Clomipramine Hydrochloride (clomipramine hydrochloride 25 mg) Dailymed
Generic: clomipramine hydrochloride is used for the treatment of Depressive Disorder Myocardial Infarction Obsessive-Compulsive Disorder Pain Panic Disorder
IMPRINT: LP 167
SHAPE: capsule
COLOR: orange
All Imprints
clomipramine hydrochloride capsule - lp 166 capsule yellow
clomipramine hydrochloride capsule - lp 167 capsule orange
clomipramine hydrochloride capsule - lp 165 capsule orange
Boxed Warning
Boxed Warning Section
Go PRO for all pill images
Boxed Warning Section
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of clomipramine hydrochloride or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressant compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality , or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Clomipramine hydrochloride is not approved for use in pediatric patients except for patients with obsessive compulsive disorder (OCD) (see WARNINGS, Clinical Worsening and Suicide Risk; PRECAUTIONS, Information for Patients; and PRECAUTIONS, Pediatric Use).
Description
Clomipramine Hydrochloride Capsules, USP is an antiobsessional drug that belongs to the class (dibenzazepine) of pharmacologic agents known as tricyclic antidepressants. Clomipramine Hydrochloride Capsules, USP is available as capsules of 25, 50, and 75 mg for oral administration.
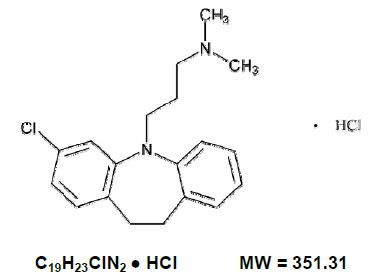
Clomipramine hydrochloride USP is 3-chloro-5-[3-(dimethylamino) propyl]-10,11-dihydro 5H-dibenz [b,f] azepine monohydrochloride, and its structural formula is:
Clomipramine hydrochloride, USP is a white to slightly yellow crystalline powder. It is freely soluble in water and methylene chloride; soluble in alcohol.
Inactive Ingredients. D&C Yellow No. 10, FD&C Blue No. 1, (25 mg only), FD&C Red No. 40, (25 mg and 75 mg only), gelatin, magnesium stearate, pregelatinized starch, titanium dioxide.
Clinical Pharmacology
Pharmacodynamics
Clomipramine (CMI) is presumed to influence obsessive and compulsive behaviors through its effects on serotonergic neuronal transmission. The actual neurochemical mechanism is unknown, but CMI’s capacity to inhibit the reuptake of serotonin (5-HT) is thought to be important.
Pharmacokinetics
Absorption/Bioavailability – CMI from Clomipramine hydrochloride capsules, USP is as bioavailable as CMI from a solution. The bioavailability of CMI from capsules is not significantly affected by food.
In a dose proportionality study involving multiple CMI doses, steady-state plasma concentrations (Css) and area-under-plasma-concentration-time curves (AUC) of CMI and CMI’s major active metabolite, desmethylclomipramine (DMI), were not proportional to dose over the ranges evaluated, i.e., between 25 to 100 mg/day and between 25 to 150 mg/day, although Css and AUC are approximately linearly related to dose between 100 to 150 mg/day. The relationship between dose and CMI/DMI concentrations at higher daily doses has not been systematically assessed, but if there is significant dose dependency at doses above 150 mg/day, there is the potential for dramatically higher Css and AUC even for patients dosed within the recommended range. This may pose a potential risk to some patients (see WARNINGS and PRECAUTIONS, Drug Interactions).
After a single 50 mg oral dose, maximum plasma concentrations of CMI occur within 2 to 6 hours (mean, 4.7 hr) and range from 56 ng/mL to 154 ng/mL (mean, 92 ng/mL). After multiple daily doses of 150 mg of Clomipramine Hydrochloride, steady-state maximum plasma concentrations range from 94 ng/mL to 339 ng/mL (mean, 218 ng/mL) for CMI and from 134 ng/mL to 532 ng/mL (mean, 274 ng/mL) for DMI. Additional information from a rising dose study of doses up to 250 mg suggests that DMI may exhibit nonlinear pharmacokinetics over the usual dosing range. At a dose of Clomipramine hydrochloride capsules, USP, 200 mg, subjects who had a single blood sample taken approximately 9 to 22 hours, (median 16 hours), after the dose had plasma concentrations of up to 605 ng/mL for CMI, 781 ng/mL for DMI, and 1386 ng/mL for both.
Distribution – CMI distributes into cerebrospinal fluid (CSF) and brain and into breast milk. DMI also distributes into CSF, with a mean CSF/plasma ratio of 2.6. The protein binding of CMI is approximately 97%, principally to albumin, and is independent of CMI concentration. The interaction between CMI and other highly protein-bound drugs has not been fully evaluated, but may be important (see PRECAUTIONS, Drug Interactions).
Metabolism – CMI is extensively biotransformed to DMI and other metabolites and their glucuronide conjugates. DMI is pharmacologically active, but its effects on OCD behaviors are unknown. These metabolites are excreted in urine and feces, following biliary elimination. After a 25 mg radiolabeled dose of CMI in two subjects, 60% and 51%, respectively, of the dose were recovered in the urine and 32% and 24%, respectively, in feces. In the same study, the combined urinary recoveries of CMI and DMI were only about 0.8% to 1.3% of the dose administered. CMI does not induce drug- metabolizing enzymes, as measured by antipyrine half-life.
Elimination – Evidence that the Css and AUC for CMI and DMI may increase disproportionately with increasing oral doses suggests that the metabolism of CMI and DMI may be capacity limited. This fact must be considered in assessing the estimates of the pharmacokinetic parameters presented below, as these were obtained in individuals exposed to doses of 150 mg. If the pharmacokinetics of CMI and DMI are nonlinear at doses above 150 mg, their elimination half-lives may be considerably lengthened at doses near the upper end of the recommended dosing range (i.e., 200 mg/day to 250 mg/day). Consequently, CMI and DMI may accumulate, and this accumulation may increase the incidence of any dose- or plasma-concentration-dependent adverse reactions, in particular seizures (see WARNINGS).
