Bacitracin Zinc and Polymyxin B Sulfate Dailymed
Generic: bacitracin zinc and polymyxin b sulfate is used for the treatment of Empyema Enterocolitis, Pseudomembranous Kidney Diseases Pneumonia Staphylococcal Infections Streptococcal Infections Eye Infections, Bacterial Skin Diseases, Bacterial Urinary Bladder Diseases Escherichia coli Infections Gastrointestinal Diseases Haemophilus Infections Klebsiella Infections Otitis Externa Pseudomonas Infections Skin Diseases Wounds and Injuries Bacteremia Meningitis, Bacterial Soft Tissue Infections
Go PRO for all pill images
Description
Bacitracin zinc and polymyxin B sulfate ophthalmic ointment, USP is a sterile antimicrobial ointment formulated for ophthalmic use.
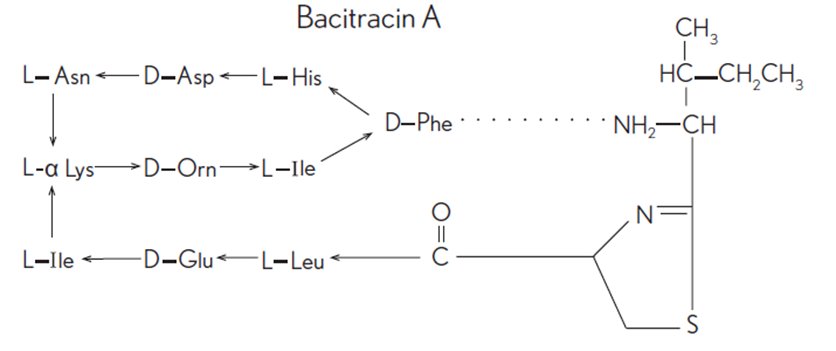
Bacitracin zinc is the zinc salt of bacitracin, a mixture of related cyclic polypeptides (mainly bacitracin A) produced by the growth of an organism of the licheniformis group of Bacillus subtilis var Tracy. It has a potency of not less than 40 bacitracin units/mg. The structural formula for bacitracin A is:
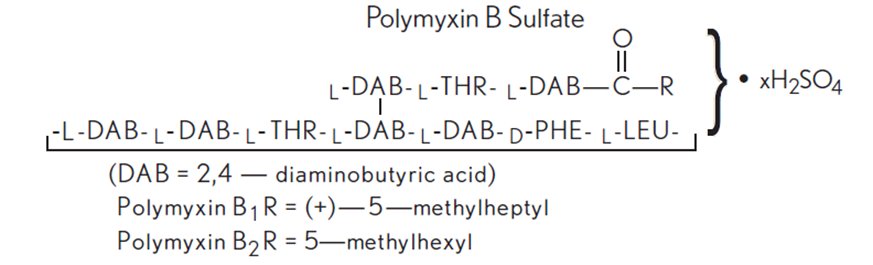
Polymyxin B sulfate is the sulfate salt of polymyxin B1 and B2, which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units/mg, calculated on an anhydrous basis. The structural formulae are:
Each gram contains: Actives: bacitracin zinc equal to 500 bacitracin units and polymyxin B sulfate equal to 10,000 polymyxin B units; Inactives: mineral oil and white petrolatum.
Clinical Pharmacology
Polymyxin B sulfate attacks gram-negative bacilli, including virtually all strains of Pseudomonas aeruginosa and Haemophilus influenzae species.
Bacitracin is active against most gram-positive bacilli and cocci including hemolytic streptococci.
Indications And Usage
For the treatment of superficial ocular infections involving the conjunctiva and/or cornea caused by organisms susceptible to bacitracin zinc and polymyxin B sulfate.
Contraindications
This product is contraindicated in those individuals who have shown hypersensitivity to any of its components.
Warnings
Ophthalmic ointments may retard corneal healing.
Precautions
As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. Appropriate measures should be taken if this occurs.
Adverse Reactions
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Dosage And Administration
Apply the ointment every 3 or 4 hours for 7 to 10 days, depending on the severity of the infection.
FOR OPHTHALMIC USE ONLY
How Supplied
Bacitracin zinc and polymyxin B sulfate ophthalmic ointment, USP is available in tubes with an ophthalmic tip applicator in the following size:
NDCÂ 24208-555-55Â -Â 3.5 g tube

Storage:
Store between 15°C to 25°C (59°F to 77°F). KEEP TIGHTLY CLOSED.
KEEP OUT OF REACH OF CHILDREN.
Distributed by: Bausch & Lomb Americas Inc.Bridgewater, NJ 08807 USA
Manufactured by: Bausch & Lomb IncorporatedTampa, FL 33637 USA
© 2023 Bausch & Lomb Incorporated or its affiliates
Revised: January 2023
9130705 (Folded) 9130605 (Flat)
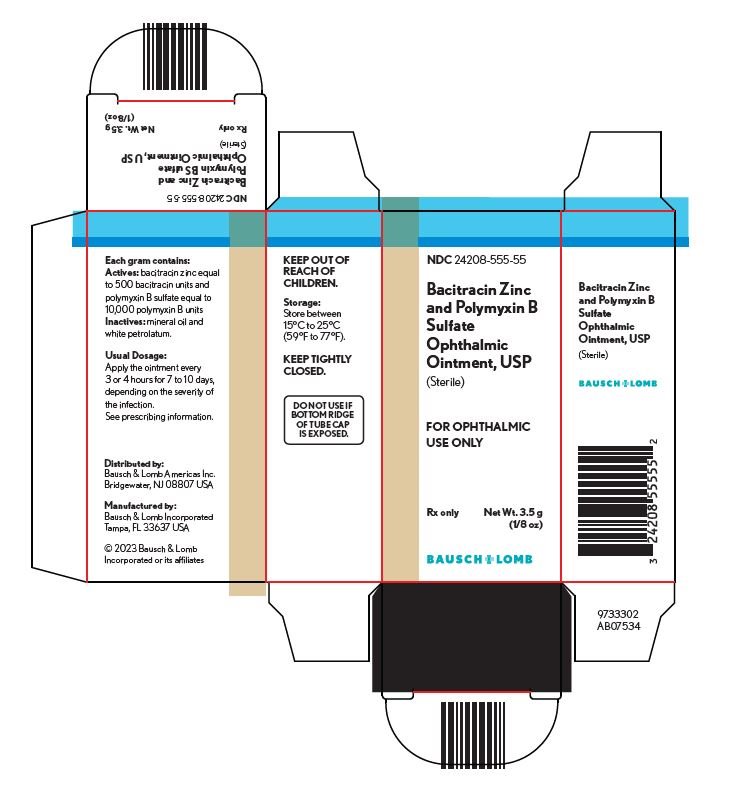
Principal Display Panel
NDC 24208-555-55
Bacitracin Zinc and Polymyxin B Sulfate Ophthalmic Ointment, USP (Sterile)
FOR OPHTHALMIC USE ONLY
Rx only
Net Wt. 3.5 g (1/8 oz)
BAUSCH + LOMB
9733302AB07534
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site

