Arazlo Dailymed
Generic: tazarotene is used for the treatment of Acne Vulgaris Pregnancy Psoriasis
Go PRO for all pill images
1 Indications And Usage
ARAZLO ®(tazarotene) lotion, 0.045% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
ARAZLO is a retinoid indicated for the topical treatment of acne vulgaris in patients 9 years of age and older. (1 )
2 Dosage And Administration
Apply a thin layer of ARAZLO to the affected areas once daily. Avoid the eyes, mouth, paranasal creases, and mucous membranes. If ARAZLO gets in or near eyes, rinse thoroughly with water.
ARAZLO is for topical use only. Not for oral, ophthalmic, or intravaginal use.
Wash hands thoroughly after applying ARAZLO.
Avoid concomitant use with oxidizing agents, such as benzoyl peroxide. If the concomitant use of ARAZLO with oxidizing agents is required, apply each at different times of the day (e.g. one in the morning and the other in the evening) [see Drug Interactions (7)] .
Use effective sunscreens and wear protective clothing while using ARAZLO [see Warnings and Precautions (5.3)] .
- Apply a thin layer of ARAZLO to the affected areas once daily. Avoid the eyes, mouth, paranasal creases, and mucous membranes. (
2 )- Not for oral, ophthalmic, or intravaginal use. (
2 )
3 Dosage Forms And Strengths
Lotion, 0.045%
Each gram of ARAZLO contains 0.45 mg (0.045%) tazarotene in a white to off-white topical lotion.
Lotion, 0.045% (3 )
4 Contraindications
ARAZLO is contraindicated in pregnancy. ARAZLO may cause fetal harm when administered to a pregnant patient [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)] .
ARAZLO is contraindicated in pregnancy. (4 ,8.1 )
5 Warnings And Precautions
- Embryofetal Toxicity: May cause fetal harm when administered during pregnancy. Patients of childbearing potential should have a negative pregnancy test within 2 weeks prior to initiating treatment and use effective contraception during treatment. (
5.1 )- Skin Irritation: Pain, dryness, exfoliation, erythema, and pruritus may occur with use of ARAZLO. Avoid application to eczematous or sunburned skin. (
5.2 )- Photosensitivity and Risk for Sunburn: Minimize exposure to sunlight and sunlamps. Use sunscreen and protective clothing when sun exposure cannot be avoided. Administer with caution if the patient is also taking drugs known to be photosensitizers. (
5.3 )5.1 Embryofetal Toxicity
Based on data from animal reproduction studies, retinoid pharmacology and the potential for systemic absorption, ARAZLO may cause fetal harm when administered to a pregnant patient and is contraindicated during pregnancy .Safety in pregnant patients has not been established. The potential risk to the fetus outweighs the potential benefit to the mother; therefore, discontinue ARAZLO as soon as pregnancy is recognized.
Tazarotene elicits malformations and developmental effects associated with retinoids after topical and oral administration to pregnant rats and rabbits during organogenesis. However, limited case reports of pregnancy in females enrolled in clinical trials for ARAZLO have not reported a clear association with tazarotene and major birth defects or miscarriage risk [see Contraindications (4), Use in Specific Populations (8.1)] .
Systemic exposure to tazarotenic acid is dependent upon the extent of the body surface area treated. In patients treated topically over sufficient body surface area, exposure could be in the same order of magnitude as in orally treated animals. Tazarotene is a teratogenic substance in animals, and it is not known what level of exposure is required for teratogenicity in humans.
Advise pregnant patients of the potential risk to a fetus. Obtain a pregnancy test within 2 weeks prior to ARAZLO therapy. Initiate ARAZLO therapy during a menstrual period. Advise patients of childbearing potential to use effective contraception during treatment with ARAZLO [see Dosage and Administration (2), Use in Specific Populations (8.3)] .
