Dexmedetomidine Hydrochloride Dailymed
Generic: dexmedetomidine hydrochloride is used for the treatment of Pain Psychomotor Agitation
Go PRO for all pill images
Intramuscular and Intravenous use in Dogs
Intramuscular use in Cats
Sedative, Analgesic, Preanesthetic
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description:
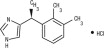
Dexmedetomidine Hydrochloride Injection is a synthetic alpha2-adrenoceptor agonist with sedative and analgesic properties. The chemical name is (+)-4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole monohydrochloride. It is a white, or almost white, crystalline, water soluble substance having a molecular weight of 236.7. The molecular formula is C13H16N2 •HCl and the structural formula is:
Each mL of Dexmedetomidine Hydrochloride Injection contains 0.5 mg dexmedetomidine hydrochloride, 1.6 mg methylparaben, 0.2 mg propylparaben, 9.0 mg sodium chloride, water for injection, q.s.
Indications:
Dexmedetomidine Hydrochloride Injection is indicated for use as a sedative and analgesic in dogs and cats to facilitate clinical examinations, clinical procedures, minor surgical procedures, and minor dental procedures. Dexmedetomidine Hydrochloride Injection is also indicated for use as a preanesthetic to general anesthesia in dogs and cats.
Dosage And Administration:
Dogs: Sedation and Analgesia: 500 mcg/m 2 intramuscularly (IM) or 375 mcg/m 2 intravenously (IV).
Preanesthesia: 125 or 375 mcg/m 2 IM.
The choice of preanesthetic dose depends on the duration and severity of the procedure, as well as the anesthetic regime. The following two tables may be used to determine the correct dexmedetomidine dosage. Note that the mcg/kg dosagedecreases as body weight increases. For example, dogs weighing 2 kg are dosed at 28.1 mcg/kg dexmedetomidine IV, compared to dogs weighing 80 kg that are dosed at 8.7 mcg/kg. Due to the small volume of administration, accurate dosing is not possible in dogs weighing less than 2 kg (4.4 lb).
Table 1: CANINE SEDATION/ANALGESIA DOSE TABLE: Intravenous (IV) and Intramuscular (IM) dosing on the basis of body weight Dexmedetomidine Hydrochloride Injection 0.5 mg/mL Sedation/analgesia in dogs DogWeight Dexmedetomidine375 mcg/m 2 IV Dexmedetomidine 500 mcg/m 2 IM lbs kg mcg/kg ml mcg/kg mL 4.4 to 7 2 to 3 28.1 0.12 40 0.15 7.1 to 9 3.1 to 4 25 0.15 35 0.2 9.1 to 11 4.1 to 5 23 0.2 30 0.3 11.1 to 22 5.1 to 10 19.6 0.29 25 0.4 22.1 to 29 10.1 to 13 16.8 0.38 23 0.5 29.1 to 33 13.1 to 15 15.7 0.44 21 0.6 33.1 to 44 15.1 to 20 14.6 0.51 20 0.7 44.1 to 55 20.1 to 25 13.4 0.6 18 0.8 55.1 to 66 25.1 to 30 12.6 0.69 17 0.9 66.1 to 73 30.1 to 33 12 0.75 16 1 73.1 to 81 33.1 to 37 11.6 0.81 15 1.1 81.1 to 99 37.1 to 45 11 0.9 14.5 1.2 99.1 to 110 45.1 to 50 10.5 0.99 14 1.3 110.1 to 121 50.1 to 55 10.1 1.06 13.5 1.4 121.1 to 132 55.1 to 60 9.8 1.13 13 1.5 132.1 to 143 60.1 to 65 9.5 1.19 12.8 1.6 143.1 to 154 65.1 to 70 9.3 1.26 12.5 1.7 154.1 to 176 70.1 to 80 9 1.35 12.3 1.8 >176 >80 8.7 1.42 12 1.9
Table 2: CANINE PREANESTHESIA DOSE TABLE: Intramuscular (IM) dosing on the basis of body weight Dexmedetomidine Hydrochloride Injection 0.5 mg/mL Preanesthesia in dogs DogWeight Dexmedetomidine125 mcg/m 2 IM Dexmedetomidine375 mcg/m 2 IM lbs kg mcg/kg mL mcg/kg mL 4.4 to 7 2 to 3 9.4 0.04 28.1 0.12 7.1 to 9 3.1 to 4 8.3 0.05 25 0.15 9.1 to 11 4.1 to 5 7.7 0.07 23 0.2 11.1 to 22 5.1 to 10 6.5 0.1 19.6 0.29 22.1 to 29 10.1 to 13 5.6 0.13 16.8 0.38 29.1 to 33 13.1 to 15 5.2 0.15 15.7 0.44 33.1 to 44 15.1 to 20 4.9 0.17 14.6 0.51 44.1 to 55 20.1 to 25 4.5 0.2 13.4 0.6 55.1 to 66 25.1 to 30 4.2 0.23 12.6 0.69 66.1 to 73 30.1 to 33 4 0.25 12 0.75 73.1 to 81 33.1 to 37 3.9 0.27 11.6 0.81 81.1 to 99 37.1 to 45 3.7 0.3 11 0.9 99.1 to 110 45.1 to 50 3.5 0.33 10.5 0.99 110.1 to 121 50.1 to 55 3.4 0.35 10.1 1.06 121.1 to 132 55.1 to 60 3.3 0.38 9.8 1.13 132.1 to 143 60.1 to 65 3.2 0.4 9.5 1.19 143.1 to 154 65.1 to 70 3.1 0.42 9.3 1.26 154.1 to 176 70.1 to 80 3 0.45 9 1.35 >176 >80 2.9 0.47 8.7 1.42
The use of dexmedetomidine as a preanesthetic markedly reduces anesthetic requirements in dogs. Injectable induction drug requirements for intubation will be reduced between 30% and 60%, depending on the choice of anesthetic and the dexmedetomidine preanesthetic dose. The concentration of inhalation maintenance anesthetic will be reduced between 40% and 60%, depending on the dose of dexmedetomidine. The anesthetic dose should always be titrated against the response of the patient. The choice of anesthetic is left to the discretion of the veterinarian.
