STEGLATRO (ertugliflozin pidolate 5 mg) Dailymed
Generic: ertugliflozin is used for the treatment of Kidney Failure, Chronic Renal Insufficiency
IMPRINT: 701
SHAPE: triangle
COLOR: pink
All Imprints
ertugliflozin 15 mg oral tablet [steglatro] - 702 triangle red
ertugliflozin 5 mg oral tablet [steglatro] - 701 triangle pink
Go PRO for all pill images
Recent Major Changes Section
Indications and Usage ( 1 )09/2023 Dosage and Administration ( 2.1 ,2.2 ,2.3 )09/2023 Contraindications ( 4 )09/2023 Warnings and Precautions ( 5.1 )09/2023
1 Indications And Usage
STEGLATRO¬ģ is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
STEGLATRO is a sodium glucose co-transporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1 )
Limitations of Use:
Not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus. (1 )
Limitations of Use
Not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus [see Warnings and Precautions (5.1)].
2 Dosage And Administration
- Assess renal function before initiating and as clinically indicated. (
2.1 )- Correct volume depletion before initiating STEGLATRO. (
2.1 )- Recommended starting dose is 5 mg once daily, taken in the morning, with or without food. (
2.2 )- Increase dose to 15 mg once daily in those tolerating STEGLATRO and needing additional glycemic control. (
2.2 )- Use is not recommended in patients with an eGFR less than 45 mL/min/1.73 m2. (
2.2 )- Withhold STEGLATRO for at least 4 days, if possible, prior to major surgery or procedures associated with prolonged fasting. (
2.3 )2.1 Prior to Initiation of STEGLATRO
- Assess renal function prior to initiation of STEGLATRO and as clinically indicated [see Warnings and Precautions (5.3)].
Assess volume status. In patients with volume depletion, correct this condition before initiating STEGLATRO [see Warnings and Precautions (5.3), Use in Specific Populations (8.5, 8.6)]. 2.2 Recommended Dosage
- The recommended starting dose of STEGLATRO is 5 mg once daily, taken in the morning, with or without food.
- For additional glycemic control, the dose may be increased to 15 mg once daily in patients tolerating STEGLATRO.
- Use of STEGLATRO is not recommended in patients with an eGFR less than 45 mL/min/1.73 m2.
2.3 Temporary Interruption for Surgery
Withhold STEGLATRO for at least 4 days, if possible, prior to major surgery or procedures associated with prolonged fasting. Resume STEGLATRO when the patient is clinically stable and has resumed oral intake [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].
3 Dosage Forms And Strengths
- Tablets: 5 mg, pink, triangular-shaped debossed with "701" on one side and plain on the other side.
- Tablets: 15 mg, red, triangular-shaped debossed with "702" on one side and plain on the other side.
Tablets: 5 mg and 15 mg
4 Contraindications
- Hypersensitivity to ertugliflozin or any excipient in STEGLATRO, reactions such as angioedema have occurred [see Adverse Reactions (6.2)].
- Hypersensitivity to ertugliflozin or any of the excipients in STEGLATRO. (
4 )
5 Warnings And Precautions
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis: Consider ketone monitoring in patients at risk for ketoacidosis, as indicated. Assess for ketoacidosis regardless of presenting blood glucose levels and discontinue STEGLATRO if ketoacidosis is suspected. Monitor patients for resolution of ketoacidosis before restarting. (
5.1 )- Lower Limb Amputation: Consider factors that may increase the risk of amputation before initiating STEGLATRO. Monitor patients for infections or ulcers of lower limbs, and discontinue if these occur. (
5.2 )- Volume Depletion: May result in acute kidney injury. Before initiating, assess and correct volume status in patients with renal impairment or low systolic blood pressure, elderly patients, or patients on diuretics. Monitor for signs and symptoms during therapy. (
5.3 )- Urosepsis and Pyelonephritis: Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated. (
5.4 )- Hypoglycemia: Consider a lower dose of insulin or insulin secretagogue to reduce risk of hypoglycemia when used in combination. (
5.5 )- Necrotizing Fasciitis of the Perineum (Fournier's Gangrene): Serious, life-threatening cases have occurred in both females and males. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment. (
5.6 )- Genital Mycotic Infections: Monitor and treat if indicated. (
5.7 )5.1 Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
In patients with type 1 diabetes mellitus, STEGLATRO significantly increases the risk of diabetic ketoacidosis, a life-threatening event, beyond the background rate. In placebo-controlled trials of patients with type 1 diabetes mellitus, the risk of ketoacidosis was markedly increased in patients who received sodium glucose transporter 2 (SGLT2) inhibitors compared to patients who received placebo; this risk may be greater with higher doses. STEGLATRO is not indicated for glycemic control in patients with type 1 diabetes mellitus.