5.2 Skin Irritation
Patients using ARAZLO may experience application site pain, dryness, exfoliation, erythema, and pruritus. Depending upon severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of the application of ARAZLO, or discontinue use. Therapy can be resumed, or the frequency of application can be increased, as the patient becomes able to tolerate treatment.
Avoid use of concomitant medications and cosmetics that have a strong drying effect. It is recommended to postpone treatment with ARAZLO until the drying effects of these products subside.
Avoid application of ARAZLO to eczematous or sunburned skin.
5.3 Photosensitivity and Risk for Sunburn
Because of heightened burning susceptibility, minimize unprotected exposure to ultraviolet light including sunlight and sunlamps during the use of ARAZLO. Warn patients who normally experience high levels of sun exposure and those with inherent sensitivity to sun to exercise caution. Use sunscreen products and protective clothing over treated areas when sun exposure cannot be avoided. Patients with sunburn should be advised not to use ARAZLO until fully recovered.
ARAZLO should be administered with caution if the patient is taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the increased possibility of augmented photosensitivity.
Weather extremes, such as wind or cold, may be more irritating to patients using ARAZLO.
6 Adverse Reactions
The following serious adverse reactions are discussed in more detail in other sections of the labeling:
- Embryofetal toxicity [see Warnings and Precautions (5.1)]
- Photosensitivity and Risk of Sunburn [see Warnings and Precautions (5.3)]
- The most common adverse reactions (occurring in ≥1% of the ARAZLO group and greater than the vehicle group) were application site reactions; pain, dryness, exfoliation, erythema and pruritus. (
6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In 2 multicenter, randomized, double-blind, vehicle-controlled clinical trials, subjects age 9 years and older applied ARAZLO or vehicle once daily for 12 weeks. The majority of subjects were White (74%) and female (66%). Approximately 22% were Hispanic/Latino and 42% were younger than 18 years of age, fourteen of 779 subjects (1.8%) treated with ARAZLO were between 9 years to less than 12 years of age. Adverse reactions reported by ≥1% of subjects treated with ARAZLO and more frequently than subjects treated with vehicle are summarized in Table 1. Most adverse reactions were mild to moderate in severity. Severe adverse reactions represented 1.3% of the subjects treated. Overall, 2.4% (19/779) of subjects discontinued ARAZLO because of local skin reactions.
Table 1:Adverse Reactions Reported by ≥1% of the ARAZLO Group and More Frequently than the Vehicle Group
Adverse Reactions N (%) ARAZLO N=779 Vehicle N=791
Application site painApplication site pain defined as application site stinging, burning or pain
41 (5)
2 (<1)
Application site dryness
30 (4)
1 (<1)
Application site exfoliation
16 (2)
0 (0)
Application site erythema
15 (2)
0 (0)
Application site pruritus
10 (1)
0 (0)
Skin irritation was evaluated by active assessment of erythema, scaling, itching, burning and stinging, with grades for none, mild, moderate, or severe. The maximum severity generally peaked at Week 2 of therapy and decreased thereafter. The percentage of subjects with these signs and symptoms at any post-baseline visit are summarized in Table 2.
Table 2: Incidence of Local Cutaneous Irritation at any Post-Baseline Visit
ARAZLO Lotion
N=774
Mild/Moderate/Severe
Vehicle Lotion
N=789
Mild/Moderate/Severe
Erythema
49%
38%
Scaling
51%
23%
Itching
29%
14%
Burning
30%
6%
Stinging
22%
5%
7 Drug Interactions
No formal drug-drug interaction studies were conducted with ARAZLO.
Concomitant use with oxidizing agents, as benzoyl peroxide, may cause degradation of tazarotene and may reduce the clinical efficacy of tazarotene.
In a trial of 27 healthy female subjects, between the ages of 20–55 years, receiving a combination oral contraceptive tablet containing 1 mg norethindrone and 35 mcg ethinyl estradiol, the concomitant use of tazarotene administered as 1.1 mg orally (mean ± SD C maxand AUC 0-24of tazarotenic acid were 28.9 ± 9.4 ng/mL and 120.6 ± 28.5 ng•hr/mL, respectively) did not affect the pharmacokinetics of norethindrone and ethinyl estradiol over a complete cycle.