After a 150 mg dose, the half-life of CMI ranges from 19 hours to 37 hours (mean, 32 hr) and that of DMI ranges from 54 hours to 77 hours (mean, 69 hr). Steady-state levels after multiple dosing are typically reached within 7 to 14 days for CMI. Plasma concentrations of the metabolite exceed the parent drug on multiple dosing. After multiple dosing with 150 mg/day, the accumulation factor for CMI is approximately 2.5 and for DMI is 4.6. Importantly, it may take two weeks or longer to achieve this extent of accumulation at constant dosing because of the relatively long elimination half- lives of CMI and DMI (see DOSAGE AND ADMINISTRATION). The effects of hepatic and renal impairment on the disposition of Clomipramine hydrochloride capsules, USP have not been determined.
Interactions – Co-administration of haloperidol with CMI increases plasma concentrations of CMI. Co-administration of CMI with phenobarbital increases plasma concentrations of phenobarbital (see PRECAUTIONS, Drug Interactions). Younger subjects (18 to 40 years of age) tolerated CMI better and had significantly lower steady- state plasma concentrations, compared with subjects over 65 years of age. Children under 15 years of age had significantly lower plasma concentration/dose ratios, compared with adults. Plasma concentrations of CMI were significantly lower in smokers than in nonsmokers.
Indications And Usage
Clomipramine Hydrochloride capsules, USP is indicated for the treatment of obsessions and compulsions in patients with Obsessive-Compulsive Disorder (OCD). The obsessions or compulsions must cause marked distress, be time-consuming, or significantly interfere with social or occupational functioning, in order to meet the DSM-III-R (circa 1989) diagnosis of OCD.
Obsessions are recurrent, persistent ideas, thoughts, images, or impulses that are egodystonic. Compulsions are repetitive, purposeful, and intentional behaviors performed in response to an obsession or in a stereotyped fashion, and are recognized by the person as excessive or unreasonable.
The effectiveness of Clomipramine hydrochloride capsules, USP for the treatment of OCD was demonstrated in multicenter, placebo-controlled, parallel-group studies, including two 10-week studies in adults and one 8-week study in children and adolescents 10 to 17 years of age. Patients in all studies had moderate-to-severe OCD (DSM-III), with mean baseline ratings on the Yale-Brown Obsessive Compulsive Scale (YBOCS) ranging from 26 to 28 and a mean baseline rating of 10 on the NIMH Clinical Global Obsessive Compulsive Scale (NIMH-OC). Patients taking CMI experienced a mean reduction of approximately 10 on the YBOCS, representing an average improvement on this scale of 35% to 42% among adults and 37% among children and adolescents. CMI-treated patients experienced a 3.5 unit decrement on the NIMH-OC. Patients on placebo showed no important clinical response on either scale. The maximum dose was 250 mg/day for most adults and 3 mg/kg/day (up to 200 mg) for all children and adolescents.
The effectiveness of Clomipramine hydrochloride capsules, USP for long-term use (i.e., for more than 10 weeks) has not been systematically evaluated in placebo-controlled trials. The physician who elects to use Clomipramine hydrochloride for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient (see DOSAGE AND ADMINISTRATION).
Contraindications
Clomipramine hydrochloride capsule, USP is contraindicated in patients with a history of hypersensitivity to Clomipramine hydrochloride or other tricyclic antidepressants.
Monoamine Oxidase Inhibitors (MAOIs)
The use of MAOIs intended to treat psychiatric disorders with Clomipramine hydrochloride capsules, USP or within 14 days of stopping treatment with Clomipramine hydrochloride is contraindicated because of an increased risk of serotonin syndrome. The use of Clomipramine hydrochloride capsules, USP within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated (see WARNINGS and DOSAGE AND ADMINISTRATION).
Starting Clomipramine hydrochloride capsules, USP in a patient who is being treated with linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome (see WARNINGS and DOSAGE AND ADMINISTRATION).
Myocardial Infarction
Clomipramine hydrochloride capsules, USP is contraindicated during the acute recovery period after a myocardial infarction.
Warnings
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long- standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
Table 1 Age Range Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated Increases Compared to Placebo <18 14 additional cases 18-24 5 additional cases Decreases Compared to Placebo 25-64 1 fewer case ≥65 6 fewer case
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for clomipramine hydrochloride should be written for the smallest quantity of capsules consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder – A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that clomipramine hydrochloride is not approved for use in treating bipolar depression.
Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported with SNRIs and SSRIs, including Clomipramine hydrochloride capsules, USP alone but particularly with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort) and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular changes (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome.
The concomitant use of Clomipramine hydrochloride capsules, USP with MAOIs intended to treat psychiatric disorders is contraindicated. Clomipramine hydrochloride capsules, USP should also not be started in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. All reports with methylene blue that provided information on the route of administration involved intravenous administration in the dose range of 1 mg/kg to 8 mg/kg. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection) or at lower doses. There may be circumstances when it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking Clomipramine hydrochloride capsules, USP. Clomipramine hydrochloride capsules, USP should be discontinued before initiating treatment with the MAOI (see CONTRAINDICATIONS and DOSAGE AND ADMINISTRATION).
If concomitant use of Clomipramine hydrochloride capsules, USP with other serotonergic drugs, including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, buspirone, tryptophan, and St. John’s Wort is clinically warranted, patients should be made aware of a potential increased risk for serotonin syndrome, particularly during treatment initiation and dose increases.
Treatment with Clomipramine hydrochloride capsules, USP and any concomitant serotonergic agents should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including Clomipramine hydrochloride capsules, USP may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
Seizures
During premarket evaluation, seizure was identified as the most significant risk of Clomipramine hydrochloride use.
The observed cumulative incidence of seizures among patients exposed to Clomipramine hydrochloride at doses up to 300 mg/day was 0.64% at 90 days, 1.12% at 180 days, and 1.45% at 365 days. The cumulative rates correct the crude rate of 0.7% (25 of 3519 patients) for the variable duration of exposure in clinical trials.
