CEFPROZIL (cefprozil 250 mg) Dailymed
Generic: cefprozil is used for the treatment of Bronchitis Escherichia coli Infections Haemophilus Infections Klebsiella Infections Otitis Media Pharyngitis Proteus Infections Sinusitis Staphylococcal Infections Streptococcal Infections Skin Diseases, Bacterial Moraxellaceae Infections
IMPRINT: LUPIN 250
SHAPE: oval
COLOR: orange
All Imprints
cefprozil 500 mg - lupin 500 oval white
cefprozil 250 mg - lupin 250 oval orange
Go PRO for all pill images
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefprozil tablets and other antibacterial drugs, cefprozil tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Description
Cefprozil is a semi-synthetic broad-spectrum cephalosporin antibiotic.
Cefprozil is a cis and trans isomeric mixture (‚Č•90% cis). The chemical name for the monohydrate is (6R,7R)-7-[(R)-2-Amino-2-(p-hydroxyphenyl)acetamido]-8-oxo-3-propenyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid monohydrate, and the structural formula is:
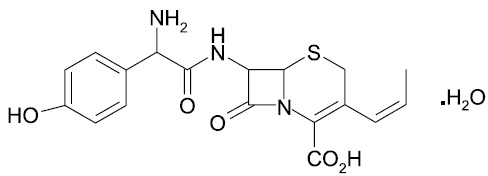
Cefprozil is a white to yellowish powder with a molecular formula for the monohydrate of C18H19N3O5S.H2O and a molecular weight of 407.45.
Cefprozil tablets are intended for oral administration.
Cefprozil tablets contain cefprozil equivalent to 250 mg or 500 mg of anhydrous cefprozil. In addition, each tablet contains the following inactive ingredients: magnesium stearate, methylcellulose, microcrystalline cellulose, sodium starch glycolate and titanium dioxide. The 250 mg tablet also contains FD & C Yellow No. 6 Aluminum Lake.
Clinical Pharmacology
The pharmacokinetic data were derived from the capsule formulation; however, bioequivalence has been demonstrated for the oral solution, capsule, tablet, and suspension formulations under fasting conditions.
Following oral administration of cefprozil to fasting subjects, approximately 95% of the dose was absorbed. The average plasma half-life in normal subjects was 1.3 hours, while the steady-state volume of distribution was estimated to be 0.23 L/kg. The total body clearance and renal clearance rates were approximately 3 mL/min/kg and 2.3 mL/min/kg, respectively.
Average peak plasma concentrations after administration of 250 mg, 500 mg, or 1 g doses of cefprozil to fasting subjects were approximately 6.1, 10.5, and 18.3 mcg/mL, respectively, and were obtained within 1.5 hours after dosing. Urinary recovery accounted for approximately 60% of the administered dose. (See Table.)
Dosage (mg)  Mean Plasma Cefprozil  8 hour Urinary Excretion (%)  Concentrations (mcg/mL) Data represent mean values of 12 healthy volunteers. Peak appx. 1.5 h  4 h  8 h  250 mg  6.1  1.7  0.2  60%  500 mg  10.5  3.2  0.4  62%  1000 mg  18.3  8.4  1.0  54%  During the first 4 hour period after drug administration, the average urine concentrations following 250 mg, 500 mg, and 1 g doses were approximately 700 mcg/mL, 1000 mcg/mL, and 2900 mcg/mL, respectively.
Administration of cefprozil with food did not affect the extent of absorption (AUC) or the peak plasma concentration (Cmax) of cefprozil. However, there was an increase of 0.25 to 0.75 hours in the time to maximum plasma concentration of cefprozil (Tmax).
The bioavailability of the capsule formulation of cefprozil was not affected when administered 5 minutes following an antacid.
Plasma protein binding is approximately 36% and is independent of concentration in the range of 2 mcg/mL to 20 mcg/mL.