The impact of tazarotene on the pharmacokinetics of progestin only oral contraceptives (i.e., minipills) has not been evaluated.
8 Use In Specific Populations
8.1 Pregnancy
Risk Summary
ARAZLO is contraindicated in pregnancy.
There are no available data on ARAZLO use in pregnant patients to inform a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Based on data from animal reproduction studies, retinoid pharmacology, and the potential for systemic absorption, ARAZLO may cause fetal harm when administered to a pregnant patient and is contraindicated during pregnancy. The potential risk to the fetus outweighs the potential benefit to the mother; therefore, ARAZLO should be discontinued as soon as pregnancy is recognized.
In animal reproduction studies with pregnant rats, reduced fetal body weights and reduced skeletal ossification were observed after topical administration of a tazarotene gel formulation during the period of organogenesis at a dose equivalent to the maximum recommended human dose (MRHD) (based on AUC comparison). In animal reproduction studies with pregnant rabbits, single incidences of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies were observed after topical administration of a tazarotene gel formulation at 15 times the MRHD (based on AUC comparison) (see Data).
In animal reproduction studies with pregnant rats and rabbits, malformations, fetal toxicity, developmental delays, and/or behavioral delays were observed after oral administration of tazarotene during the period of organogenesis at doses 1 and 30 times, respectively, the MRHD (based on AUC comparison). In pregnant rats, decreased litter size, decreased numbers of live fetuses, decreased fetal body weights, and increased malformations were observed after oral administration of tazarotene prior to mating through early gestation at doses 6 times the MRHD (based on AUC comparison) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of major birth defects, loss, and other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In an embryofetal development study in rats, a tazarotene gel formulation, 0.5% (0.25 mg/kg/day tazarotene) was topically administered to pregnant rats during gestation days 6 through 17. Reduced fetal body weights and reduced skeletal ossification occurred at this dose (equivalent to the MRHD based on AUC comparison). In an embryofetal development study in rabbits, a tazarotene gel formulation, 0.5% (0.25 mg/kg/day tazarotene) was topically administered to pregnant rabbits during gestation days 6 through 18. Single incidences of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies were noted at this dose (15 times the MRHD based on AUC comparison).
When tazarotene was given orally to animals, developmental delays were seen in rats; malformations and post- implantation loss were observed in rats and rabbits at doses producing 1 and 30 times, respectively, the MRHD (based on AUC comparison).
In female rats orally administered 2 mg/kg/day of tazarotene from 15 days before mating through gestation day 7, classic developmental effects of retinoids including decreased number of implantation sites, decreased litter size, decreased numbers of live fetuses, and decreased fetal body weights were observed at this dose (6 times the MRHD based on AUC comparison). A low incidence of retinoid-related malformations was observed at this dose.
In a pre- and postnatal development toxicity study, topical administration of a tazarotene gel formulation (0.125 mg/kg/day) to pregnant female rats from gestation day 16 through lactation day 20 reduced pup survival, but did not affect the reproductive capacity of the offspring. Based on data from another study, the systemic drug exposure in the rat at this dose would be equivalent to the MRHD (based on AUC comparison).
8.2 Lactation
Risk Summary
There are no data on the presence of tazarotene or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. After single topical doses of a 14C-tazarotene gel formulation to the skin of lactating rats, radioactivity was detected in rat milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ARAZLO and any potential adverse effects on the breastfed child from ARAZLO.
Clinical Considerations
To minimize potential exposure to the breastfed infant via breast milk, use ARAZLO for the shortest duration possible while breastfeeding. Advise breastfeeding patients not to apply ARAZLO directly to the nipple and areola to prevent direct infant exposure.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for patients of childbearing potential within 2 weeks prior to initiating ARAZLO therapy which should begin during a menstrual period.