Sedation, Analgesia and Preanesthesia: 40 mcg/kg intramuscularly (IM).
Cats:
This dose can also be used as a preanesthetic and has been shown to markedly reduce anesthetic requirements in cats. Injectable anesthetic drug requirements for intubation were reduced up to 49%, depending on the choice of induction drug. The concentration of inhalation maintenance anesthetic was reduced between 35% and 44%, depending on the choice of induction drug. The anesthetic dose should always be titrated against the response of the patient.
The following table may be used to determine the correct dexmedetomidine dosage for cats based on body weight.
Table 3: FELINE DOSE TABLE: Intramuscular (IM) dosing on the basis of body weight in cats Dexmedetomidine Hydrochloride Injection 0.5 mg/mL Sedation/analgesia and preanesthesia in cats CatWeight Dexmedetomidine40 mcg/kg IM lbs kg mcg/kg mL 2 to 4 1 to 2 40 0.1 4.1 to 7 2.1 to 3 40 0.2 7.1 to 9 3.1 to 4 40 0.3 9.1 to 13 4.1 to 6 40 0.4 13.1 to 15 6.1 to 7 40 0.5 15.1 to 18 7.1 to 8 40 0.6 18.1 to 22 8.1 to 10 40 0.7
It is recommended that dogs and cats be fasted for 12 hours before treatment with Dexmedetomidine Hydrochloride Injection. An eye lubricant should be applied to cats to prevent corneal desiccation that may result from a reduction in the blink reflex. Following injection of Dexmedetomidine Hydrochloride Injection, the animal should be allowed to rest quietly for 15 minutes; sedation and analgesia occur within 5 to 15 minutes, with peak effects at 30 minutes after dexmedetomidine.
Contraindications:
Do not use Dexmedetomidine Hydrochloride Injection in dogs or cats with cardiovascular disease, respiratory disorders, liver or kidney diseases, or in conditions of shock, severe debilitation, or stress due to extreme heat, cold or fatigue.
As with all alpha2-adrenoceptor agonists, the potential for isolated cases of hypersensitivity, including paradoxical response (excitation), exists.
Warnings:
Human safety: Not for human use. Keep out of reach of children.
Dexmedetomidine Hydrochloride can be absorbed following direct exposure to skin, eyes, or mouth, and may cause irritation. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing.
Appropriate precautions should be taken while handling and using filled syringes. Accidental topical (including ocular) exposure, oral exposure, or exposure by injection could cause adverse reactions, including sedation, hypotension, and bradycardia. Seek medical attention immediately.
Users with cardiovascular disease (for example, hypertension or ischemic heart disease) should take special precautions to avoid any exposure to this product.
Caution should be exercised when handling sedated animals. Handling or any other sudden stimuli, including noise, may cause a defense reaction in an animal that appears to be heavily sedated.
The safety data sheet (SDS) contains more detailed occupational safety information. To report adverse reactions in users or to obtain a copy of the SDS for this product call 1-800-932-5676.
Note to physician: This product contains an alpha2-adrenergic agonist.
Animal safety: Dexmedetomidine should not be administered in the presence of preexisting hypotension, hypoxia, or bradycardia. Due to the pronounced cardiovascular effects of dexmedetomidine, only clinically healthy dogs and cats (ASA classes I and II) should be treated. Animals should be frequently monitored for cardiovascular function and body temperature during sedation or anesthesia. Dexmedetomidine sedation is not recommended for cats with respiratory disease.