Type 2 diabetes mellitus and pancreatic disorders (e.g., history of pancreatitis or pancreatic surgery) are also risk factors for ketoacidosis. There have been postmarketing reports of fatal events of ketoacidosis in patients with type 2 diabetes mellitus using SGLT2 inhibitors.
Precipitating conditions for diabetic ketoacidosis or other ketoacidosis include under-insulinization due to insulin dose reduction or missed insulin doses, acute febrile illness, reduced caloric intake, ketogenic diet, surgery, volume depletion, and alcohol abuse.
Signs and symptoms are consistent with dehydration and severe metabolic acidosis and include nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. Blood glucose levels at presentation may be below those typically expected for diabetic ketoacidosis (e.g., less than 250 mg/dL). Ketoacidosis and glucosuria may persist longer than typically expected. Urinary glucose excretion persists for 4 days after discontinuing STEGLATRO [see Clinical Pharmacology (12.2)]; however, there have been postmarketing reports of ketoacidosis and/or glucosuria lasting greater than 6 days and some up to 2 weeks after discontinuation of SGLT2 inhibitors.
Consider ketone monitoring in patients at risk for ketoacidosis if indicated by the clinical situation. Assess for ketoacidosis regardless of presenting blood glucose levels in patients who present with signs and symptoms consistent with severe metabolic acidosis. If ketoacidosis is suspected, discontinue STEGLATRO, promptly evaluate, and treat ketoacidosis, if confirmed. Monitor patients for resolution of ketoacidosis before restarting STEGLATRO.
Withhold STEGLATRO, if possible, in temporary clinical situations that could predispose patients to ketoacidosis. Resume STEGLATRO when the patient is clinically stable and has resumed oral intake [see Dosage and Administration (2.3)].
Educate all patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue STEGLATRO and seek medical attention immediately if signs and symptoms occur.
5.2 Lower Limb Amputation
In a long-term cardiovascular outcomes study [see Clinical Studies 14.2], in patients with type 2 diabetes and established cardiovascular disease, the occurrence of non-traumatic lower limb amputations was reported with event rates of 4.7, 5.7, and 6.0 events per 1000 patient-years in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg treatment arms, respectively.
Amputation of the toe and foot were most frequent (81 out of 109 patients with lower limb amputations). Some patients had multiple amputations, some involving both lower limbs.
Lower limb infections, gangrene, and diabetic foot ulcers were the most common precipitating medical events leading to the need for an amputation. Patients with amputations were more likely to be male, have higher A1C (%) at baseline, have a history of peripheral arterial disease, amputation or peripheral revascularization procedure, diabetic foot, and to have been taking diuretics or insulin.
Across seven STEGLATRO clinical trials, non-traumatic lower limb amputations were reported in 1 (0.1%) patient in the comparator group, 3 (0.2%) patients in the STEGLATRO 5 mg group, and 8 (0.5%) patients in the STEGLATRO 15 mg group.