There was no evidence of accumulation of cefprozil in the plasma in individuals with normal renal function following multiple oral doses of up to 1000 mg every 8 hours for 10 days.
In patients with reduced renal function, the plasma half-life may be prolonged up to 5.2 hours depending on the degree of the renal dysfunction. In patients with complete absence of renal function, the plasma half-life of cefprozil has been shown to be as long as 5.9 hours. The half-life is shortened during hemodialysis. Excretion pathways in patients with markedly impaired renal function have not been determined. (See PRECAUTIONS and DOSAGE AND ADMINISTRATION .)
In patients with impaired hepatic function, the half-life increases to approximately 2 hours. The magnitude of the changes does not warrant a dosage adjustment for patients with impaired hepatic function.
Healthy geriatric volunteers (‚Č•65 years old) who received a single 1 g dose of cefprozil had 35% to 60% higher AUC and 40% lower renal clearance values compared with healthy adult volunteers 20 to 40 years of age. The average AUC in young and elderly female subjects was approximately 15% to 20% higher than in young and elderly male subjects. The magnitude of these age- and gender-related changes in the pharmacokinetics of cefprozil is not sufficient to necessitate dosage adjustments.
Adequate data on CSF levels of cefprozil are not available.
Comparable pharmacokinetic parameters of cefprozil are observed between pediatric patients (6 months to 12 years) and adults following oral administration of selected matched doses. The maximum concentrations are achieved at 1 to 2 hours after dosing.
The plasma elimination half-life is approximately 1.5 hours. In general, the observed plasma concentrations of cefprozil in pediatric patients at the 7.5, 15, and 30 mg/kg doses are similar to those observed within the same time frame in normal adult subjects at the 250, 500, and 1000 mg doses, respectively. The comparative plasma concentrations of cefprozil in pediatric patients and adult subjects at the equivalent dose level are presented in the table below.
Mean (SD) Plasma Cefprozil  an=11  bn=5  cn=9  dn=11
Concentrations¬†(mcg/mL)¬† Population¬† Dose¬† 1¬†h¬† 2¬†h¬† 4¬†h¬† 6¬†h¬† T¬Ĺ¬†(h)¬† children¬† 7.5¬†mg/kg¬† 4.70¬† 3.99¬† 0.91¬† 0.23¬†a 0.94¬† (n=18)¬† (1.57)¬† (1.24)¬† (0.30)¬† (0.13)¬† (0.32)¬† adults¬† 250¬†mg¬† 4.82¬† 4.92¬† 1.70b 0.53¬† 1.28¬† (n=12)¬† (2.13)¬† (1.13)¬† (0.53)¬† (0.17)¬† (0.34)¬† children¬† 15¬†mg/kg¬† 10.86¬† 8.47¬† 2.75¬† 0.61c 1.24¬† (n=19)¬† (2.55)¬† (2.03)¬† (1.07)¬† (0.27)¬† (0.43)¬† adults¬† 500¬†mg¬† 8.39¬† 9.42¬† 3.18d 1.00d 1.29¬† (n=12)¬† (1.95)¬† (0.98)¬† (0.76)¬† (0.24)¬† (0.14)¬† children¬† 30¬†mg/kg¬† 16.69¬† 17.61¬† 8.66¬† --¬† 2.06¬† (n=10)¬† (4.26)¬† (6.39)¬† (2.70)¬† (0.21)¬† adults¬† 1000¬†mg¬† 11.99¬† 16.95¬† 8.36¬† 2.79¬† 1.27¬† (n=12)¬† (4.67)¬† (4.07)¬† (4.13)¬† (1.77)¬† (0.12)¬† Microbiology:
Cefprozil has in vitro activity against a broad range of gram-positive and gram-negative bacteria. The bactericidal action of cefprozil results from inhibition of cell-wall synthesis. Cefprozil has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic Gram-Positive Microorganisms:
Staphylococcus aureus
(including ß-lactamase-producing strains)
NOTE: Cefprozil is inactive against methicillin-resistant staphylococci.