The use of dexmedetomidine as a preanesthetic in dogs and cats significantly reduces the amount of induction and maintenance anesthetic requirements. Careful patient monitoring during anesthetic induction and maintenance is necessary to avoid anesthetic overdose.
Precautions:
dexmedetomidine for the prevention of the dexmedetomidine-induced reduction in heart rate. Therefore, the routine use of anticholinergics simultaneously with, or after dexmedetomidine in dogs or cats, is not recommended (see ANIMAL SAFETY).
Apnea may occur with dexmedetomidine use. In the event of apnea, additional oxygen should be supplied. Administration of atipamezole to dogs is warranted when apnea is accompanied by bradycardia and cyanotic mucous membranes.
Adverse reaction reports for dexmedetomidine in cats include rare events of severe dyspnea and respiratory crackles diagnosed as acute pulmonary edema. Dyspnea due to the delayed onset of pulmonary edema could develop in rare instances up to three days after dexmedetomidine administration. Some of these acute and delayed pulmonary edema cases have resulted in death although this was not observed in the feline clinical field studies with dexmedetomidine.
In dogs, intramuscular atipamezole may be routinely used to rapidly reverse the effects of dexmedetomidine. Since analgesic as well as sedative effects will be reversed, pain management may need to be addressed.
In cats, atipamezole has not been evaluated as a routine dexmedetomidine reversal agent. In cats, cases of dyspnea following atipamezole administration have been reported.
Dexmedetomidine has not been evaluated in the presence of other preanesthetics in cats. Although not observed in the feline field studies, death has been reported in cats receiving dexmedetomidine in conjunction with ketamine and butorphanol.
Analgesia resulting from preanesthetic dexmedetomidine may not provide adequate pain control during the postoperative or postprocedural period. Additional pain management should be addressed as needed.
Following administration of dexmedetomidine, a decrease in body temperature is likely to occur unless externally maintained. Once established, hypothermia may persist longer than sedation and analgesia. To prevent hypothermia, treated animals should be kept warm and at a constant temperature during the procedure, and until full recovery.
Nervous or excited animals with high levels of endogenous catecholamines may exhibit a reduced pharmacological response to alpha2-adrenoceptor agonists like dexmedetomidine (ineffectiveness). In agitated animals, the onset of sedative/analgesic effects could be slowed, or the depth and duration of effects could be diminished or nonexistent. Therefore, allow dogs and cats to rest quietly for 10 to 15 minutes after injection. Repeat dosing has not been evaluated.
Administration of anticholinergic agents in dogs or cats at the same time or after dexmedetomidine could lead to adverse cardiovascular effects (secondary tachycardia, prolonged hypertension, and cardiac arrhythmias1, 2, 3). However, an anticholinergic drug may be administered to dogs at least 10 minutes before
Spontaneous muscle contractions (twitching) can be expected in some dogs sedated with dexmedetomidine.
Dexmedetomidine has been evaluated only in fasted dogs; therefore, its effects on fed dogs (for example, the occurrence of vomiting) have not been characterized. In cats, there is a high frequency of vomition whether fed or fasted; therefore, fasting is recommended to reduce stomach contents.
Dexmedetomidine has not been evaluated in dogs younger than 16 weeks of age, in cats younger than 12 weeks of age, or in geriatric dogs and cats.
Dexmedetomidine has not been evaluated for use in breeding, pregnant, or lactating dogs or cats.
Adverse Reactions:
Canine sedation/anaigesia field study: In the field study safety analysis, 106 dogs received dexmedetomidine and 107 received medetomidine. Dogs ranged from 16 weeks to 16 years of age, representing 49 breeds. The following table shows the number of dogs displaying each clinical observation (some dogs experienced more than one adverse reaction).
Table 4: Adverse reactions during the canine sedation/anaigesia field study DexmedetomidineTotal n=106 MedetomidineTotal n=107 Ausculted unidentified arrhythmias 19 20 Severe bradycardia requiring treatment 1 1 Apnea requiring treatment 1 0 Slow onset of sedation (exceeding 30 minutes) 1 1 Ineffectiveness (dog standing throughout the study) 3 2 Severe hypothermia requiring treatment 2 0 Prolonged recovery 1 4
The occurrence of ausculted unidentified arrhythmias (some at multiple time points) decreased following the administration of atipamezole.
Canine preanesthesia field study: The preanesthesia field study safety analysis included 192 dogs, between 5 months and 15 years of age, representing 43 breeds enrolled for elective procedures conducted under general anesthesia. The following table shows the number of dogs within a treatment group that showed each clinical sign (dogs may have experienced more than one adverse reaction).