Before initiating STEGLATRO, consider factors in the patient history that may predispose them to the need for amputations, such as a history of prior amputation, peripheral vascular disease, neuropathy and diabetic foot ulcers. Counsel patients about the importance of routine preventative foot care. Monitor patients receiving STEGLATRO for signs and symptoms of infection (including osteomyelitis), new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue STEGLATRO if these complications occur.
5.3 Volume Depletion
STEGLATRO can cause intravascular volume contraction which may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine [see Adverse Reactions (6.1)]. There have been postmarketing reports of acute kidney injury, some requiring hospitalization and dialysis, in patients with type 2 diabetes mellitus receiving SGLT2 inhibitors, including STEGLATRO. Patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2) [see Use in Specific Populations (8.6)], elderly patients, patients with low systolic blood pressure, or patients on loop diuretics may be at increased risk for volume depletion or hypotension. Before initiating STEGLATRO in patients with one or more of these characteristics, assess volume status and renal function. In patients with volume depletion, correct this condition before initiating STEGLATRO. Monitor for signs and symptoms of volume depletion, and renal function after initiating therapy.
5.4 Urosepsis and Pyelonephritis
There have been postmarketing reports of serious urinary tract infections, including urosepsis and pyelonephritis, requiring hospitalization in patients receiving SGLT2 inhibitors. Treatment with SGLT2 inhibitors increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated [see Adverse Reactions (6.1)].
5.5 Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Insulin and insulin secretagogues (e.g., sulfonylurea) are known to cause hypoglycemia. STEGLATRO may increase the risk of hypoglycemia when used in combination with insulin and/or an insulin secretagogue [see Adverse Reactions (6.1)]. Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with STEGLATRO.
5.6 Necrotizing Fasciitis of the Perineum (Fournier's Gangrene)
Reports of necrotizing fasciitis of the perineum (Fournier's Gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in postmarketing surveillance in patients with diabetes mellitus receiving SGLT2 inhibitors, including STEGLATRO. Cases have been reported in females and males. Serious outcomes have included hospitalization, multiple surgeries, and death.
Patients treated with STEGLATRO presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue STEGLATRO, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
5.7 Genital Mycotic Infections
STEGLATRO increases the risk of genital mycotic infections. Patients who have a history of genital mycotic infections or who are uncircumcised are more likely to develop genital mycotic infections [see Adverse Reactions (6.1)]. Monitor and treat appropriately.
6 Adverse Reactions
The following important adverse reactions are described elsewhere in the labeling:
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes and Other Ketoacidosis [see Warnings and Precautions (5.1)]
- Lower Limb Amputation [see Warnings and Precautions (5.2)]
- Volume Depletion [see Warnings and Precautions (5.3)]
- Urosepsis and Pyelonephritis [see Warnings and Precautions (5.4)]
- Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues [see Warnings and Precautions (5.5)]
- Necrotizing Fasciitis of the Perineum (Fournier's Gangrene) [see Warnings and Precautions (5.6)]
- Genital Mycotic Infections [see Warnings and Precautions (5.7)]
Most common adverse reactions (incidence ‚Č• 5%) were female genital mycotic infections. (6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch .
6.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Trials Evaluating STEGLATRO 5 and 15 mg
The data in Table 1 are derived from a pool of three 26-week, placebo-controlled trials. STEGLATRO was used as monotherapy in one trial and as add-on therapy in two trials [see Clinical Studies (14)]. These data reflect exposure of 1,029 patients to STEGLATRO with a mean exposure duration of approximately 25 weeks. Patients received STEGLATRO 5 mg (N=519), STEGLATRO 15 mg (N=510), or placebo (N=515) once daily. The mean age of the population was 57 years and 2% were older than 75 years of age. Fifty-three percent (53%) of the population was male and 73% were Caucasian, 15% were Asian, and 7% were Black or African American. At baseline the population had diabetes for an average of 7.5 years, had a mean HbA1c of 8.1%, and 19.4% had established microvascular complications of diabetes. Baseline renal function (mean eGFR 88.9 mL/min/1.73 m2) was normal or mildly impaired in 97% of patients and moderately impaired in 3% of patients.