Streptococcus pneumoniae
Streptococcus pyogenes
Aerobic Gram-Negative Microorganisms:
Haemophilus influenzae
(including ß-lactamase-producing strains)
Moraxella (Branhamella) catarrhalis
(including ß-lactamase-producing strains)
The following in vitro data are available; however, their clinical significance is unknown. Cefprozil exhibits in vitro minimum inhibitory concentrations (MICs) of 8 mcg/mL or less against most (‚Č•90%) strains of the following microorganisms; however, the safety and effectiveness of cefprozil in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Aerobic Gram-Positive Microorganisms:
Enterococcus durans
Enterococcus faecalis
Listeria monocytogenes
Staphylococcus epidermidis
Staphylococcus saprophyticus
Staphylococcus warneri
Streptococcus agalactiae
Streptococci (Groups C, D, F, and G)
viridans group Streptococci
NOTE: Cefprozil is inactive against Enterococcus faecium .
Aerobic Gram-Negative Microorganisms:
Citrobacter diversus
Escherichia coli
Klebsiella pneumoniae
Neisseria gonorrhoeae
(including ß-lactamase-producing strains)
Proteus mirabilis
Salmonella spp.
Shigella spp .
Vibrio spp.
NOTE: Cefprozil is inactive against most strains of Acinetobacter, Enterobacter, Morganella morganii, Proteus vulgaris, Providencia, Pseudomonas, and Serratia.
Anaerobic Microorganisms:
Prevotella (Bacteroides) melaninogenicus
Clostridium difficile
Clostridium perfringens
Fusobacterium spp.
Peptostreptococcus spp.
Propionibacterium acnes
NOTE: Most strains of the Bacteroides fragilis group are resistant to cefprozil.
Susceptibility Tests:
Dilution Techniques: Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1,2 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of cefprozil powder. The MIC values should be interpreted according to the following criteria:
MIC¬† ( mcg / mL )¬† ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† Interpretation¬† ‚ȧ8¬† ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Susceptible¬†(S)¬† 16¬† ¬†¬†¬†¬†¬†¬†¬†¬†¬†Intermediate¬†(I)¬† ‚Č•32¬† ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Resistant¬†(R)¬† A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard cefprozil powder should provide the following MIC values:
    Microorganism MIC  ( mcg / mL )          Enterococcus faecalis ATCC 29212  4 to 16          Escherichia coli ATCC 25922  1 to 4          Haemophilus influenzae ATCC 49766  1 to 4          Staphylococcus aureus ATCC 29213  0.25 to 1          Streptococcus pneumoniae ATCC 49619  0.25 to 1  Diffusion Techniques: Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure3 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30 mcg cefprozil to test the susceptibility of microorganisms to cefprozil.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30-mcg cefprozil disk should be interpreted according to the following criteria:
Zone¬† diameter¬† ( mm )¬† Interpretation¬† ‚Č•18¬† ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Susceptible¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†(S)¬† 15¬†to¬†17¬† ¬†¬†¬†¬†¬†¬†¬†¬†Intermediate¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†(I)¬† ‚ȧ14¬† ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†Resistant¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†(R)¬† Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for cefprozil.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30-mcg cefprozil disk should provide the following zone diameters in these laboratory test quality control strains.
           Microorganism  Zone  diameter  ( mm )            Escherichia coli ATCC 25922  21 to 27            Haemophilus influenzae ATCC 49766  20 to 27            Staphylococcus aureus ATCC 25923  27 to 33            Streptococcus pneumoniae ATCC 49619  25 to 32 
Indications And Usage
Cefprozil tablets are indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions uled below:
Upper Respiratory Tract:
Pharyngitis/Tonsillitis:
Caused by Streptococcus pyogenes.