Although dose appears to be a predictor of seizure, there is a confounding of dose and duration of exposure, making it difficult to assess independently the effect of either factor alone. The ability to predict the occurrence of seizures in subjects exposed to doses of CMI greater than 250 mg is limited, given that the plasma concentration of CMI may be dose-dependent and may vary among subjects given the same dose. Nevertheless, prescribers are advised to limit the daily dose to a maximum of 250 mg in adults and 3 mg/kg (or 200 mg) in children and adolescents ( see DOSAGE AND ADMINISTRATION).
Caution should be used in administering Clomipramine hydrochloride capsules, USP to patients with a history of seizures or other predisposing factors, e.g., brain damage of varying etiology, alcoholism, and concomitant use with other drugs that lower the seizure threshold.
Rare reports of fatalities in association with seizures have been reported by foreign postmarketing surveillance, but not in U.S. clinical trials. In some of these cases, Clomipramine hydrochloride capsules, USP had been administered with other epileptogenic agents; in others, the patients involved had possibly predisposing medical conditions. Thus a causal association between Clomipramine hydrochloride treatment and these fatalities has not been established.
Physicians should discuss with patients the risk of taking Clomipramine hydrochloride capsules, USP while engaging in activities in which sudden loss of consciousness could result in serious injury to the patient or others, e.g., the operation of complex machinery, driving, swimming, climbing.
DRESS Rare cases of drug rash with eosinophilia and systemic symptoms (DRESS) have been reported with use of clomipramine. In the event of severe acute reactions such as DRESS, discontinue clomipramine therapy immediately and institute appropriate treatment.
Precautions
General
Suicide – Since depression is a commonly associated feature of OCD, the risk of suicide must be considered. Prescriptions for Clomipramine hydrochloride capsules, USP should be written for the smallest quantity of capsules consistent with good patient management, in order to reduce the risk of overdose.
Cardiovascular Effects – Modest orthostatic decreases in blood pressure and modest tachycardia were each seen in approximately 20% of patients taking Clomipramine hydrochloride capsules, USP in clinical trials; but patients were frequently asymptomatic. Among approximately 1400 patients treated with CMI in the premarketing experience who had ECGs, 1.5% developed abnormalities during treatment, compared with 3.1% of patients receiving active control drugs and 0.7% of patients receiving placebo. The most common ECG changes were PVCs, ST-T wave changes, and intraventricular conduction abnormalities. These changes were rarely associated with significant clinical symptoms. Nevertheless, caution is necessary in treating patients with known cardiovascular disease, and gradual dose titration is recommended.
Psychosis, Confusion, and Other Neuropsychiatric Phenomena – Patients treated with Clomipramine hydrochloride capsules, USP have been reported to show a variety of neuropsychiatric signs and symptoms including delusions, hallucinations, psychotic episodes, confusion, and paranoia. Because of the uncontrolled nature of many of the studies, it is impossible to provide a precise estimate of the extent of risk imposed by treatment with Clomipramine hydrochloride. As with tricyclic antidepressants to which it is closely related, Clomipramine hydrochloride capsules, USP may precipitate an acute psychotic episode in patients with unrecognized schizophrenia.
Mania/Hypomania – During premarketing testing of Clomipramine hydrochloride capsules, USP in patients with affective disorder, hypomania or mania was precipitated in several patients. Activation of mania or hypomania has also been reported in a small proportion of patients with affective disorder treated with marketed tricyclic antidepressants, which are closely related to Clomipramine hydrochloride.
Hepatic Changes – During premarketing testing, Clomipramine hydrochloride capsules, USP was occasionally associated with elevations in SGOT and SGPT (pooled incidence of approximately 1% and 3%, respectively) of potential clinical importance (i.e., values greater than 3 times the upper limit of normal). In the vast majority of instances, these enzyme increases were not associated with other clinical findings suggestive of hepatic injury; moreover, none were jaundiced. Rare reports of more severe liver injury, some fatal, have been recorded in foreign post marketing experience. Caution is indicated in treating patients with known liver disease, and periodic monitoring of hepatic enzyme levels is recommended in such patients.
Hematologic Changes – Although no instances of severe hematologic toxicity were seen in the premarketing experience with Clomipramine hydrochloride capsules, USP there have been postmarketing reports of leukopenia, agranulocytosis, thrombocytopenia, anemia, and pancytopenia in association with Clomipramine hydrochloride capsules, USP use. As is the case with tricyclic antidepressants to which Clomipramine hydrochloride is closely related, leukocyte and differential blood counts should be obtained in patients who develop fever and sore throat during treatment with Clomipramine hydrochloride capsules, USP.
Central Nervous System – More than 30 cases of hyperthermia have been recorded by nondomestic postmarketing surveillance systems. Most cases occurred when Clomipramine hydrochloride capsules, USP was used in combination with other drugs. When Clomipramine hydrochloride capsules, USP and a neuroleptic were used concomitantly, the cases were sometimes considered to be examples of a neuroleptic malignant syndrome.
Sexual Dysfunction – The rate of sexual dysfunction in male patients with OCD who were treated with Clomipramine hydrochloride capsules, USP in the premarketing experience was markedly increased compared with placebo controls (i.e., 42% experienced ejaculatory failure and 20% experienced impotence, compared with 2.0% and 2.6%, respectively, in the placebo group). Approximately 85% of males with sexual dysfunction chose to continue treatment.
Hyponatremia – Hyponatremia has occurred as a result of treatment with clomipramine. In many cases, hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Elderly patients may be at greater risk of developing hyponatremia with a serotonergic antidepressant. Also, patients taking diuretics or who are otherwise volume-depleted can be at greater risk. Discontinuation of Clomipramine Hydrochloride in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted. Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which can lead to falls. More severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
Weight Changes – In controlled studies of OCD, weight gain was reported in 18% of patients receiving Clomipramine hydrochloride capsules, USP, compared with 1% of patients receiving placebo. In these studies, 28% of patients receiving Clomipramine Hydrochloride had a weight gain of at least 7% of their initial body weight, compared with 4% of patients receiving placebo. Several patients had weight gains in excess of 25% of their initial body weight. Conversely, 5% of patients receiving Clomipramine hydrochloride capsules, USP and 1% receiving placebo had weight losses of at least 7% of their initial body weight.
Electroconvulsive Therapy – As with closely related tricyclic antidepressants, concurrent administration of Clomipramine hydrochloride capsules, USP with electroconvulsive therapy may increase the risks; such treatment should be limited to those patients for whom it is essential, since there is limited clinical experience.