Table 5: Adverse reactions during the canine preanesthesia field study Treatment Groups InductionAnesthetic: Propofol Barbiturate Preanesthetic Dose: 0 mcg/m 2 n=32 125 mcg/m 2 n=32 375 mcg/m 2 n-32 0 mcg/m 2 n=32 125 mcg/m 2 n=32 375 mcg/m 2 n=32 Emesis 4 7 4 2 3 6 Ventricular premature contractions 0 2 0 4 1 0 Diarrhea 1 0 0 3 1 1 Self trauma 0 2 1 2 1 0 Severe bradycardia 0 0 1 0 0 1 Tachycardia 0 0 0 1 1 0 Urinaryincontinence 0 0 0 0 0 1
Other clinical signs observed in dogs treated with dexmedetomidine include decreased respiratory rate and hypothermia.
Feline sedation/analgesia field study: The field study safety analysis included 242 cats (122 received dexmedetomidine; 120 received xylazine), 6 months to 17 years of age, and representing 19 breeds. The following table shows the number of cats reported with an adverse reaction (cats may have experienced more than one adverse reaction).
Table 6: Adverse reactions during the feline field study Dexmedetomidinen = 122 Xylazinen = 120 Vomiting 70 82 Urinary incontinence 6 11 Hypersalivation 4 5 Involuntary defecation 4 1 Hypothermia 2 1 Diarrhea 2 0 Arrhythmia 1 2 Corneal ulcer 1 0 Cyanosis 1 0 Dyspnea 1 0
The most frequently observed adverse reaction was vomiting in both fasted and fed cats. Other infrequent clinical signs observed in cats treated with dexmedetomidine included fatigue, anorexia, cystitis, and peripheral vascular disorder.
One incidence of dyspnea was reported, 43 minutes after dexmedetomidine administration during an oral examination/dental procedure. Prior to dexmedetomidine, the cat was free of clinical signs, but had a history of asthma and respiratory infection. The cat responded successfully to treatment.
Feline preanesthesia field study: The field study safety analysis included 184 cats (116 received dexmedetomidine; 68 received saline), 12 weeks to 16 years of age, and representing 11 breeds. The following table shows the number of cats reported with an adverse reaction (cats may have experienced more than one adverse reaction).
Table 7: Adverse reactions during the feline preanesthesia field study Induction Anesthetic Ketamine Propofol Preanesthetic Salinen=37 Dexmedetomidinen=64 Salinen=31 Dexmedetomidinen=52 Emesis 2 20 1 12 Pale mucous membranes 11 9 Decreased body temperature 4 Retching 1 1 3 Heart Murmur 2 Loose Stool 2 Corneal Injury 1 Apnea 1 Behavioral change 1 Fluid in endotracheal tube 1
One case of apnea was reported in a cat that received ketamine as the induction agent. This cat required artificial ventilation from the start of the procedure until 30 minutes into recovery when the cat began to breathe on its own. The cat recovered without further problems.
Post Approval Experience:
The following adverse events were obtained from post-approval adverse drug events reported for dexmedetomidine hydrochloride sterile injectable solution from 2007 to 2009. Not all adverse reactions are reported. Some adverse reactions occurred when dexmedetomidine hydrochloride was used alone for sedation; most occurred when dexmedetomidine hydrochloride was used in the presence of anesthetics and/or other preanesthetics. It is not always possible to reliably estimate the frequency of an adverse event or to establish a causal relationship to the drug, especially when multiple drugs are administered. The following reported adverse events are uled in decreasing order of frequency:
Dogs: ineffective for sedation, death, bradycardia, cardiac arrest, apnea, convulsions, vomiting, prolonged sedation, elevated temperature, and delayed sedation.
Cats: ineffective for sedation, death, cardiac arrest, vomiting, apnea, prolonged sedation, hypersalivation, hypothermia, bradycardia, cyanotic mucous membranes, sedation too brief, and dyspnea.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet, contact Covetrus at 1-855-724-3461 or www.covetrus.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
Information For Owners:
Owners should notify their veterinarian immediately if their cat experiences difficulty breathing due to the rare possibility of delayed onset of pulmonary edema which has been associated with administration of alpha2-adrenergic agonists in cats.
