg/kg/day, respectively. These doses are 29 times and 4.8 times the maximum recommended human dose of 10 mg/day based on mg/m2 body surface area, respectively.
Mutagenesis
Alprazolam was negative in the in vitro Ames bacterial reverse mutation assay and DNA Damage/Alkaline Elution Assay and in vivo rat micronucleus genetic toxicology assays.
Impairment of Fertility
Alprazolam produced no impairment of fertility in rats at doses up to 5 mg/kg per day, which is approximately 5 times the maximum recommended human dose of 10 mg per day based on mg/m2 body surface area.
13.2 Animal Toxicology and/or Pharmacology
When rats were treated with alprazolam at oral doses of 3 mg/kg, 10 mg/kg, and 30 mg/kg per day (3 to 29 times the maximum recommended human dose based on mg/m2 body surface area) for 2 years, a tendency for a dose related increase in the number of cataracts was observed in females and a tendency for a dose related increase in corneal vascularization was observed in males. These lesions did not appear until after 11 months of treatment.
14 Clinical Studies
The efficacy of alprazolam extended-release tablets in the treatment of panic disorder in adults was established in two 6-week, flexible-dose, placebo-controlled studies in adult patients meeting DSM-III criteria for panic disorder. In these studies, patients were treated with alprazolam extended-release tablets in a dose range of 1 mg to 10 mg once per day. The effectiveness of alprazolam extended-release tablets was assessed on the basis of changes in various measures of panic attack frequency, on various measures of the Clinical Global Impression, and on the Overall Phobia Scale. In all, there were 7 primary efficacy measures in these studies, and alprazolam extended-release tablets was superior to placebo on all 7 outcomes in both studies. The mean dose of alprazolam extended-release tablets at the last treatment visit was 4.2 mg per day in the first study and 4.6 mg per day in the second.
In addition, there were two 8-week, fixed-dose, placebo-controlled studies of alprazolam extended-release tablets in adult patients with panic disorder, involving fixed alprazolam extended-release tablets doses of 4 mg and 6 mg/ once per day that did not show a benefit for either dose of alprazolam extended-release tablets.
Analyses of the relationship between treatment outcome and gender did not suggest any differential responsiveness on the basis of gender.
16 How Supplied/storage And Handling
Alprazolam Extended-Release Tablets USP are supplied in the following strengths and package configurations: Alprazolam Extended-Release Tablets USP, 0.5 mg are white to off-white, round, biconvex tablets with beveled edge debossed with ‚ÄėX‚Äô on one side and ‚Äė70‚Äô on the other side. ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 60¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†NDC 65862-454-60¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 1,000¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† NDC 65862-454-99¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 7,000¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† NDC 65862-454-71 Alprazolam Extended-Release Tablets USP, 1 mg are yellow colored, round, biconvex tablets with beveled edge debossed with ‚ÄėX‚Äô on one side and ‚Äė73‚Äô on the other side. The tablets may be mottled.¬† ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 60¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†NDC 65862-455-60¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 1,000¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† NDC 65862-455-99¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 7,000¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† NDC 65862-455-71 Alprazolam Extended-Release Tablets USP, 2 mg are blue colored, round, biconvex tablets with beveled edge debossed with ‚ÄėX‚Äô on one side and ‚Äė74‚Äô on the other side. The tablets may be mottled.¬† ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 60¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† NDC 65862-456-60¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 1,000¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† NDC 65862-456-99¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 7,000¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† NDC 65862-456-71 Alprazolam Extended-Release Tablets USP, 3 mg are green colored, round, biconvex tablets with beveled edge debossed with ‚ÄėX‚Äô on one side and ‚Äė75‚Äô on the other side. The tablets may be mottled.¬† ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 60¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† NDC 65862-457-60¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 1,000¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† NDC 65862-457-99¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Bottles of 7,000¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† NDC 65862-457-71 Store at 20¬į to 25¬įC (68¬į to 77¬įF); excursions permitted to 15¬į to 30¬įC (59¬į to 86¬įF) [see USP Controlled Room Temperature].
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Risks from Concomitant Use with Opioids
Advise both patients and caregivers about the risks of potentially fatal respiratory depression and sedation when alprazolam extended-release tablets are used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined [see Warnings and Precautions (5.1), Drug Interactions (7.1)]. Abuse, Misuse, and Addiction
Inform patients that the use of alprazolam extended-release tablets, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug [see Warnings and Precautions (5.2), Drug Abuse and Dependence (9.2)].
Withdrawal Reactions
Inform patients that the continued use of alprazolam extended-release tablets may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of alprazolam extended-release tablets may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of alprazolam extended-release tablets may require a slow taper [see Warnings and Precautions (5.3), Drug Abuse and Dependence (9.3)].