Contraception
Advise patients of childbearing potential to use effective contraception during treatment with ARAZLO.
8.4 Pediatric Use
Safety and effectiveness of ARAZLO for the topical treatment of acne vulgaris have been established in pediatric patients age 9 years and older based on evidence from two multicenter, randomized, double-blind, parallel-group, vehicle-controlled, 12-week clinical trials and an open-label pharmacokinetic study. A total of 300 pediatric subjects aged 9 to less than 17 years received ARAZLO in the clinical studies [see Clinical Pharmacology (12.3)and Clinical Studies (14)] .
The safety and effectiveness of ARAZLO in pediatric patients below the age of 9 years have not been established.
8.5 Geriatric Use
Clinical trials of ARAZLO did not include sufficient numbers of subjects age 65 years and older to determine whether they respond differently from younger subjects.
10 Overdosage
Oral ingestion of the drug may lead to the same adverse effects as those associated with excessive oral intake of Vitamin A (hypervitaminosis A) or other retinoids. If oral ingestion occurs, monitor the patient closely and administer appropriate supportive measures, as necessary.
11 Description
ARAZLO (tazarotene) is a white to off-white lotion containing 0.045% tazarotene by weight for topical administration.
Tazarotene is a member of the acetylenic class of retinoids. The chemical name for tazarotene is 6-[(3,4-Dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl]-3-pyridinecarboxylic acid ethyl ester. The structural formula for tazarotene is represented below:
Tazarotene:
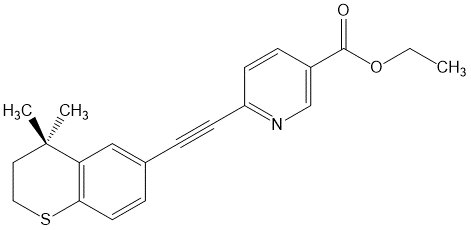
Molecular Formula: C 21H 21NO 2S Molecular Weight: 351.46
Each gram of ARAZLO contains 0.45 mg (0.045%) tazarotene in a white to off-white lotion base consisting of carbomer copolymer type B, carbomer homopolymer type A, diethyl sebacate, edetate disodium dihydrate, light mineral oil, methylparaben, propylparaben, purified water, sodium hydroxide, sorbitan monooleate and sorbitol solution, 70%.
12 Clinical Pharmacology
12.1 Mechanism of Action
Tazarotene is a retinoid prodrug which is converted to its active form, tazarotenic acid, the carboxylic acid of tazarotene, by deesterification. Tazarotenic acid binds to all three members of the retinoic acid receptor (RAR) family: RARα, RARβ and RARγ, but shows relative selectivity for RARβ, and RARγ and may modify gene expression. The clinical significance of these findings for the treatment of acne vulgaris is unknown.
12.3 Pharmacokinetics
Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, tazarotenic acid. Tazarotenic acid is highly bound to plasma proteins (greater than 99%).
Systemic exposure following topical application of ARAZLO was evaluated in 28 subjects in an open-label, randomized, pharmacokinetic study. Subjects aged 9 years and older with moderate to severe acne applied approximately 4 grams of ARAZLO to the entire face (excluding eyes and lips), neck, upper chest, upper back and shoulders once daily for 14 Days.
The majority of collected samples had concentrations below the limit of quantification (LOQ) for tazarotene (0.005 ng/mL). The mean C maxand mean AUC (0-t)values for tazarotene from quantifiable samples were 0.007 ng/mL and 0.164 ng•hr/mL on Day 14 to 15, respectively. The mean C maxand AUC (0-t)of tazarotene in subjects aged 9 to less than 12 years was approximately 3.7 and 3.6 fold higher, respectively, compared to that observed in subjects 12 years and older.