Clinical Pharmacology:
Dexmedetomidine is a potent non-narcotic alpha2-adrenoceptor agonist which produces sedation and analgesia. These effects are dose dependent in depth and duration. Blood pressure is initially increased due to peripheral vasoconstriction, subsequently dropping to normal or slightly below normal levels. Vasoconstriction may cause mucous membranes to appear pale or mildly cyanotic. This initial vasopressor response is accompanied by a compensatory marked decrease in heart rate mediated by a vagal baroreceptor. The peripheral pulse may feel weak and a transient change in the conductivity of the cardiac muscle may occur, as evidenced by first and second degree atrioventricular blocks. Other arrhythmias may occur. Dexmedetomidine also decreases the respiratory rate and decreases body temperature. The magnitude and duration of the decrease in body temperature is dose dependent. Dexmedetomidine causes depression of gastrointestinal motility due to decrease in smooth muscle activity, increases in blood glucose levels due to inhibition of insulin release, and increases in production of urine. Spontaneous muscle contractions (twitching) can be expected in some dogs sedated with dexmedetomidine. Vomiting in cats has been associated with alpha2-adrenergic agonist central stimulation of the brain4
Effectiveness:
Canine sedation/analgesia field study: Dexmedetomidine was evaluated in a masked, controlled, multi-site field study, using parallel treatment groups. Effectiveness was evaluated in 200 (of 213) healthy client-owned dogs, ranging in age between 16 weeks and 16 years of age, and in size between 4.8 lbs and 141 lbs (2.2 kg and 64 kg). Dogs admitted to veterinary clinics for various procedures requiring sedation and/or analgesia received either dexmedetomidine or medetomidine once, by IV or IM injection. Procedures included dental care, radiography, minor skin tumor removal, and treatment of otitis.
Sedation and analgesia occurred within 5 minutes after IV dexmedetomidine, and within 15 minutes after IM dexmedetomidine, with peak effects approximately at 15 or 30 minutes, respectively. Effects waned by approximately two hours after IV administration, and by three hours using the IM route. Dexmedetomidine and medetomidine showed comparable clinical effects.
Cardiac rhythms were evaluated by auscultation. Bradycardia occurred within 5 to 15 minutes after IV dexmedetomidine or medetomidine, and within 15 to 30 minutes after either drug given IM. Sixty-four dexmedetomidine-treated dogs and 50 medetomidine-treated dogs were observed with bradycardia.
Adverse reactions during the field study included ausculted unidentified arrhythmias, apnea, hypothermia, and ineffectiveness (see ADVERSE REACTIONS).
Eleven dogs received concomitant medication during the field study, including amoxicillin, cephalexin, triamcinolone, methyl-prednisolone acetate, neomycin, nystatin, thiostrepton, acepromazine, atropine, and atipamezole.
The results of this field study demonstrate that dexmedetomidine produces satisfactory levels of sedation and analgesia for clinical examinations and procedures, minor surgical procedures, and minor dental procedures.
Canine preanesthesia field study: The use of dexmedetomidine as a preanesthetic was evaluated in a controlled, multi-site field study, using parallel treatment groups. Effectiveness was evaluated in 192 healthy, client-owned dogs, between 5 months and 15 years of age, weighing 4 to 196 lbs (2 kg to 89 kg). Dogs received IM dexmedetomidine or saline as a preanesthetic to general anesthesia. All dogs were induced by an injectable anesthetic; half of the dogs were maintained with an inhalation anesthetic. Procedures included orchiectomy, ovariohysterectomy, skin surgery, radiography, physical examination, dental procedures, ear cleaning, anal sac treatment, and grooming.
Compared to saline controls, dexmedetomidine IM reduced induction drug requirements by 30 to 36% (at 125 mcg/m2) and by 38 to 61% (at 375 mcg/m2). Inhalation anesthetic requirements were 40 to 60% less for dexmedetomidine-preanesthetized dogs. The number of dogs with clinical signs of pain was less for at least 30 minutes after the procedure in dogs treated with 375 mcg/m2 dexmedetomidine, compared to saline controls.
Recovery times were dose dependent, averaging 15 to 32 minutes to extubation and 71 to 131 minutes to standing recovery (longer times correspond to higher dexmedetomidine dose). Recovery times also depended on the induction anesthetic. Recovery times following barbiturate induction were longer (30 minutes to extubation and 118 minutes to standing), compared to dogs induced with propofol (23 minutes to extubation and 84 minutes to standing).
Cardiac arrhythmias were monitored by ECG. Dexmedetomidine-treated dogs were more frequently observed with at least one incidence of arrhythmia compared to saline controls. The most commonly observed arrhythmias were bradycardia, 1st and 2nd degree AV block, and sinus arrest. Other less frequently observed arrhythmias included ventricular premature complexes (VPCs), supraventricular premature complexes, 3rd degree AV block, and sinus pause.
Adverse events included bradycardia, tachycardia, VPCs, vomiting, diarrhea, urinary incontinence, and self trauma (see ADVERSE REACTIONS).
The results of the preanesthesia field study demonstrate that dexmedetomidine provided anesthetic dose-sparing, sedation, and analgesia during procedures conducted under general anesthesia.
Feline sedation/analgesia field study: Dexmedetomidine hydrochloride was evaluated in a masked, controlled, multiple site field study, using parallel treatment groups. Effectiveness was evaluated in 242 client-owned cats, ranging in age between 6 months and 17 years, and in size between 2.3 and 9.6 kg (5 and 21 lbs). Cats admitted to veterinary clinics for various procedures requiring restraint, sedation, and/or analgesia were randomized to treatment group and given dexmedetomidine (122 cats) or xylazine (120 cats) once by IM injection. Procedures performed using dexmedetomidine included dental care, radiography, minor superficial surgery, otitis treatment, blood or urine sample collection, tattooing, microchip placement, and grooming.