Surgery – Prior to elective surgery with general anesthetics, therapy with Clomipramine hydrochloride capsules, USP should be discontinued for as long as is clinically feasible, and the anesthetist should be advised.
Use in Concomitant Illness – As with closely related tricyclic antidepressants, Clomipramine hydrochloride capsules, USP should be used with caution in the following:
- Hyperthyroid patients or patients receiving thyroid medication, because of the possibility of cardiac toxicity;
- Patients with increased intraocular pressure, a history of narrow-angle glaucoma, or urinary retention, because of the anticholinergic properties of the drug;
- Patients with tumors of the adrenal medulla (e.g., pheochromocytoma, neuroblastoma) in whom the drug may provoke hypertensive crises;
- Patients with significantly impaired renal function.
Withdrawal Symptoms – A variety of withdrawal symptoms have been reported in association with abrupt discontinuation of Clomipramine hydrochloride capsules, USP, including dizziness, nausea, vomiting, headache, malaise, sleep disturbance, hyperthermia, and irritability. In addition, such patients may experience a worsening of psychiatric status. While the withdrawal effects of Clomipramine hydrochloride capsules, USP have not been systematically evaluated in controlled trials, they are well known with closely related tricyclic antidepressants, and it is recommended that the dosage be tapered gradually and the patient monitored carefully during discontinuation (see DRUG ABUSE AND DEPENDENCE).
Information For Patients
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with clomipramine hydrochloride and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and other Serious Mental Illness, and Suicidal Thoughts or Actions” is available for clomipramine hydrochloride. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking clomipramine hydrochloride.
Clinical Worsening and Suicide Risk – Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient’s prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient’s presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Physicians are advised to discuss the following issues with patients for whom they prescribe Clomipramine hydrochloride capsules, USP:
(1) The risk of seizure (see WARNINGS);
(2) The relatively high incidence of sexual dysfunction among males (see Sexual Dysfunction);
(3) Since Clomipramine hydrochloride capsules, USP may impair the mental and/or physical abilities required for the performance of complex tasks, and since Clomipramine hydrochloride capsules, USP is associated with a risk of seizures, patients should be cautioned about the performance of complex and hazardous tasks (see WARNINGS);
(4) Patients should be cautioned about using alcohol, barbiturates, or other CNS depressants concurrently, since Clomipramine hydrochloride capsules, USP may exaggerate their response to these drugs;
(5) Patients should notify their physician if they become pregnant or intend to become pregnant during therapy;
(6) Patients should notify their physician if they are breast-feeding.
Patients should be advised that taking Clomipramine hydrochloride capsules, USP can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle-closure glaucoma. Pre existing glaucoma is almost always open-angle glaucoma because angle-closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle-closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible.
Drug Interactions
The risks of using Clomipramine hydrochloride capsules, USP in combination with other drugs have not been systematically evaluated. Given the primary CNS effects of Clomipramine hydrochloride capsules, USP, caution is advised in using it concomitantly with other CNS-active drugs (see Information for Patients). Clomipramine hydrochloride capsules, USP should not be used with MAO inhibitors (see CONTRAINDICATIONS).
Close supervision and careful adjustment of dosage are required when Clomipramine hydrochloride capsules, USP is administered with anticholinergic or sympathomimetic drugs.
Several tricyclic antidepressants have been reported to block the pharmacologic effects of guanethidine, clonidine, or similar agents, and such an effect may be anticipated with CMI because of its structural similarity to other tricyclic antidepressants.
The plasma concentration of CMI has been reported to be increased by the concomitant administration of haloperidol; plasma levels of several closely related tricyclic antidepressants have been reported to be increased by the concomitant administration of methylphenidate or hepatic enzyme inhibitors (e.g., cimetidine, fluoxetine) and decreased by the concomitant administration of hepatic enzyme inducers (e.g., barbiturates, phenytoin), and such an effect may be anticipated with CMI as well. Administration of CMI has been reported to increase the plasma levels of phenobarbital, if given concomitantly (see CLINICAL PHARMACOLOGY, Interactions).
Drugs Metabolized by P450 2D6 – The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7% to 10% of Caucasians are so-called “poor metabolizers”); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8 fold increase in plasma AUC of the TCA). In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dose of TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, paroxetine, and fluvoxamine, inhibit P450 2D6, they may vary in the extent of inhibition. Fluvoxamine has also been shown to inhibit P450 1A2, an isoform also involved in TCA metabolism. The extent to which SSRI-TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the co-administration of TCAs with any of the SSRIs and also in switching from one class to the other. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary). Concomitant use of agents in the tricyclic antidepressant class (which includes Clomipramine hydrochloride) with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant agent or the other drug. Furthermore, whenever one of these drugs is withdrawn from co-therapy, an increased dose of tricyclic antidepressant agent may be required. It is desirable to monitor TCA plasma levels whenever an agent of the tricyclic antidepressant class including Clomipramine hydrochloride capsules, USP is going to be co-administered with another drug known to be an inhibitor of P450 2D6 (and/or P450 1A2).
Because Clomipramine hydrochloride capsules, USP is highly bound to serum protein, the administration of Clomipramine hydrochloride to patients taking other drugs that are highly bound to protein (e.g., warfarin, digoxin) may cause an increase in plasma concentrations of these drugs, potentially resulting in adverse effects. Conversely, adverse effects may result from displacement of protein-bound Clomipramine hydrochloride capsules, USP by other highly bound drugs (see CLINICAL PHARMACOLOGY, Distribution).
Monoamine Oxidase Inhibitors (MAOIs)
(See CONTRAINDICATIONS, WARNINGS, and DOSAGE AND ADMINISTRATION)
Serotonergic Drugs
(See CONTRAINDICATIONS, WARNINGS, and DOSAGE AND ADMINISTRATION)
Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenicity was found in two 2-year bioassays in rats at doses up to 100 mg/kg, which is 24 and 4 times the maximum recommended human daily dose (MRHD) on a mg/kg and mg/m2 basis, respectively, or in a 2-year bioassay in mice at doses up to 80 mg/kg, which is 20 and 1.5 times the MRHD on a mg/kg and mg/m2 basis, respectively.