Effects on Driving and Operating Machinery
Advise patients not to drive a motor vehicle or operate heavy machinery while taking alprazolam extended-release tablets due to its CNS depressant effects. Also advise patients to avoid use of alcohol or other CNS depressants while taking alprazolam extended-release tablets [see Warnings and Precautions (5.3)].
Patients with Depression
Advise patients, their families and caregivers to look for signs of suicidality or worsening depression, and to inform the patient’s healthcare provider immediately [see Warnings and Precautions (5.6)].
Concomitant Medications
Advise patients to inform their healthcare provider of all medicines they take, including prescription and nonprescription medications, vitamins and herbal supplements [see Drug Interactions (7)]. Pregnancy Advise pregnant females that use of alprazolam extended-release tablets late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns [see Warnings and Precautions (5.8), Use in Specific Populations (8.1)]. Instruct patients to inform their healthcare provider if they are pregnant. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to alprazolam extended-release tablets during pregnancy [see Use in Specific Populations (8.1)]. Lactation Advise patients that breastfeeding is not recommended during treatment with alprazolam extended-release tablets [see Use in Specific Populations (8.2)]. Dispense with Medication Guide available at: www.aurobindousa.com/medication-guides.
Spl Medguide Section
MEDICATION GUIDE Alprazolam Extended-Release Tablets USP CIV (al pra' zoe lam) What is the most important information I should know about alprazolam extended-release tablets? Do not drive or operate heavy machinery until you know how taking alprazolam extended-release tablets with opioids affects you.
- Alprazolam extended-release tablets are a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death. Get emergency help right away if any of the following happens:  
- shallow or slowed breathing 
- breathing stops (which may lead to the heart stopping)
- excessive sleepiness (sedation)
   
- Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines including alprazolam extended-release tablets which can lead to overdose and serious side effects including coma and death.  
- Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including alprazolam extended-release tablets. These serious side effects may also include delirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.
- You can develop an addiction even if you take alprazolam extended-release tablets as prescribed by your healthcare provider.
- Take alprazolam extended-release tablets exactly as your healthcare provider prescribed.
- Do not share your alprazolam extended-release tablets with other people.
- Keep alprazolam extended-release tablets in a safe place and away from children.
- Physical dependence and withdrawal reactions. Alprazolam extended-release tablets can cause physical dependence and withdrawal reactions.  
- Do not suddenly stop taking alprazolam extended-release tablets. Stopping alprazolam extended-release tablets suddenly can cause serious and life-threatening side effects, including, unusual movements, responses, or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of the following symptoms.
- Some people who suddenly stop benzodiazepines, have symptoms that can last for several weeks to more than 12 months, including, anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.
- Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
- Do not take more alprazolam extended-release tablets than prescribed or take alprazolam extended-release tablets for longer than prescribed.
What are alprazolam extended-release tablets?
- Alprazolam extended-release tablets are a prescription medicine used to treat panic disorder, with or without a fear of places and situations that might cause panic, helplessness, or embarrassment (agoraphobia)
- Alprazolam extended-release tablets are a federal controlled substance (C-IV) because it contains alprazolam that can be abused or lead to dependence. Keep alprazolam extended-release tablets in a safe place to prevent misuse and abuse. Selling or giving away alprazolam extended-release tablets may harm others and is against the law. Tell your healthcare provider if you have abused or been dependent on alcohol, prescription medicines or street drugs.
- It is not known if alprazolam extended-release tablets are safe and effective in children.
- Elderly patients are especially susceptible to dose related adverse effects when taking alprazolam extended-release tablets.
- It is not known if alprazolam extended-release tablets are safe and effective in the treatment of panic disorder for use longer than 8 weeks.
Do not take alprazolam extended-release tablets if:
- you are allergic to alprazolam, other benzodiazepines, or any of the ingredients in alprazolam extended-release tablets. See the end of this Medication Guide for a complete ul of ingredients in alprazolam extended-release tablets.
- you are taking antifungal medicines including ketoconazole and itraconazole
Before you take alprazolam extended-release tablets, tell your healthcare provider about all of your medical conditions, including if you: Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking alprazolam extended-release tablets with certain other medicines can cause side effects or affect how well alprazolam extended-release tablets or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.  
- have or have had depression, mood problems, or suicidal thoughts or behavior
- have liver or kidney problems
- have lung disease or breathing problems
- are pregnant or plan to become pregnant.
- Taking alprazolam extended-release tablets late in pregnancy may cause your baby to have symptoms of sedation (breathing problems, sluggishness, low muscle tone), and/or withdrawal symptoms (jitteriness, irritability, restlessness, shaking, excessive crying, feeding problems).
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with alprazolam extended-release tablets.