Sedation and analgesia occurred within 5 to 15 minutes and peak effects were observed 30 minutes after dexmedetomidine. The procedure was easily performed in 91% of cats beginning 30 minutes after dexmedetomidine. Sedative and analgesic effects waned by three hours after dexmedetomidine.
Signs of sedation were deeper for cats receiving dexmedetomidine compared to those receiving xylazine. No clinically relevant differences were observed between dexmedetomidine and xylazine with respect to analgesia or physiological variables. Heart rate, respiratory rate, and rectal temperature decreased. Bradycardia was observed within 5 to 15 minutes and heart rates of ≤70 beats/minute were seen in 18% of cats. The most commonly observed arrhythmias assessed with ECG were atrioventricular dissociation and escape rhythms, followed by a few incidences of premature complexes and one incidence of atrioventricular block. Oxygen saturation, mucous membrane color, capillary refill time, pulse character, respiratory depth and pattern, and response of the animal to injection were clinically satisfactory. All cats recovered from changes induced by dexmedetomidine.
Ninety-seven adverse events were reported after dexmedetomidine. The most frequently reported adverse reactions included vomiting (70), urinary incontinence (6), hypersalivation (4), involuntary defecation (4), hypothermia (2), and diarrhea (2) (see ADVERSE REACTIONS).
The results of this field study demonstrate that dexmedetomidine produces satisfactory levels of sedation and analgesia for clinical examinations and procedures, minor surgical procedures, and minor dental procedures.
Feline preanesthesia field study: The use of dexmedetomidine as a preanesthetic was evaluated in a masked, controlled, multi-site field study, using parallel treatment groups. Effectiveness was evaluated in 182 healthy, client-owned cats, between 12 weeks and 16 years of age, weighing 2.10 to 18.8 lbs (0.9 kg to 8.5 kg). Preanesthetic/induction drug regimens included saline/ketamine, dexmedetomidine/ketamine, saline/propofol, and dexmedetomidine/propofol. All cats were intubated prior to the procedure. Inhalant anesthesia (isoflurane) was added during longer procedures (>15 minutes) and could be added during shorter procedures if the veterinarian deemed it necessary. Procedures included ovariohysterectomy, orchiectomy, onychectomy, and dental cleaning.
Dexmedetomidine IM administered at 40 mcg/kg prior to induction with ketamine resulted in a significantly higher proportion of cats that were successfully intubated compared to saline (success rates of 89.5% and 10.7%, respectively).
Cats preanesthetized with dexmedetomidine IM required 48.9% less propofol for successful intubation compared to cats that received saline. Inhalant anesthetic requirements were 35 to 44% less for dexmedetomidine preanesthetized cats. Recovery times following ketamine and propofol induction averaged 36 and 38 minutes to extubation and 161 and 131 minutes to standing, respectively for dexmedetomidine-treated groups.
Dexmedetomidine (followed by ketamine or propofol) resulted in the following ECG abnormalities (in decreasing order of frequency): sinus bradycardia, sinus arrhythmia, 1st degree atrioventricular (AV) block, long QT interval, sinus pauses, ventricular premature depolarizations, 2nd degree AV block, escape beats/rhythms, and supraventricular premature depolarizations. Dexmedetomidine-treated cats had a lower mean heart rate, respiratory rate, and body temperature compared to saline controls continuing through the recovery period.
Sixty-six adverse events were reported after dexmedetomidine. The most frequently reported adverse events were: vomiting (32), pale mucous membranes (20), decreased body temperature (4), and retching (4). (see ADVERSE REACTIONS).
Animal Safety:
Canine safety study: In the multiple dose safety study, dexmedetomidine was administered at 0, 1, 3 or 5 times (x) the recommended IV and IM doses on 3 consecutive days to a total of 36 healthy, young beagles. Two additional groups were given a 3x dose of dexmedetomidine (IV or IM) followed by three 1x doses of the reversal agent, atipamezole, every 30 minutes. This was repeated for a total of 3 days. No deaths occurred during the study.
1x dose group: At the recommended dose, sedation lasted less than 3 hours. During sedation, muscle twitches occurred intermittently, and decreases in temperature, respiratory rate and heart rate were observed in all animals. A slow pupil response to light was seen transiently about 15 minutes after dosing in one of twelve dogs. Second degree atrioventricular (AV) blocks were observed in one of twelve dogs.
3x dose group: At 3 times the recommended dose, the duration of sedation was between two and eight hours. During sedation, muscle twitches occurred, and temperature, respiratory rate, and heart rate decreased in all dogs. The pupillary light reflex was transiently decreased for up to 90 minutes in four of twelve dogs. Vomiting was seen in two of twelve dogs. One dog experienced first and second degree AV blocks; second degree AV block was observed in three of twelve dogs. Elevated concentrations of alanine aminotransferase (ALT) were observed in one dog, without histological changes to the liver.