In reproduction studies, no effects on fertility were found in rats given up to 24 mg/kg, which is 6 times, and approximately equal to, the MRHD on a mg/kg and mg/m2 basis, respectively.
Pregnancy
No teratogenic effects were observed in studies performed in rats and mice at doses up to 100 mg/kg, which is 24 times the maximum recommended human daily dose (MRHD) on a mg/kg basis and 4 times (rats) and 2 times (mice) the MRHD on a mg/m2 basis. Slight nonspecific embryo/fetotoxic effects were seen in the offspring of treated rats given 50 and 100 mg/kg and of treated mice given 100 mg/kg.
There are no adequate or well-controlled studies in pregnant women. Withdrawal symptoms, including jitteriness, tremor, and seizures, have been reported in neonates whose mothers had taken Clomipramine hydrochloride capsules, USP until delivery. Clomipramine hydrochloride capsules, USP should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Clomipramine hydrochloride capsules, USP has been found in human milk. Because of the potential for adverse reactions, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in the pediatric population other than pediatric patients with OCD have not been established (see BOX WARNING and WARNINGS, Clinical Worsening and Suicide Risk). Anyone considering the use of Clomipramine hydrochloride capsules, USP in a child or adolescent must balance the potential risks with the clinical need.
In a controlled clinical trial in children and adolescents (10 to 17 years of age), 46 outpatients received Clomipramine hydrochloride capsules, USP for up to 8 weeks. In addition, 150 adolescent patients have received Clomipramine hydrochloride capsules, USP in open-label protocols for periods of several months to several years. Of the 196 adolescents studied, 50 were 13 years of age or less and 146 were 14 to 17 years of age. The adverse reaction profile in this age group (see ADVERSE REACTIONS) is similar to that observed in adults.
The risks, if any, that may be associated with Clomipramine hydrochloride’s extended use in children and adolescents with OCD have not been systematically assessed. The evidence supporting the conclusion that Clomipramine hydrochloride capsules, USP is safe for use in children and adolescents is derived from relatively short term clinical studies and from extrapolation of experience gained with adult patients. In particular, there are no studies that directly evaluate the effects of long term Clomipramine hydrochloride capsules, USP use on the growth, development, and maturation of children and adolescents. Although there is no evidence to suggest that Clomipramine Hydrochloride adversely affects growth, development or maturation, the absence of such findings is not adequate to rule out a potential for such effects in chronic use.
The safety and effectiveness in pediatric patients below the age of 10 have not been established. Therefore, specific recommendations cannot be made for the use of Clomipramine hydrochloride capsules, USP in pediatric patients under the age of 10.
Geriatric Use
Clinical studies of Clomipramine hydrochloride capsules, USP did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger subjects; 152 patients at least 60 years of age participating in various U.S. clinical trials received Clomipramine hydrochloride capsules, USP for periods of several months to several years. No unusual age-related adverse events were identified in this population. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
Clomipramine Hydrochloride has been associated with cases of clinically significant hyponatremia. Elderly patients may be at greater risk for this adverse reaction (seePRECAUTIONS, Hyponatremia ).
Adverse Reactions
Commonly Observed
The most commonly observed adverse events associated with the use of Clomipramine hydrochloride capsules, USP and not seen at an equivalent incidence among placebo-treated patients were gastrointestinal complaints, including dry mouth, constipation, nausea, dyspepsia, and anorexia; nervous system complaints, including somnolence, tremor, dizziness, nervousness, and myoclonus; genitourinary complaints, including changed libido, ejaculatory failure, impotence, and micturition disorder; and other miscellaneous complaints, including fatigue, sweating, increased appetite, weight gain, and visual changes.
Leading to Discontinuation of Treatment
Approximately 20% of 3616 patients who received Clomipramine hydrochloride capsules, USP in U.S. premarketing clinical trials discontinued treatment because of an adverse event. Approximately one-half of the patients who discontinued (9% of the total) had multiple complaints, none of which could be classified as primary. Where a primary reason for discontinuation could be identified, most patients discontinued because of nervous system complaints (5.4%), primarily somnolence. The second-most-frequent reason for discontinuation was digestive system complaints (1.3%), primarily vomiting and nausea.
There was no apparent relationship between the adverse events and elevated plasma drug concentrations.
Incidence in Controlled Clinical Trials
The following table enumerates adverse events that occurred at an incidence of 1% or greater among patients with OCD who received Clomipramine hydrochloride capsules, USP in adult or pediatric placebo-controlled clinical trials. The frequencies were obtained from pooled data of clinical trials involving either adults receiving Clomipramine hydrochloride capsules, USP (N=322) or placebo (N=319) or children treated with Clomipramine hydrochloride (N=46) or placebo (N=44). The prescriber should be aware that these figures cannot be used to predict the incidence of side effects in the course of usual medical practice, in which patient characteristics and other factors differ from those that prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, provide the physician with a basis for estimating the relative contribution of drug and nondrug factors to the incidence of side effects in the populations studied.