Tazarotenic acid concentrations were measurable in the majority of samples following single and repeated topical administration of ARAZLO (LOQ = 0.005 ng/mL). The mean C maxand AUC (0-t)values for tazarotenic acid from quantifiable samples were 0.365 ng/mL and 5.72 ng•hr/mL on Days 14 to 15, respectively. The mean C maxand AUC (0-t)of tazarotenic acid in subjects aged 9 to less than 12 years was approximately 2.4 and 2.3 fold higher, respectively, compared to that observed in subjects 12 years and older.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A long-term study of tazarotene following oral administration of 0.025, 0.050, and 0.125 mg/kg/day to rats showed no indications of increased carcinogenic risks. Based on pharmacokinetic data from a shorter-term study in rats, the highest dose of 0.125 mg/kg/day was anticipated to give systemic exposure in the rat equivalent to the MRHD (based on AUC comparison).
A long-term study with topical application of up to 0.1% of tazarotene in a gel formulation in mice terminated at 88 weeks showed that dose levels of 0.05, 0.125, 0.25, and 1 mg/kg/day (reduced to 0.5 mg/kg/day for males after 41 weeks due to severe dermal irritation) revealed no apparent carcinogenic effects when compared to vehicle control animals. Tazarotenic acid systemic exposures at the highest dose was 7 times the MRHD (based on AUC comparison).
Tazarotene was non-mutagenic in the Ames assay and did not produce structural chromosomal aberrations in human lymphocytes. Tazarotene was non-mutagenic in CHO/HGPRT mammalian cell forward gene mutation assay and was non-clastogenic in an in vivo mouse micronucleus test.
No impairment of fertility occurred in rats when male animals were treated for 70 days prior to mating and female animals were treated for 14 days prior to mating and continuing through gestation and lactation with topical doses of a tazarotene gel formulation up to 0.125 mg/kg/day. Based on data from another study, the systemic drug exposure in the rat at the highest dose was equivalent to the MRHD (based on AUC comparison).
No impairment of mating performance or fertility was observed in male rats treated for 70 days prior to mating with oral doses of tazarotene up to 1 mg/kg/day which produced a systemic exposure 4 times the MRHD (based on AUC comparison).
No impairment of mating performance or fertility was observed in female rats treated for 15 days prior to mating and continuing through gestation day 7 with oral doses of tazarotene up to 2 mg/kg/day. However, there was a significant decrease in the number of estrous stages and an increase in developmental effects at that dose which produced a systemic exposure 6 times the MRHD (based on AUC comparison).
14 Clinical Studies
The safety and efficacy of once daily use of ARAZLO for the treatment of acne vulgaris were assessed in two multicenter, randomized, double-blind clinical trials in subjects 9 years and older with facial acne vulgaris. Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 20 to 50 inflammatory lesions (papules, pustules, and nodules), 25 to 100 non-inflammatory lesions (open and closed comedones) and two or fewer facial nodules. The majority of subjects were White (74%) and female (66%). Approximately 22% were Hispanic/Latino and 42% were younger than 18 years of age. The efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 12. Success on the EGSS was defined as at least a 2-grade improvement from Baseline and an EGSS score of clear (0) or almost clear (1). Table 3 uls the efficacy results for trials 1 (NCT03168321) and 2 (NCT03168334).
Table 3: Efficacy Results at Week 12
Trial 1
ARAZLO Lotion N=402
Vehicle N=411
Treatment Difference
(95% Confidence Interval)
EGSS
Clear or Almost Clear and
25.5%
13%
12.5% (7.1%, 17.9%)
2-Grade Reduction from Baseline
Non-Inflammatory Facial Lesions
Mean Absolute Reduction
21.0
16.4
4.5 (2.6, 6.4)
Mean Percent Reduction
51.4%
41.5%
Inflammatory Facial Lesions
Mean Absolute Reduction
15.6
12.4
3.3 (1.9, 4.7)
Mean Percent Reduction
55.5%
45.7%
Trial 2
ARAZLO Lotion N=397
Vehicle N=404
EGSS
Clear or Almost Clear and
2-Grade Reduction from Baseline
29.6%
17.3%
12.3% (6.5%, 18.1%)
Non-Inflammatory Facial Lesions
Mean Absolute Reduction
24.6
16.6
8.1 (5.9, 10.2)
Mean Percent Reduction
60%
41.6%
Inflammatory Facial Lesions
Mean Absolute Reduction
16.7
13.4
3.2 (1.9, 4.5)
Mean Percent Reduction
59.5%
49%
16 How Supplied/storage And Handling
ARAZLO (tazarotene) Lotion, 0.045% is a white to off-white lotion supplied in a white aluminum tube as follows:
- 45 g (NDC 0187-2098-45)
Storage and Handling Conditions
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from freezing.
