Cefotaxime Dailymed
Generic: cefotaxime injection is used for the treatment of Acinetobacter Infections Pelvic Inflammatory Disease Bacteroides Infections Bone Diseases, Infectious Clostridium Infections Escherichia coli Infections Fusobacterium Infections Gonorrhea Haemophilus Infections Klebsiella Infections Peritonitis Proteus Infections Pseudomonas Infections Salmonella Infections Staphylococcal Infections Streptococcal Infections Urinary Tract Infections Serratia Infections Meningitis, Bacterial Skin Diseases, Bacterial Pneumonia, Bacterial Soft Tissue Infections Sepsis
Go PRO for all pill images
Important Prescribing Information
January 13, 2023
Temporary Importation of Cefotaxime for Injection to Address Drug Shortage
Dear Healthcare Professional:
Due to the current critical shortage of Cefotaxime for Injection products in the United States (U.S.) market, SteriMax Inc. (SteriMax), in conjunction with Provepharm, Inc. (Provepharm) and Direct Success, Inc. (Direct Success) is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of the drug. SteriMax has initiated temporary importation of non-FDA approved Cefotaxime for Injection (1 g/vial, and 2 g/vial) into the U.S. market. The Cefotaxime for Injection from SteriMax is marketed in Canada and is manufactured at an FDA-inspected facility that complies with current Good Manufacturing Practice requirements.
At this time, no other entity except Provepharm or its distributor Direct Success is authorized by the FDA to import or distribute SteriMax’s Cefotaxime for Injection in the United States. FDA has not approved SteriMax’s Cefotaxime for Injection in the United States.
Effective immediately, Provepharm will distribute the following presentations of SteriMax’s Cefotaxime for Injection to address the critical shortage:
Note: DIN refers to Drug Identification Number for products approved by Health Canada SteriMax Cefotaxime for Injection  1 g/vial (as cefotaxime sodium)  DIN: 02434091 (Canada) NDC 21586-011-2  2 g/vial (as cefotaxime sodium)  DIN: 02434105 (Canada) NDC 21586-012-2
The barcode on the imported product label may not register accurately on the U.S. scanning systems. Institutions should manually input the imported product information into their systems and confirm that the barcode, if scanned, provides correct information. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