5x dose group: At 5 times the recommended dose, the duration of sedation was between four and eight hours. Muscle twitches, decreases in temperature, respiratory rates, and heart rates were seen in all dogs. No pupil response was noted in six of twelve dogs (IV) for up to 1.5 hours; decreased transient pupillary light reflex was seen for up to 60 minutes in two of twelve dogs (IM). Vomiting was seen in one of twelve dogs. First and second degree AV blocks were observed in one of twelve dogs. Elevated concentrations of ALT were observed in 3 of 12 dogs, without histological changes to the liver.
Dexmedetomidine demonstrated dose dependent effects related to its pharmacology when administered IV or IM to healthy dogs at doses up to five times the recommended dose.
Canine safety study with an anticholinergic: In another laboratory safety study, one of three doses of an IM anticholinergic drug or saline was administered 10 minutes before, at the same time, or 15 minutes after 500 mcg/m2 IM dexmedetomidine. The anticholinergic drug was given for the prevention or treatment of dexmedetomidine-induced reduction in heart rate. In a crossover design, 18 dogs were used in a total of 72 trials, to evaluate the safety of dexmedetomidine used with an anticholinergic drug.
Dogs were instrumented for the accumulation of continuous ECG data. The following arrhythmias were recorded during the study (some dogs experienced more than one arrhythmia).
Table 8: Arrhythmias recorded during the canine laboratory safety study* * Table does not relate arrhythmias to the presence or absence of anticholinergic.
Type of arrhythmia Number of dogs (of 18) Second degree AV block 18 Supraventricular tachycardia (SVT) or SVPCs 16 Ventricular escape beats 16 Ventricular premature contractions 14 Third degree AV block 6 Idioventricular rhythm 1 Paroxysmal VT 1 Ventricular bigeminy; SVPCs; pulse alternans 1 Junctional escape beat 1
The occurrence of arrhythmias was not related to the presence or absence of the anticholinergic drug. Arrhythmias were transient (although frequent over time in some dogs), returning toward baseline levels within 55 minutes after dexmedetomidine. No dogs required treatment related to these arrhythmias, and none of these arrhythmias persisted or adversely affected the overall clinical status of any dog in the study.
Dexmedetomidine without anticholinergic: Without the anticholinergic drug, and in addition to arrhythmias, dexmedetomidine produced clinically relevant sedation accompanied by a statistically significant reduction in heart rate, respiratory rate, cardiac output, pulmonary arterial temperature, and mixed venous oxygen tension. A statistically significant increase in arterial blood pressure, pulmonary capillary wedge pressure, central venous pressure, and systemic vascular resistance was noted. No dogs experienced hypotension. Dexmedetomidine tended to increase pulmonary vascular resistance. Dexmedetomidine alone had no statistically significant effect on mean pulmonary arterial pressure, arterial pH, arterial carbon dioxide tension, and arterial oxygen tension.
Dexmedetomidine plus anticholinergic: Either of the two higher anticholinergic doses was effective in the prevention or treatment of the dexmedetomidine-induced reduction in heart rate. Anticholinergic (higher doses) given after dexmedetomidine caused marked increases in the occurrence of various cardiac arrhythmias, especially second degree AV block. When the higher doses of anticholinergic drug were given at the same time or 15 minutes after dexmedetomidine, large increases in heart rate (p<0.01) and blood pressure (p<0.05) were seen. Increases were dose related; the highest anticholinergic dose elicited more frequent arrhythmias and larger increases in heart rate and blood pressure.
In conclusion, moderate doses of anticholinergic drug given prior to dexmedetomidine performed best for the prevention of dexmedetomidine-induced reduction of heart rate in dogs. The routine use of anticholinergics given simultaneously with, or after dexmedetomidine, is not recommended.