Incidence of Treatment-Emergent Adverse Experience in Placebo-Controlled Clinical Trials (Percentage of Patients Reporting Event) Adults Children and Adolescents Body System/ Adverse Event Events reported by at least 1% of Clomipramine hydrochloride capsules, USP patients are included. Clomipramine hydrochloride(N=322) Placebo (N=319) Clomipramine hydrochloride (N=46) Placebo (N=44) Nervous System Somnolence 54 16 46 11 Tremor 54 2 33 2 Dizziness 54 14 41 14 Headache 52 41 28 34 Insomnia 25 15 11 7 Libido change 21 3 - - Nervousness 18 2 4 2 Myoclonus 13 - 2 - Increased appetite 11 2 - 2 Paresthesia 9 3 2 2 Memory impairment 9 1 7 2 Anxiety 9 4 2 - Twitching 7 1 4 5 Impaired concentration 5 2 - - Depression 5 1 - - Hypertonia 4 1 2 - Sleep disorder 4 - 9 5 Psychosomatic disorder 3 - - - Yawning 3 - - - Confusion 3 - 2 - Speech disorder 3 - - - Abnormal dreaming 3 - - 2 Agitation 3 - - - Migraine 3 - - - Depersonalization 2 - 2 - Irritability 2 2 2 - Emotional lability 2 - - 2 Panic reaction 1 - 2 - Aggressive reaction - - 2 - Paresis - - 2 - Skin and Appendages Increased sweating 29 3 9 - Rash 8 1 4 2 Pruritus 6 - 2 2 Dermatitis 2 - - 2 Acne 2 2 - 5 Dry skin 2 - - 5 Urticaria 1 - - - Abnormal skin odor - - 2 - Digestive System Dry mouth 84 17 63 16 Constipation 47 11 22 9 Nausea 33 14 9 11 Dyspepsia 22 10 13 2 Diarrhea 13 9 7 5 Anorexia 12 - 22 2 Abdominal pain 11 9 13 16 Vomiting 7 2 7 - Flatulence 6 3 - 2 Tooth disorder 5 - - - Gastrointestinal disorder 2 - - 2 Dysphagia 2 - - - Esophagitis 1 - - - Eructation - - 2 2 Ulcerative stomatitis - - 2 - Body as a Whole Fatigue 39 18 35 9 Weight increase 18 1 2 - Flushing 8 - 7 - Hot flushes 5 - 2 - Chest pain 4 4 7 - Fever 4 - 2 7 Allergy 3 3 7 5 Pain 3 2 4 2 Local edema 2 4 - - Chills 2 1 - - Weight decrease - - 7 - Otitis media - - 4 5 Asthenia - - 2 - Halitosis - - 2 - Cardiovascular System Postural hypotension 6 - 4 - Palpitation 4 2 4 - Tachycardia 4 - 2 - Syncope - - 2 - Respiratory System Pharyngitis 14 9 - 5 Rhinitis 12 10 7 9 Sinusitis 6 4 2 5 Coughing 6 6 4 5 Bronchospasm 2 - 7 2 Epistaxis 2 - - 2 Dyspnea - - 2 - Laryngitis - 1 2 - Urogenital System Male and Female Patients Combined Micturition disorder 14 2 4 2 Urinary tract infection 6 1 - - Micturition frequency 5 3 - - Urinary retention 2 - 7 - Dysuria 2 2 - - Cystitis 2 - - - Female Patients Only (N=182) (N=167) (N=10) (N=21) Dysmenorrhea 12 14 10 10 Lactation (nonpuerperal) 4 - - - Menstrual disorder 4 2 - - Vaginitis 2 - - - Leukorrhea 2 - - - Breast enlargement 2 - - - Breast pain 1 - - - Amenorrhea 1 - - - Male Patients Only (N=140) (N=152) (N=36) (N=23) Ejaculation failure 42 2 6 - Impotence 20 3 - - Special Senses Abnormal vision 18 4 7 2 Taste perversion 8 - 4 - Tinnitus 6 - 4 - Abnormal lacrimation 3 2 - - Mydriasis 2 - - - Conjunctivitis 1 - - - Anisocoria - - 2 - Blepharospasm - - 2 - Ocular allergy - - 2 - Vestibular disorder - - 2 2 Musculoskeletal Myalgia 13 9 - - Back pain 6 6 - - Arthralgia 3 5 - - Muscle weakness 1 - 2 - Hemic and Lymphatic Purpura 3 - - - Anemia - - 2 2 Metabolic and Nutritional Thirst 2 2 - 2
Other Events Observed During the Premarketing Evaluation of Clomipramine hydrochloride capsules, USP
During clinical testing in the U.S., multiple doses of Clomipramine hydrochloride capsules, USP were administered to approximately 3600 subjects. Untoward events associated with this exposure were recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of untoward events into a smaller number of standardized event categories.
In the tabulations that follow, a modified World Health Organization dictionary of terminology has been used to classify reported adverse events. The frequencies presented, therefore, represent the proportion of the 3525 individuals exposed to Clomipramine hydrochloride capsules, USP who experienced an event of the type cited on at least one occasion while receiving Clomipramine hydrochloride. All events are included except those already uled in the previous table, those reported in terms so general as to be uninformative, and those in which an association with the drug was remote. It is important to emphasize that although the events reported occurred during treatment with Clomipramine hydrochloride capsules, USP, they were not necessarily caused by it.
Events are further categorized by body system and uled in order of decreasing frequency according to the following definitions: frequent adverse events are those occurring on one or more occasions in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in less than 1/1000 patients.
Body as a Whole – Infrequent - general edema, increased susceptibility to infection, malaise. Rare - dependent edema, withdrawal syndrome.
Cardiovascular System – Infrequent - abnormal ECG, arrhythmia, bradycardia, cardiac arrest, extrasystoles, pallor. Rare - aneurysm, atrial flutter, bundle branch block, cardiac failure, cerebral hemorrhage, heart block, myocardial infarction, myocardial ischemia, peripheral ischemia, thrombophlebitis, vasospasm, ventricular tachycardia.
Digestive System – Infrequent - abnormal hepatic function, blood in stool, colitis, duodenitis, gastric ulcer, gastritis, gastroesophageal reflux, gingivitis, glossitis, hemorrhoids, hepatitis, increased saliva, irritable bowel syndrome, peptic ulcer, rectal hemorrhage, tongue ulceration, tooth caries. Rare - cheilitis, chronic enteritis, discolored feces, gastric dilatation, gingival bleeding, hiccup, intestinal obstruction, oral/pharyngeal edema, paralytic ileus, salivary gland enlargement.
Endocrine System – Infrequent - hypothyroidism. Rare - goiter, gynecomastia, hyperthyroidism.
Hemic and Lymphatic System – Infrequent - lymphadenopathy. Rare - leukemoid reaction, lymphoma-like disorder, marrow depression.
Metabolic and Nutritional Disorder – Infrequent - dehydration, diabetes mellitus, gout, hypercholesterolemia, hyperglycemia, hyperuricemia, hypokalemia. Rare - fat intolerance, glycosuria.
Musculoskeletal System – Infrequent - arthrosis. Rare - dystonia, exostosis, lupus erythematosus rash, bruising, myopathy, myositis, polyarteritis nodosa, torticollis.