In addition, the packaging of the imported product does not include serialization information. SteriMax’s Cefotaxime for Injection does not meet the Drug Supply Chain Security Act (DSCSA) requirements for the Interoperable Exchange of Information for Tracing of Human, Finished Prescription Drugs.
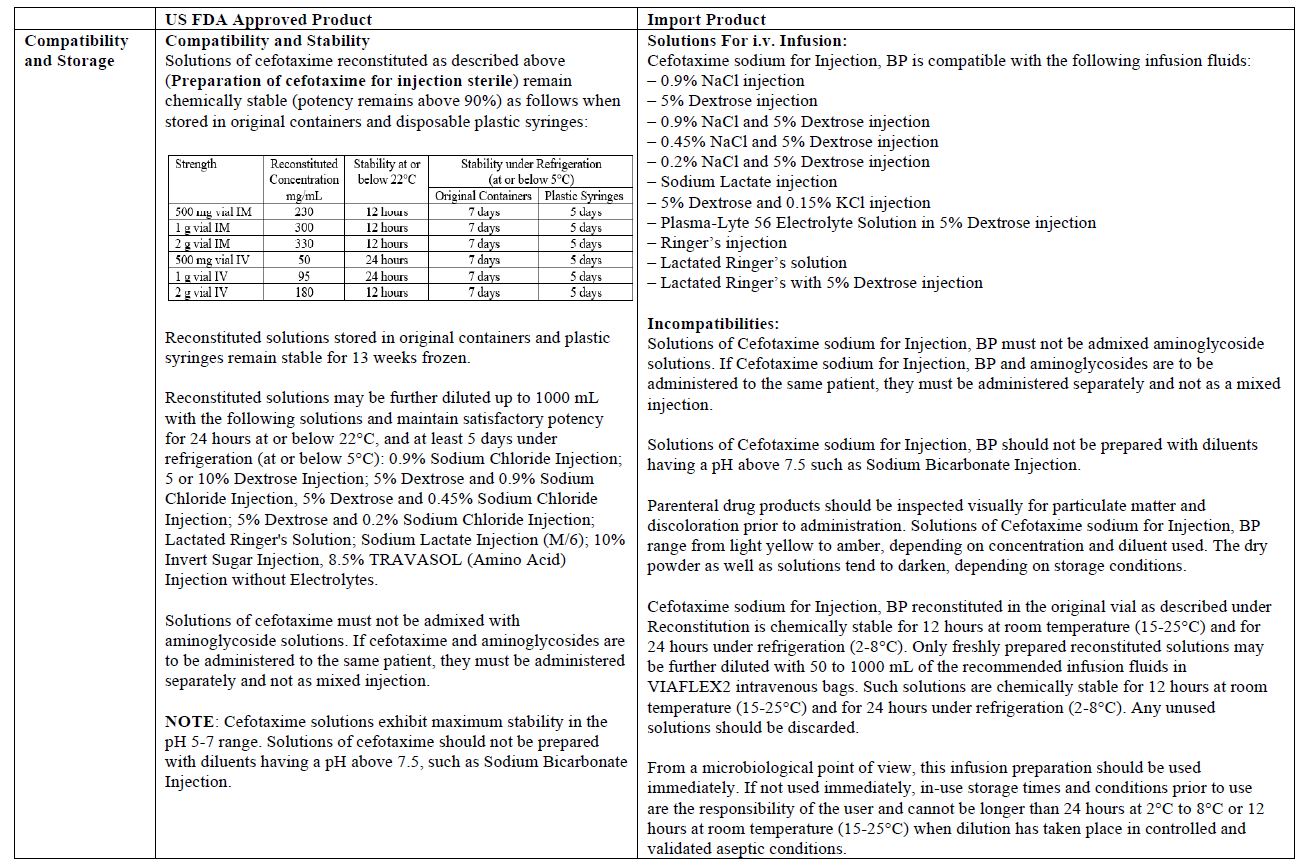
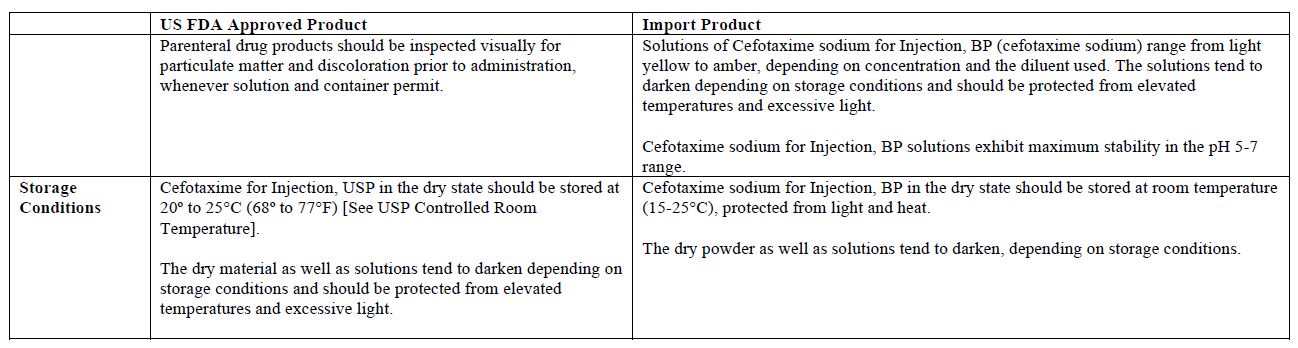
The vial and carton labels will display the text used and approved for marketing the products in Canada with both English and French translations. It is important to note that there are differences in the format and content of the labeling between the US approved product and SteriMax’s Cefotaxime for Injection. Please see the product comparison tables at the end of this letter.
Cefotaxime for Injection is available only by prescription in the U.S. Please refer to the package insert for the FDA-approved Cefotaxime for Injection drug product for full prescribing information.
Finally, please ensure that your staff and others in your institution who may be involved in the administration of Cefotaxime for Injection receive a copy of this letter and review the information.
If you have any questions about the information contained in this letter, any quality related problems, or questions on the use of SteriMax’s Cefotaxime for Injection, please contact SteriMax Inc. Customer Service at 1-800-881-3550.
To place an order, please contact Direct Success at Distribution@DSuccess.com or 1-877-404-3338.
Healthcare providers should report adverse events associated with the use of SteriMax’s Cefotaxime for Injection to Provepharm at 1-833-684-3234.
Adverse events or quality problems experienced with the use of this product may also be reported to the FDA’s MedWatch Adverse Event Reporting Program either online, by regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
We remain at your disposal to answer any questions you may have about our product; and provide more information if needed.
Sincerely,

Ritesh AcharyaExecutive Vice President, Scientific AffairsSteriMax Inc.

Cefotaxime For Injection - 1 G Per Vial
Sterile/Stérile DIN 02234091
cefoTAXime sodium for Injection BP
1 g per vial
Cefotaxime sodium powder for solution
Intramuscular or Intravenous Use
Antibiotic/Antibiotique
LATEX FREE/SANS LATEX
STERIMAX

Cefotaxime For Injection - 2 G Per Vial
Sterile/Stérile DIN 02434105
cefoTAXime sodium for Injection BP
2 g per vial
Cefotaxime sodium powder for solution
Intramuscular or Intravenous Use
Antibiotic/Antibiotique
LATEX FREE/SANS LATEX
STERIMAX

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