NOTE: The usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever, is penicillin given by the intramuscular route. Cefprozil is generally effective in the eradication of Streptococcus pyogenes from the nasopharynx; however, substantial data establishing the efficacy of cefprozil in the subsequent prevention of rheumatic fever are not available at present.
Otitis Media:
Caused by Streptococcus pneumoniae, Haemophilus influenzae (including ß-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including ß-lactamase-producing strains). (See CLINICAL STUDIES.)
NOTE: In the treatment of otitis media due to ß-lactamase producing organisms, cefprozil had bacteriologic eradication rates somewhat lower than those observed with a product containing a specific ß-lactamase inhibitor. In considering the use of cefprozil, lower overall eradication rates should be balanced against the susceptibility patterns of the common microbes in a given geographic area and the increased potential for toxicity with products containing ß-lactamase inhibitors.
Acute Sinusitis:
Caused by Streptococcus pneumoniae, Haemophilus influenzae (including ß-lactamase producing strains), and Moraxella (Branhamella) catarrhalis (including ß-lactamase-producing strains).
Lower Respiratory Tract:
Acute Bacterial Exacerbation of Chronic Bronchitis:
Caused by Streptococcus pneumoniae, Haemophilus influenzae (including ß-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including ß-lactamase-producing strains).
Skin and Skin Structure:
Uncomplicated Skin and Skin-Structure Infections:
Caused by Staphylococcus aureus (including penicillinase-producing strains) and Streptococcus pyogenes. Abscesses usually require surgical drainage.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefprozil tablets and other antibacterial drugs, cefprozil tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Contraindications
Cefprozil is contraindicated in patients with known allergy to the cephalosporin class of antibiotics.
Warnings
BEFORE THERAPY WITH CEFPROZIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFPROZIL, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-SENSITIVITY AMONG ß-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFPROZIL OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Cefprozil, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD.                        Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Precautions
General:
Prescribing cefprozil in the absence of proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
In patients with known or suspected renal impairment (see DOSAGE AND ADMINISTRATION ),careful clinical observation and appropriate laboratory studies should be done prior to and during therapy. The total daily dose of cefprozil should be reduced in these patients because high and/or prolonged plasma antibiotic concentrations can occur in such individuals from usual doses. Cephalosporins, including cefprozil, should be given with caution to patients receiving concurrent treatment with potent diuretics since these agents are suspected of adversely affecting renal function.
Prolonged use of cefprozil may result in the overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Cefprozil should be prescribed with caution in individuals with a history of gastrointestinal disease particularly colitis.
Positive direct Coombs' tests have been reported during treatment with cephalosporin antibiotics.
Information for Patients:
Patients should be counseled that antibacterial drugs including cefprozil should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefprozil are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefprozil or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug Interactions:
Nephrotoxicity has been reported following concomitant administration of aminoglycoside antibiotics and cephalosporin antibiotics. Concomitant administration of probenecid doubled the AUC for cefprozil.
The bioavailability of the capsule formulation of cefprozil was not affected when administered 5 minutes following an antacid.
Drug/Laboratory Test Interactions:
Cephalosporin antibiotics may produce a false positive reaction for glucose in the urine with copper reduction tests (Benedict‚Äôs or Fehling‚Äôs solution or with Clinitest¬ģ tablets), but not with enzyme-based tests for glycosuria (e.g., Clinistix¬ģ). A false negative reaction may occur in the ferricyanide test for blood glucose. The presence of cefprozil in the blood does not interfere with the assay of plasma or urine creatinine by the alkaline picrate method.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Long term in vivo studies have not been performed to evaluate the carcinogenic potential of cefprozil.
Cefprozil was not found to be mutagenic in either the Ames Salmonella or E. coli WP2 urvA reversion assays or the Chinese hamster ovary cell HGPRT forward gene mutation assay and it did not induce chromosomal abnormalities in Chinese hamster ovary cells or unscheduled DNA synthesis in rat hepatocytes in vitro. Chromosomal aberrations were not observed in bone marrow cells from rats dosed orally with over 30 times the highest recommended human dose based upon mg/m2.