Nervous System – Frequent - abnormal thinking, vertigo. Infrequent - abnormal coordination, abnormal EEG, abnormal gait, apathy, ataxia, coma, convulsions, delirium, delusion, dyskinesia, dysphonia, encephalopathy, euphoria, extrapyramidal disorder, hallucinations, hostility, hyperkinesia, hypnagogic hallucinations, hypokinesia, leg cramps, manic reaction, neuralgia, paranoia, phobic disorder, psychosis, sensory disturbance, somnambulism, stimulation, suicidal ideation, suicide attempt, teeth- grinding. Rare - anticholinergic syndrome, aphasia, apraxia, catalepsy, cholinergic syndrome, choreoathetosis, generalized spasm, hemiparesis, hyperesthesia, hyperreflexia, hypoesthesia, illusion, impaired impulse control, indecisiveness, mutism, neuropathy, nystagmus, oculogyric crisis, oculomotor nerve paralysis, schizophrenic reaction, stupor, suicide.
Respiratory System – Infrequent - bronchitis, hyperventilation, increased sputum, pneumonia. Rare - cyanosis, hemoptysis, hypoventilation, laryngismus.
Skin and Appendages – Infrequent - alopecia, cellulitis, cyst, eczema, erythematous rash, genital pruritus, maculopapular rash, photosensitivity reaction, psoriasis, pustular rash, skin discoloration. Rare - chloasma, folliculitis, hypertrichosis, piloerection, seborrhea, skin hypertrophy, skin ulceration.
Special Senses – Infrequent - abnormal accommodation, deafness, diplopia, earache, eye pain, foreign body sensation, hyperacusis, parosmia, photophobia, scleritis, taste loss. Rare - blepharitis, chromatopsia, conjunctival hemorrhage, exophthalmos, glaucoma, keratitis, labyrinth disorder, night blindness, retinal disorder, strabismus, visual field defect.
Urogenital System – Infrequent - endometriosis, epididymitis, hematuria, nocturia, oliguria, ovarian cyst, perineal pain, polyuria, prostatic disorder, renal calculus, renal pain, urethral disorder, urinary incontinence, uterine hemorrhage, vaginal hemorrhage. Rare - albuminuria, anorgasmy, breast engorgement, breast fibroadenosis, cervical dysplasia, endometrial hyperplasia, premature ejaculation, pyelonephritis, pyuria, renal cyst, uterine inflammation, vulvar disorder.
Postmarketing Experience The following adverse drug reaction has been reported during post-approval use of Clomipramine hydrochloride capsules, USP. Because this reaction is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate frequency.
Eye Disorders – Angle-closure glaucoma.
Immune System Disorders- Drug Rash with Eosinophilia and Systemic Symptoms(DRESS)
Metabolism and Nutrition Disorders – Hyponatremia.
Endocrine Disorders – Syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Drug Abuse And Dependence
Clomipramine hydrochloride capsules, USP has not been systematically studied in animals or humans for its potential for abuse, tolerance, or physical dependence. While a variety of withdrawal symptoms have been described in association with Clomipramine hydrochloride capsules, USP discontinuation (see PRECAUTIONS, Withdrawal Symptoms), there is no evidence for drug-seeking behavior, except for a single report of potential Clomipramine hydrochloride capsules, USP abuse by a patient with a history of dependence on codeine, benzodiazepines, and multiple psychoactive drugs. The patient received Clomipramine hydrochloride capsules, USP for depression and panic attacks and appeared to become dependent after hospital discharge
Despite the lack of evidence suggesting an abuse liability for Clomipramine hydrochloride capsules, USP in foreign marketing, it is not possible to predict the extent to which Clomipramine hydrochloride capsules, USP might be misused or abused once marketed in the U.S. Consequently, physicians should carefully evaluate patients for a history of drug abuse and follow such patients closely.
Overdosage
Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic overdose. Therefore, hospital monitoring is required as soon as possible.
Human Experience
In U.S. clinical trials, 2 deaths occurred in 12 reported cases of acute overdosage with Clomipramine hydrochloride capsules, USP either alone or in combination with other drugs. One death involved a patient suspected of ingesting a dose of 7000 mg. The second death involved a patient suspected of ingesting a dose of 5750 mg. The 10 nonfatal cases involved doses of up to 5000 mg, accompanied by plasma levels of up to 1010 ng/mL. All 10 patients completely recovered. Among reports from other countries of Clomipramine hydrochloride overdose, the lowest dose associated with a fatality was 750 mg. Based upon postmarketing reports in the United Kingdom, CMI’s lethality in overdose is considered to be similar to that reported for closely related tricyclic compounds marketed as antidepressants.
Manifestations
Signs and symptoms vary in severity depending upon factors such as the amount of drug absorbed, the age of the patient, and the time elapsed since drug ingestion. Critical manifestations of overdose include cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic toxicity. Other CNS manifestations may include drowsiness, stupor, ataxia, restlessness, agitation, delirium, severe perspiration, hyperactive reflexes, muscle rigidity, and athetoid and choreiform movements. Cardiac abnormalities may include tachycardia, signs of congestive heart failure, and in very rare cases, cardiac arrest. Respiratory depression, cyanosis, shock, vomiting, hyperpyrexia, mydriasis, and oliguria or anuria may also be present.
Management
Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient’s airway, establish an intravenous line, and initiate gastric decontamination. A minimum of 6 hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary.
If signs of toxicity occur at any time during this period, extended monitoring is required. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient.
Gastrointestinal Decontamination – All patients suspected of tricyclic overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. Emesis is contraindicated.
Cardiovascular – A maximal limb-lead QRS duration of ≥ 0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH >7.60 or a pCO2 <20 mmHg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium, or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in tricyclic poisoning.
CNS – In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
Psychiatric Follow-up – Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
Pediatric Management – The principles of management of child and adult overdosages are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
Dosage And Administration
The treatment regimens described below are based on those used in controlled clinical trials of Clomipramine hydrochloride capsules, USP in 520 adults, and 91 children and adolescents with OCD. During initial titration, Clomipramine hydrochloride should be given in divided doses with meals to reduce gastrointestinal side effects. The goal of this initial titration phase is to minimize side effects by permitting tolerance to side effects to develop or allowing the patient time to adapt if tolerance does not develop.
