Amiglyde-V Dailymed
Generic: amikacin sulfate
Go PRO for all pill images
Caution
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Description
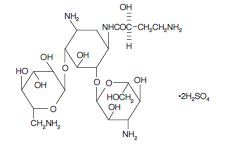
Amikacin sulfate is a semi-synthetic aminoglycosideantibiotic derived from kanamycin. It is C22H43N5O13•2H2SO4, D-streptamine, 0-3-amino-3-deoxy-α-D-glucopyranosyl-(1→6)-0-[6-amino-6- deoxy-α-D-glucopyranosyl-(1→4)]-N1-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-, (S)-, sulfate (1:2) (salt).
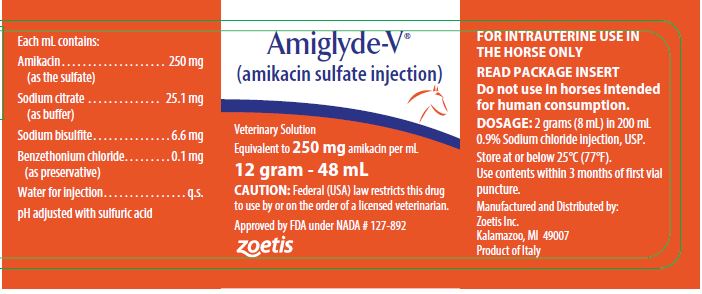
The dosage form supplied is a sterile, colorless solution.The solution contains, in addition to amikacin sulfate, USP, 2.5% sodium citrate, USP with pH adjusted to 4.5 with sulfuric acid and 0.66% sodium bisulfite added. The multi-dose 12 gram–48 mL vial contains 0.01% benzethonium chloride, USP as a preservative.
Action
Antibacterial Activity The effectiveness of AMIGLYDE-V (amikacin sulfate injection) in infections caused by Escherichia coli, Pseudomonas sp and Klebsiella sp has been demonstrated clinically in the horse. In addition, the following microorganisms have been shown to be susceptible to amikacin in vitro1, although the clinical significance of this action has not been demonstrated in animals: • Enterobacter sp• Proteus mirabilis• Proteus sp (indole positive)• Serratia marcescens• Salmonella sp• Shigella sp• Providencia sp• Citrobacter freundii• Listeria monocytogenesureus (both penicillin-resistant and penicillin-sensitive)
The aminoglycoside antibiotics in general have limitedactivity against gram-positive pathogens, although Staphylococcus aureus and Listeria monocytogenes are susceptible to amikacin as noted above.
Amikacin has been shown to be effective against
many aminoglycoside-resistant strains due to its
ability to resist degradation by aminoglycoside
inactivating enzymes known to affect gentamicin,
tobramycin and kanamycin2.
Clinical Pharmacology
Endometrial Tissue Concentrations Comparisons of amikacin activity in endometrial biopsy tissue following intrauterine infusion with that following intramuscular injection of AMIGLYDE-V in mares demonstrate superior endometrial tissue concentrations when the drug is administered bythe intrauterine route.Intrauterine infusion of 2 grams AMIGLYDE-V daily for three consecutive days in mares results in peak concentrations typically exceeding 40 mcg/g of endometrial biopsy tissue within one hour after infusion. Twenty-four hours after each treatment amikacin activity is still detectable at concentrations averaging 2 to 4 mcg/g. However, the drug is not appreciably absorbed systemically following intrauterine infusion. Endometrial tissue concentrations following intramuscular injection are roughly parallel, but are typically somewhat lowerthan corresponding serum concentrations of amikacin.
Safety
AMIGLYDE-V is non-irritating to equine endometrialtissue when infused into the uterus as directed (see ADMINISTRATION AND DOSAGE). In laboratoryanimals as well as equine studies, the drug was generally found not to be irritating when injected intravenously, subcutaneously or intramuscularly.Although amikacin, like other aminoglycosides, is potentially nephrotoxic, ototoxic and neurotoxic, parenteral (intravenous) administration of AMIGLYDE-V (amikacin sulfate injection) twice daily at dosages of up to 10 mg/lb for 15 consecutive days in horses resulted in no clinical, laboratory or histopathologic evidence of toxicity.Intrauterine infusion of 2 grams of AMIGLYDE-V8 hours prior to breeding by natural service did not impair fertility in mares. Therefore, mares should not be bred for at least 8 hours following uterine infusion.
Indications
AMIGLYDE-V is indicated for the treatment of uterineinfections (endometritis, metritis and pyometra) in mares, when caused by susceptible organisms including Escherichia coli, Pseudomonas sp and Klebsiella sp. The use of AMIGLYDE-V in eliminating infections caused by the above organisms has been shown clinically to improve fertility in infected mares.While nearly all strains of Escherichia coli, Pseudomonas sp and Klebsiella sp, including those that are resistant to gentamicin, kanamycin or otheraminoglycosides, are susceptible to amikacin at levels achieved following treatment, it is recommended that the invading organism be cultured and its susceptibility demonstrated as a guide to therapy. Amikacin susceptibility discs, 30 mcg, should be used for determining in vitro susceptibility.
Administrationand Dosage
For treatment of uterine infections in mares, 2 grams(8 mL) of AMIGLYDE-V, mixed with 200 mL 0.9% Sodium chloride injection, USP and aseptically infused into the uterus daily for three consecutive days, has been found to be the most efficacious dosage.
Contraindications
There are no known contraindications for the use ofAMIGLYDE-V in horses other than a history of hypersensitivity to amikacin.
Precautions
Although AMIGLYDE-V is not absorbed to an appreciableextent following intrauterine infusion, concurrent use of other aminoglycosides should               be avoided because of the potential for additive effects.
.
Adverse Reactions
No adverse reactions or other side effects have been reported.
Warnings
Do not use in horses intended for human consumption.
In vitro studies have demonstrated that when sperm are exposed to the preservative which is present in the 48 mL
vials (250 mg/mL) sperm viability is impaired.
How Supplied
AMIGLYDE-V (amikacin sulfate injection) Veterinary
Solution is supplied as a colorless solution which is stable
when stored at or below 25°C (77°F). Use contents within
3 months of first vial puncture.
48 mL vial, 250 mg/mL
Store at or below 25°C (77°F).
References
1. Price, K.E., et al: Microbiological Evaluation of BB-K8, a New Semisynthetic Aminoglycoside. J Antibiot 25: 709–731, 1972.
2. Davies, J., Courvalin, P.: Mechanisms of Resistance to Aminoglycosides. Am J Med 62: 868–872, 1977.
Approved by FDA under NADA # 127-892 zoetis Manufactured and Distributed by:Zoetis Inc.Kalamazoo, MI 49007
Revised: July 2019
40028299
Principal Display Panel - Carton Label
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site