Impairment of fertility was not observed in male or female rats given oral doses of cefprozil up to 18.5 times the highest recommended human dose based upon mg/m2.
Pregnancy:
Teratogenic Effects- Pregnancy Category B:
Reproduction studies have been performed in rabbits, mice, and rats using oral doses of cefprozil of 0.8, 8.5, and 18.5 times the maximum daily human dose (1000 mg) based upon mg/m2, and have revealed no harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery:
Cefprozil has not been studied for use during labor and delivery. Treatment should only be given if clearly needed.
Nursing Mothers:
Small amounts of cefprozil (<0.3% of dose) have been detected in human milk following administration of a single 1 gram dose to lactating women. The average levels over 24 hours ranged from 0.25 to 3.3 mcg/mL. Caution should be exercised when cefprozil tablets are administered to a nursing woman, since the effect of cefprozil on nursing infants is unknown.
Pediatric Use:
(See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION.)
The safety and effectiveness of cefprozil in the treatment of otitis media have been established in the age groups 6 months to 12 years. Use of cefprozil for the treatment of otitis media is supported by evidence from adequate and well-controlled studies of cefprozil in pediatric patients. (See CLINICAL STUDIES)
The safety and effectiveness of cefprozil in the treatment of pharyngitis/tonsillitis or uncomplicated skin and skin-structure infections have been established in the age groups 2 to 12 years. Use of cefprozil for the treatment of these infections is supported by evidence from adequate and well-controlled studies of cefprozil in pediatric patients.
The safety and effectiveness of cefprozil in the treatment of acute sinusitis have been established in the age groups 6 months to 12 years. Use of cefprozil in these age groups is supported by evidence from adequate and well-controlled studies of cefprozil in adults.
Safety and effectiveness in pediatric patients below the age of 6 months have not been established for the treatment of otitis media or acute sinusitis, or below the age of 2 years for the treatment of pharyngitis/tonsillitis or uncomplicated skin and skin-structure infections. However, accumulation of other cephalosporin antibiotics in newborn infants (resulting from prolonged drug half-life in this age group) has been reported.
Geriatric Use:
Of the more than 4500 adults treated with cefprozil in clinical studies, 14% were 65 years and older, while 5% were 75 years and older. When geriatric patients received the usual recommended adult doses, their clinical efficacy and safety were comparable to clinical efficacy and safety in nongeriatric adult patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals to the effects of cefprozil cannot be excluded (see CLINICAL PHARMACOLOGY).
Cefprozil is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function. See DOSAGE AND ADMINISTRATION for dosing recommendations for patients with impaired renal function.
Adverse Reactions
The adverse reactions to cefprozil are similar to those observed with other orally administered cephalosporins. Cefprozil was usually well tolerated in controlled clinical trials. Approximately 2% of patients discontinued cefprozil therapy due to adverse events.
The most common adverse effects observed in patients treated with cefprozil are:
Gastrointestinal:
Diarrhea (2.9%), nausea (3.5%), vomiting (1%), and abdominal pain (1%).
Hepatobiliary:
Elevations of AST (SGOT) (2%), ALT (SGPT) (2%), alkaline phosphatase (0.2%), and bilirubin values (<0.1%). As with some penicillins and some other cephalosporin antibiotics, cholestatic jaundice has been reported rarely.
Hypersensitivity:
Rash (0.9%), urticaria (0.1%). Such reactions have been reported more frequently in children than in adults. Signs and symptoms usually occur a few days after initiation of therapy and subside within a few days after cessation of therapy.
CNS:
Dizziness (1%), hyperactivity, headache, nervousness, insomnia, confusion, and somnolence have been reported rarely (<1%). All were reversible.
Hematopoietic:
Decreased leukocyte count (0.2%), eosinophilia (2.3%).