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Embryofetal Toxicity
Inform patients of childbearing potential of the potential risk to a fetus. To avoid pregnancy, advise these patients to use effective contraception during treatment with ARAZLO. Advise patients to discontinue the medication if pregnant and to inform their healthcare provider of a known or suspected pregnancy [see Contraindications (4.1), Warnings and Precautions (5.1), and Use in Specific Populations (8.1), (8.3)] .
Lactation
Advise patients to use ARAZLO for the shortest duration possible while breastfeeding. Advise breastfeeding patients not to apply ARAZLO directly to the nipple and areola to avoid direct infant exposure [see Use in Specific Populations (8.2)] .
Skin Irritation
Advise patients to avoid applying ARAZLO to eczematous or sunburned skin. If undue irritation occurs, reduce frequency of application, temporarily interrupt treatment, or discontinue use. Treatment may be resumed once irritation subsides [see Warnings and Precautions (5.2)] .
Photosensitivity and Risk of Sunburn
Advise patients to minimize exposure to sunlight and sunlamps; recommend the use of sunscreen products and protective apparel (e.g., wide-brimmed hat) when sun exposure cannot be avoided. Advise patients to avoid using ARAZLO if also taking other medicines that may increase sensitivity to sunlight [see Warnings and Precautions (5.3)] .
Distributed by: Bausch Health US, LLC Bridgewater, NJ 08807 USA
Manufactured by: Bausch Health Companies Inc. Laval, Quebec H7L 4A8, Canada
Patented. See https://patents.ortho-dermatologics.com for US patent information. ARAZLO is a trademark of Bausch Health Companies Inc. or its affiliates. © 2023 Bausch Health Companies Inc. or its affiliates 9701202
Patient Information
PATIENT INFORMATION ARAZLO ®(ah-RAZ-low) (tazarotene) lotion, for topical use
Important information:ARAZLO is for use on skin only. Do not use ARAZLO in your eyes, mouth, the corners of your nose, or vagina.
What is the most important information I should know about ARAZLO?
ARAZLO may cause birth defects if used during pregnancy.
You must not be pregnant when you start using ARAZLO or become pregnant during treatment with ARAZLO.
- For people who are able to get pregnant:
- Your healthcare provider should order a pregnancy test for you within 2 weeks before you begin treatment with ARAZLO to be sure that you are not pregnant. Your healthcare provider will decide when to do the test.
- Begin treatment with ARAZLO during a normal menstrual period.
- Use an effective form of birth control (contraception) during treatment with ARAZLO. Talk with your healthcare provider about birth control options that may be used to prevent pregnancy during treatment with ARAZLO.
- Stop using ARAZLO and tell your healthcare provider right away if you become pregnant during treatment with ARAZLO.
What is ARAZLO?
ARAZLO is a prescription medicine used on the skin (topical) to treat people 9 years of age and older with acne vulgaris.
It is not known if ARAZLO is safe and effective in children under 9 years of age.
Do not use ARAZLO if youare pregnant or plan to become pregnant. See, “What is the most important information I should know about ARAZLO?” at the beginning of this leaflet.