Feline safety study. In a multiple dose safety study, dexmedetomidine hydrochloride was administered intramuscularly (IM) at 1x, 3x, and 5x (40, 120, and 200 mcg/kg) the recommended dose of 40 mcg/kg on 3 consecutive days to healthy cats, 6 to 8 months old. A control group received the product vehicle as a placebo (0x). No mortality was observed. The depth and duration of sedation was dose dependent, lasting approximately 2 hours in the 1x group, 2 to 4 hours in the 3x group, and greater than 8 hours in the 5x group. The lowest recorded individual heart rate was 60 beats/minute and occurred in the 5x dose group (2 cats). Cardiac arrhythmias characterized by isolated junctional escape complexes with episodes of junctional escape rhythm were observed during periods of low heart rate or following sinus pauses in all dexmedetomidine dose groups. In most cases the arrhythmia was no longer observed after 1 to 2 hours. Atrioventricular block was not observed. Incidences of arrhythmias were not related to dose; however, more cats were affected by cardiac arrhythmias on the third day of treatment, compared to the first two days of the study. The decrease in respiratory rate, but not the duration, was dose dependent. The rectal temperature decreased in all dexmedetomidine-treated groups, with the lowest temperatures in the 5x group at 8 hours on all three days. Two cats vomited (40 and 120 mcg/kg). Corneal opacity was noted in all dexmedetomidine-dose groups, was transient, related to dose and duration of sedation, and was attributed to lack of lubrication with decreased blinking during sedation. Hematology and blood chemistry were unaffected by treatment. Injection site tolerance was good, with mild inflammatory lesions representative of the IM injection procedure. Gross and histological examination of all other tissues did not reveal any abnormalities related to dexmedetomidine hydrochloride administration.
Dexmedetomidine demonstrated dose dependent effects related to its pharmacology when administered IM to healthy cats at doses up to five times the recommended dose.
Feline acute tolerance study: IM dexmedetomidine hydrochloride was administered once at 10x (400 mcg/kg) the recommended dose of 40 mcg/kg to 3 female and 3 male 7 month old cats. No mortality was observed. Sedation was observed within 15 minutes of dosing and lasted for at least 4 hours with full recovery noted between 8 and 24 hours after dosing. Transient observations of corneal dehydration and opacity, miosis, pale skin and gingiva, salivation, and watery ocular discharge were observed in some animals. Vomiting was observed 7 to 11 hours after dosing in all but one animal. Decreases in heart rate accompanied by prolonged PQ and QT intervals were most pronounced 2 to 4 hours after dosing. No atrioventricular (AV) blocks or escape rhythms were noted. In one cat, incidental and reversible premature junctional complexes were seen at 1 and 2 hours after dosing which were considered secondary to bradycardia. Slightly lower respiratory rate and reduced rectal temperature were observed 4 to 8 hours after dosing. Observations had returned to normal by 24 hours after dosing. Mild inflammatory lesions observed histologically at the injection site were representative of the IM injection procedure. No treatment related changes were observed in hematology. Mild elevations in some clinical ALT, AST, and CK, were observed 24 hours after dosing, with a trend towards recovery by 48 hours. Total protein, albumin and globulin levels were slightly lowered in one cat 48 hours after dosing.
Storage:
Store at controlled room temperature 20° to 25°C (68° to 77°F). Protect from freezing. Use contents within 90 days of first puncture.
How Supplied:
Dexmedetomidine Hydrochloride Injection is supplied in 10 mL, multi-dose vials containing 0.5 mg of dexmedetomidine hydrochloride per mL.
References:
- Ko JCH, Fox SMF, Mandsager RE. Effects of preemptive atropine administration on incidence of medetomidine-induced bradycardia in dogs. J Am Vet Med Assoc 2001; 218:52-58.
- Alibhai HIK, Clarke KW, Lee YH, et al. Cardiopulmonary effects of combinations of medetomidine hydrochloride and atropine sulphate in dogs. Vet Rec 1996; 138:11-13.
- Short, CE. Effects of anticholinergic treatment on the cardiac and respiratory systems in dogs sedated with medetomidine. Vet Rec 1991; 129:310-313.
- Hikasa Y, Akiba T, lino Y et al. Central alpha-adrenoceptor subtypes involved in the emetic pathway in cats. Eur J Pharmacol 1992; 229:241-251.
Approved by FDA under ANADA # 200-699.

covetrus®
Distributed by:
Covetrus North America
400 Metro Place North
Dublin, OH 43017
covetrus.com
AH-072960-01
HDE00N REV: 0221
Package Label.principal Display Panel
Principal Display Panel Text for Container Label:
covetrus logo
NDC: 11695-6967-1
Dexmedetomidine
hydrochloride injection
5 mg/10 mL (0.5 mg/mL)
Sterile injectable solution sedative,
analgesic and preanesthetic
For intramuscular and intravenous use in dogs
and for intramuscular use in cats.
Caution: Federal law restricts this drug to use
by or on the order of a licensed veterinarian.
Net contents: 10 mL
Approved by FDA under ANADA # 200-699

Package Label.principal Display Panel
Principal Display Panel Text for Carton Label:
covetrus logo
NDC: 11695-6967-1
Dexmedetomidine
hydrochloride
injection
5 mg/10 mL
(0.5 mg/mL)
Sterile injectable solution
Sedative, analgesic
and preanesthetic
For intramuscular and
intravenous use in dogs and
for intramuscular use in cats.
Caution: Federal law restricts this
drug to use by or on the order of a
licensed veterinarian.
Net contents: 10 mL
Approved by FDA under
ANADA # 200-699
Reorder #072960
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site