Renal:
Elevated BUN (0.1%), serum creatinine (0.1%).
Other:
Diaper rash and superinfection (1.5%), genital pruritus and vaginitis (1.6%).
The following adverse events, regardless of established causal relationship to cefprozil tablets, have been rarely reported during postmarketing surveillance: anaphylaxis, angioedema, colitis (including pseudomembranous colitis), erythema multiforme, fever, serumsickness like reactions, Stevens-Johnson syndrome, and thrombocytopenia.
Cephalosporin Class Paragraph:
In addition to the adverse reactions uled above which have been observed in patients treated with cefprozil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics:
Aplastic anemia, hemolytic anemia, hemorrhage, renal dysfunction, toxic epidermal necrolysis, toxic nephropathy, prolonged prothrombin time, positive Coombs’ test, elevated LDH, pancytopenia, neutropenia, agranulocytosis.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment, when the dosage was not reduced. (SeeDOSAGE AND ADMINISTRATION and OVERDOSAGE.)If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Overdosage
Single 5000 mg/kg oral doses of cefprozil caused no mortality or signs of toxicity in adult, weanling, or neonatal rats, or adult mice. A single oral dose of 3000 mg/kg caused diarrhea and loss of appetite in cynomolgus monkeys, but no mortality.
Cefprozil is eliminated primarily by the kidneys. In case of severe overdosage, especially in patients with compromised renal function, hemodialysis will aid in the removal of cefprozil from the body.
Dosage And Administration
Cefprozil tablets are administered orally.
Population / Infection  Dosage  ( mg )  Duration  ( days )  a In the treatment of infections due to Streptococcus pyogenes, cefprozil tablets should be administered for at least 10 days.
b Not to exceed recommended adult doses.
 ADULTS (13 years and older)       UPPER RESPIRATORY TRACT           Pharyngitis/Tonsillitis  500 q24h  10 a          Acute Sinusitis  250 q12h or  10           (For moderate to severe infections, the higher dose should be used)  500 q12h      LOWER RESPIRATORY TRACT          Acute Bacterial Exacerbation of Chronic Bronchitis  500 q12h  10      SKIN AND SKIN STRUCTURE           Uncomplicated Skin and Skin Structure Infections  250 q12h or  10  500 q24h or  500 q12h   CHILDREN (2 years to 12 years)      UPPER RESPIRATORY TRACT b         Pharyngitis/Tonsillitis  7.5 mg/kg q12h  10 a     SKIN AND SKIN STRUCTURE         Uncomplicated Skin and Skin Structure Infections  20 mg/kg q24h  10   INFANTS & CHILDREN (6 months to 12 years)      UPPER RESPIRATORY TRACTb         Otitis Media  15 mg/kg q12h  10          (See INDICATIONS AND USAGE and CLINICAL STUDIES)         Acute Sinusitis  7.5 mg/kg q12h or  10          (For moderate to severe infections, the higher dose should be used)  15 mg/kg q12h  Renal Impairment:
Cefprozil may be administered to patients with impaired renal function. The following dosage schedule should be used.
Creatinine  Clearance  ( mL / min )  Dosage  ( mg )  Dosing  Interval  30 to 120  standard  standard  0 to 29  Cefprozil is in part removed by hemodialysis; therefore, cefprozil should be administered after the completion of hemodialysis. 50% of standard  standard  Hepatic Impairment:
No dosage adjustment is necessary for patients with impaired hepatic function.
How Supplied
Cefprozil tablets USP, 250 mg and 500 mg are available as follows:
Each light orange, film-coated, oval tablet debossed with 'LUPIN' on one side and '250' on the other side, contains the equivalent of 250 mg anhydrous cefprozil.
Bottles of 100 Tablets                       NDC 68180-403-01
Each white, film-coated, oval tablet debossed with 'LUPIN' on one side and '500' on the other side, contains the equivalent of 500 mg anhydrous cefprozil.