Before using ARAZLO, tell your healthcare provider about all your medical conditions, including if you:
- have eczema or any other skin problems
- are breastfeeding or plan to breastfeed. It is not known if ARAZLO passes into your breast milk. If you use ARAZLO while breastfeeding, use ARAZLO for the shortest time needed. Do not apply ARAZLO directly to the nipple and the areola to avoid your child being exposed to the medicine.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Certain medicines, vitamins, or supplements may make your skin more sensitive to sunlight.Ask your healthcare provider for a ul of medicines if you are not sure.
Especially tell your healthcare providerabout any medicines (such as benzoyl peroxide) used on your skin or cosmetics you use, including moisturizers, creams, lotions, or products that can dry out your skin.
Know the medicines you take. Keep a ul of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I use ARAZLO?
- Use ARAZLO exactly as your healthcare provider tells you to use it.
- Apply a thin layer of ARAZLO to cover the affected areas 1 time each day.
- If you use other medicines on your skin such as benzoyl peroxide during treatment with ARAZLO, you should apply one in the morning and one in the evening to separate the application time.
- Do not get ARAZLO in your eyes, the corners of your nose, or in your mouth. If ARAZLO gets in your eyes, rinse them well with water. Call your healthcare provider or get medical help if you have eye irritation that does not go away.
- Wash your hands after applying ARAZLO.
What should I avoid during treatment with ARAZLO?
- Avoid sunlight, including sunlamps during treatment with ARAZLO. ARAZLO can make you more sensitive to the sun, and the light from sunlamps and tanning beds. You could get severe sunburn. Use sunscreen and wear a wide-brimmed hat and clothes that cover your skin if you have to be in sunlight.
- Avoid using cosmetics or topical medicines that may make your skin more sensitive to sunlight or make your skin dry.
- Avoid using ARAZLO on skin with eczema or sunburned skin because it may cause severe irritation.
What are the possible side effects of ARAZLO?
ARAZLO may cause serious side effects, including:
- See, “What is the most important information I should know about ARAZLO?”
- Skin irritation.ARAZLO may cause application site skin pain, dryness, flaking or peeling, redness, and itching. Tell your healthcare provider if you develop any skin irritation during treatment with ARAZLO. If you develop any of these symptoms, your healthcare provider may tell you to use a moisturizer, decrease the number of times you apply ARAZLO, or completely stop treatment with ARAZLO. Also, wind or cold weather may be more irritating to your skin during treatment with ARAZLO.
- Sensitivity to sunlight and risk of sunburn.See, “What should I avoid during treatment with ARAZLO?”
- The most common side effects of ARAZLO includeskin pain, dryness, peeling, redness, and itching.
These are not all the possible side effects of ARAZLO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ARAZLO?
- Store ARAZLO at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze.
Keep ARAZLO and all medicines out of the reach of children.
General information about the safe and effective use of ARAZLO.
Medicines are sometimes prescribed for purposes other than those uled in a Patient Information leaflet. Do not use ARAZLO for a condition for which it was not prescribed. Do not give ARAZLO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ARAZLO that is written for health professionals.
What are the ingredients in ARAZLO?
Active ingredient:tazarotene
Inactive ingredients:carbomer copolymer type B, carbomer homopolymer type A, diethyl sebacate, edetate disodium dihydrate, light mineral oil, methylparaben, propylparaben, purified water, sodium hydroxide, sorbitan monooleate and sorbitol solution, 70%
Distributed by:Bausch Health US, LLC, Bridgewater, NJ 08807 USA
Manufactured by:Bausch Health Companies Inc., Laval, Quebec H7L 4A8, Canada Patented. See https://patents.ortho-dermatologics.com for US patent information.
ARAZLO is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2023 Bausch Health Companies Inc. or its affiliates
For more information, call 1-800-321-4576.
- This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 08/2023 9701202
Package/label Principal Display Panel 45 Gram Carton
NDC0187-2098-45
For Topical Use Only Not For Eye Use Rx only
ARAZLO ® (tazarotene) Lotion, 0.045%
Net Wt. 45 g
OrthoDermatologics
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site