- There is a pregnancy registry for women who take alprazolam extended-release tablets during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. If you become pregnant during treatment with alprazolam extended-release tablets, talk to your healthcare provider about registering with the National Pregnancy Registry for Psychiatric Medications. You can register by calling 1-866-961-2388 or visiting https://womensmentalhealth.org/research/pregnancyregistry/.
- are breastfeeding or plan to breastfeed. Alprazolam passes into your breast milk.
- Talk to your healthcare provider about the best way to feed your baby if you take alprazolam extended-release tablets.
- Breastfeeding is not recommended during treatment with alprazolam extended-release tablets.
How should I take alprazolam extended-release tablets?
- See ‚ÄúWhat is the most important information I should know about alprazolam extended-release tablets?‚ÄĚ
- Take alprazolam extended-release tablets exactly as your healthcare provider tells you to take it. Your healthcare provider will tell you how much alprazolam extended-release tablets to take and when to take it.
- If you take too much alprazolam, call your healthcare provider or go to the nearest hospital emergency room right away.
- Swallow alprazolam extended-release tablets whole. Do not crush, chew or break alprazolam extended-release tablets.
What are the possible side effects of alprazolam extended-release tablets? Alprazolam extended-release tablets may cause serious side effects, including: The most common side effects of alprazolam extended-release tablets include:  
- See ‚ÄúWhat is the most important information I should know about alprazolam extended-release tablets?‚ÄĚ
- Seizures. Stopping alprazolam extended-release tablets can cause seizures and seizures that will not stop (status epilepticus).
- Mania. Alprazolam extended-release tablets may cause an increase in activity and talking (hypomania and mania) in people who have depression.
- Alprazolam extended-release tablets can make you sleepy or dizzy and can slow your thinking and motor skills.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how alprazolam extended-release tablets affects you.
- Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking alprazolam extended-release tablets without first talking to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, alprazolam extended-release tablets may make your sleepiness or dizziness much worse.
These are not all the possible side effects of alprazolam extended-release tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
- sleepiness
- changes in sex drive (libido)
- trouble saying words clearly (dysarthria)
- constipation
- problems with memory
- nausea
- problems with coordination
How should I store alprazolam extended-release tablets?
- Store alprazolam extended-release tablets at room temperature between 68¬įF to 77¬įF (20¬įC to 25¬įC)
- Keep alprazolam extended-release tablets and all medicines out of the reach of children.
- General information about the safe and effective use of alprazolam extended-release tablets.
- Medicines are sometimes prescribed for purposes other than those uled in a Medication Guide.
- Do not use alprazolam extended-release tablets for a condition for which it was not prescribed.
- Do not give alprazolam extended-release tablets to other people, even if they have the same symptoms that you have. It may harm them.
- You can ask your pharmacist or healthcare provider for information about alprazolam extended-release tablets that is written for health professionals.
What are the ingredients in alprazolam extended-release tablets? Active ingredient: alprazolam   Inactive ingredients: colloidal silicon dioxide, hypromellose, lactose monohydrate, and magnesium stearate. In addition, the 1 mg and 3 mg tablets contain D&C Yellow No. 10 aluminum lake and the 2 mg and 3 mg tablets contain FD&C Blue No. 2 lake.  For more information, call Aurobindo Pharma USA, Inc. at 1-866-850-2876  Dispense with Medication Guide available at: www.aurobindousa.com/medication-guides.  Distributed by: Aurobindo Pharma USA, Inc. 279 Princeton-Hightstown RoadEast Windsor, NJ 08520 Manufactured by: Aurobindo Pharma Limited Hyderabad-500 032, India
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 03/2023
Package Label-principal Display Panel - 0.5 Mg (60 Tablet Bottle)
NDC 65862-454-60 Rx only Alprazolam CIV Extended-Release Tablets USP 0.5 mgPHARMACIST: Dispense the Medication Guide provided separately to each patient. AUROBINDO                            60 Tablets 
Package Label-principal Display Panel -1 Mg (60 Tablet Bottle)
NDC 65862-455-60 Rx only Alprazolam CIV  Extended-Release Tablets USP 1 mgPHARMACIST: Dispense the Medication Guide provided separately to each patient.   AUROBINDO                             60 Tablets 
Package Label-principal Display Panel - 2 Mg (60 Tablet Bottle)
NDC 65862-456-60 Rx only Alprazolam CIV Extended-Release Tablets USP 2 mgPHARMACIST: Dispense the Medication Guide provided separately to each patient.  AUROBINDO                            60 Tablets 
Package Label-principal Display Panel - 3 Mg (60 Tablet Bottle)
NDC 65862-457-60 Rx only Alprazolam CIV Extended-Release Tablets USP 3 mgPHARMACIST: Dispense the Medication Guide provided separately to each patient.  AUROBINDO                            60 Tablets 
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