Bottles of 50 Tablets                         NDC 68180-404-01
Bottles of 100 Tablets                       NDC 68180-404-02
Store at 20¬į to 25¬įC (68¬į to 77¬įF) [See USP Controlled Room Temperature].
Clinical Studies
Study One:
In a controlled clinical study of acute otitis media performed in the United States where significant rates of ß-lactamase- producing organisms were found, cefprozil was compared to an oral antimicrobial agent that contained a specific ß-lactamase inhibitor. In this study, using very strict evaluability criteria and microbiologic and clinical response criteria at the 10 to 16 days post-therapy follow-up, the following presumptive bacterial eradication/clinical cure outcomes (i.e., clinical success) and safety results were obtained:
                                                                          U.S. Acute Otitis Media Study
¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† Cefprozil vs ő≤-lactamase inhibitor-containing control drug
EFFICACY:
Pathogen %  of  Cases  with  Pathogen ( n = 155 ) Outcome S .  pneumoniae 48.4% cefprozil success rate 5% better than control H .  influenzae 35.5% cefprozil success rate 17% less than control M .  catarrhalis 13.5% cefprozil success rate 12% less than control S .  pyogenes 2.6% cefprozil equivalent to control Overall 100.0% cefprozil success rate 5% less than control SAFETY:
The incidences of adverse events, primarily diarrhea and rash*, were clinically and statistically significantly higher in the control arm versus the cefprozil arm.
* The majority of these involved the diaper area in young children.
Age  Group Cefprozil Control 6 months to 2 years 21% 41% 3 to 12 years 10% 19% Study Two:
In a controlled clinical study of acute otitis media performed in Europe, cefprozil was compared to an oral antimicrobial agent that contained a specific ß-lactamase inhibitor. As expected in a European population, this study population had a lower incidence of ß-lactamase-producing organisms than usually seen in U.S. trials. In this study, using very strict evaluability criteria and microbiologic and clinical response criteria at the 10 to 16 days post-therapy follow-up, the following presumptive bacterial eradication/clinical cure outcomes (i.e., clinical success) were obtained:
                                                                  European Acute Otitis Media Study
¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† Cefprozil vs ő≤-lactamase inhibitor-containing control drug
EFFICACY:
Pathogen  %  of  Cases  with  Pathogen  ( n = 47 )  Outcome  S .  pneumoniae  51.0%  cefprozil equivalent to control  H .  influenzae  29.8%  cefprozil equivalent to control  M .  catarrhalis  6.4%  cefprozil equivalent to control  S .  pyogenes  12.8%  cefprozil equivalent to control  Overall  100.0%  cefprozil equivalent to control  SAFETY:
The incidence of adverse events in the cefprozil arm was comparable to the incidence of adverse events in the control arm (agent that contained a specific ß-lactamase inhibitor).
References
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically-Third Edition. Approved Standard NCCLS Document M7-A3, Vol.13, No. 25, NCCLS, Villanova, PA, December 1993.
- National Committee for Clinical Laboratory Standards. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria-Third Edition. Approved Standard NCCLS Document M11-A3, Vol. 13, No. 26, NCCLS, Villanova, PA, December 1993.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests -Fifth Edition. Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24, NCCLS, Villanova, PA, December 1993.
Clintest¬ģ and Clinistix¬ģ are registered trademarks of Bayer HealthCare LLC.
Manufactured for:                                                          Manufactured by                      
Lupin Pharmaceuticals, Inc.                                       Lupin Limited
Baltimore, Maryland 21202                                           Mandideep 462046
United States                                                                 INDIA
Revised: January 2017                                                   ID#: 250340
Package Label.principal Display Panel
CEFPROZIL TABLETS USP
Rx Only
250 mg
NDC 68180-403-01
100 Tablets

CEFPROZIL TABLETS USP
Rx Only
500 mg
NDC 68180-404-01
50 Tablets

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