Because both CMI and its active metabolite, DMI, have long elimination half-lives, the prescriber should take into consideration the fact that steady-state plasma levels may not be achieved until 2 to 3 weeks after dosage change (see CLINICAL PHARMACOLOGY). Therefore, after initial titration, it may be appropriate to wait 2 to 3 weeks between further dosage adjustments.
Initial Treatment/Dose Adjustment (Adults)
Treatment with Clomipramine hydrochloride capsules, USP should be initiated at a dosage of 25 mg daily and gradually increased, as tolerated, to approximately 100 mg during the first 2 weeks. During initial titration, Clomipramine hydrochloride should be given in divided doses with meals to reduce gastrointestinal side effects. Thereafter, the dosage may be increased gradually over the next several weeks, up to a maximum of 250 mg daily. After titration, the total daily dose may be given once daily at bedtime to minimize daytime sedation.
Initial Treatment/Dose Adjustment (Children and Adolescents)
As with adults, the starting dose is 25 mg daily and should be gradually increased (also given in divided doses with meals to reduce gastrointestinal side effects) during the first 2 weeks, as tolerated, up to a daily maximum of 3 mg/kg or 100 mg, whichever is smaller. Thereafter, the dosage may be increased gradually over the next several weeks up to a daily maximum of 3 mg/kg or 200 mg, whichever is smaller (see PRECAUTIONS, Pediatric Use). As with adults, after titration, the total daily dose may be given once daily at bedtime to minimize daytime sedation.
Maintenance/Continuation Treatment (Adults, Children, and Adolescents)
While there are no systematic studies that answer the question of how long to continue Clomipramine hydrochloride capsules, USP, OCD is a chronic condition and it is reasonable to consider continuation for a responding patient. Although the efficacy of Clomipramine hydrochloride capsules, USP after 10 weeks has not been documented in controlled trials, patients have been continued in therapy under double-blind conditions for up to 1 year without loss of benefit. However, dosage adjustments should be made to maintain the patient on the lowest effective dosage, and patients should be periodically reassessed to determine the need for treatment. During maintenance, the total daily dose may be given once daily at bedtime.
Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders
At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with Clomipramine hydrochloride capsules, USP. Conversely, at least 14 days should be allowed after stopping Clomipramine hydrochloride capsules, USP before starting an MAOI intended to treat psychiatric disorders (see CONTRAINDICATIONS).
Use of Clomipramine hydrochloride capsules, USP With Other MAOIs, Such as Linezolid or Methylene Blue
Do not start Clomipramine hydrochloride capsules, USP in a patient who is being treated with linezolid or intravenous methylene blue because there is increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered (see CONTRAINDICATIONS).
In some cases, a patient already receiving Clomipramine hydrochloride capsules, USP therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, Clomipramine hydrochloride capsules, USP should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for two weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with Clomipramine hydrochloride capsules, USP may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue (see WARNINGS).
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with Clomipramine hydrochloride capsules, USP is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use (see WARNINGS).
How Supplied
Clomipramine hydrochloride capsules USP Capsules 25 mg – Opaque orange cap imprinted in black with LP 167 and opaque blue body imprinted in black with LP 167.
Bottles of 30………....………...……….....…... NDC 69315-167-03Bottles of 90………....………...……….....…... NDC 69315-167-09Bottles of 100………....………...……….....…. NDC 69315-167-01
Capsules 50 mg – Opaque yellow cap and body imprinted in black with LP 166. Bottles of 30………..………...……….....…….NDC 69315-166-03Bottles of 90………....………...……….....…...NDC 69315-166-09Bottles of 100………..………...……….......… NDC 69315-166-01
Capsules 75 mg – Opaque orange cap imprinted in black with LP 165 and opaque white body imprinted in black with with LP 165. Bottles of 30……….………...……….....………NDC 69315-165-03Bottles of 100……….………...……….....……. NDC 69315-165-01
Storage – Store at 20º to 25ºC (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in well-closed, light-resistant container as defined in the USP, using a child-resistant closure.
Animal Toxicology
Phospholipidosis and testicular changes, commonly associated with tricyclic compounds, have been observed with Clomipramine hydrochloride capsules, USP. In chronic rat studies, changes related to Clomipramine hydrochloride capsules, USP consisted of systemic phospholipidosis, alterations in the testes (atrophy, mineralization) and secondary changes in other tissues. In addition, cardiac thrombosis and dermatitis/keratitis were observed in rats treated for 2 years at doses which were 24 and 10 times the maximum recommended human daily dose (MRHD), respectively, on a mg/kg basis, and 4 and 1.5 times the MRHD, respectively, on a mg/m2 basis.
Manufactured by:Leading Pharma, LLCFairfield, NJ 07004
Rev. 06 01/20
Medication Guide Clomipramine Hydrochloride Capsules Uspkloe Mip' Ra Meen Hye'' Droe Klor' Ide
Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Read the Medication Guide that comes with you or your family member’s antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines. Talk to your, or your family member’s, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
Low salt (sodium) levels in the blood. Elderly people may be at greater risk for this. Symptoms may include:
- headache
- weakness or feeling unsteady
- confusion, problems concentrating or thinking or memory problems
Visual problems
- eye pain
- changes in vision
- swelling or redness in or around the eye
Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are.
Who should not take Clomipramine hydrochloride capsules, USP?
Do not take Clomipramine hydrochloride capsules, USP if you:
- take a monoamine oxidase inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid.
- Do not take an MAOI within 2 weeks of stopping Clomipramine hydrochloride capsules, USP unless directed to do so by your physician.
- Do not start Clomipramine hydrochloride capsules, USP if you stopped taking an MAOI in the last 2 weeks unless directed to do so by your physician.
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a ul of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
CALL YOUR DOCTOR FOR MEDICAL ADVICE ABOUT SIDE EFFECTS. YOU MAY REPORT SIDE EFFECTS TO THE FDA AT 1-800-FDA-1088 OR LEADING PHARMA, LLC AT 1-844-740-7500.
Manufactured by:Leading Pharma, LLCFairfield, NJ 07004
Rev. 06 01/2020
Package Label.principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site