Table 1 shows common adverse reactions associated with the use of STEGLATRO. These adverse reactions were not present at baseline, occurred more commonly on STEGLATRO than on placebo, and occurred in at least 2% of patients treated with either STEGLATRO 5 mg or STEGLATRO 15 mg.
Table 1: Adverse Reactions Reported in ‚Č•2% of Patients with Type 2 Diabetes Mellitus Treated with STEGLATRO The three placebo-controlled studies included one monotherapy trial and two add-on combination trials with metformin or with metformin and sitagliptin. and Greater than Placebo in Pooled Placebo-Controlled Clinical Studies of STEGLATRO Monotherapy or Combination TherapyNumber (%) of Patients PlaceboN = 515 STEGLATRO 5 mgN = 519 STEGLATRO 15 mgN = 510 Female genital mycotic infections Includes: genital candidiasis, genital infection fungal, vaginal infection, vulvitis, vulvovaginal candidiasis, vulvovaginal mycotic infection, and vulvovaginitis. Percentages calculated with the number of female patients in each group as denominator: placebo (N=235), STEGLATRO 5 mg (N=252), STEGLATRO 15 mg (N=245). 3.0% 9.1% 12.2% Male genital mycotic infections Includes: balanitis candida, balanoposthitis, genital infection, and genital infection fungal. Percentages calculated with the number of male patients in each group as denominator: placebo (N=280), STEGLATRO 5 mg (N=267), STEGLATRO 15 mg (N=265). 0.4% 3.7% 4.2% Urinary tract infections Includes: cystitis, dysuria, streptococcal urinary tract infection, urethritis, urinary tract infection. 3.9% 4.0% 4.1% Headache 2.3% 3.5% 2.9% Vaginal pruritus Includes: vulvovaginal pruritus and pruritus genital. Percentages calculated with the number of female patients in each group as denominator: placebo (N=235), ertugliflozin 5 mg (N=252), ertugliflozin 15 mg (N=245). 0.4% 2.8% 2.4% Increased urination Includes: pollakiuria, micturition urgency, polyuria, urine output increased, and nocturia. 1.0% 2.7% 2.4% Nasopharyngitis 2.3% 2.5% 2.0% Back pain 2.3% 1.7% 2.5% Weight decreased 1.0% 1.2% 2.4% Thirst Includes: thirst, dry mouth, polydipsia, and dry throat. 0.6% 2.7% 1.4%
Volume Depletion
STEGLATRO causes an osmotic diuresis, which may lead to intravascular volume contraction and adverse reactions related to volume depletion, particularly in patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2). In patients with moderate renal impairment, adverse reactions related to volume depletion (e.g., dehydration, dizziness postural, presyncope, syncope, hypotension, and orthostatic hypotension) were reported in 0%, 4.4%, and 1.9% of patients treated with placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. STEGLATRO may also increase the risk of hypotension in other patients at risk for volume contraction [see Use in Specific Populations (8.5, 8.6)].
Hypoglycemia
The incidence of hypoglycemia by study is shown in Table 2.
Table 2: Incidence of Overall Overall hypoglycemic events: plasma or capillary glucose of less than or equal to 70 mg/dL. and SevereSevere hypoglycemic events: required assistance, lost consciousness, or experienced a seizure regardless of blood glucose. Hypoglycemia in Placebo-Controlled Clinical Studies in Patients with Type 2 Diabetes MellitusMonotherapy (26 weeks) Placebo(N = 153) STEGLATRO5 mg(N =156) STEGLATRO15 mg(N = 152) Overall [N (%)] 1 (0.7) 4 (2.6) 4 (2.6) Severe [N (%)] 0 (0.0) 0 (0.0) 2 (1.3) Add-on Combination Therapy with Metformin (26 weeks) Placebo(N = 209) STEGLATRO5 mg(N = 207) STEGLATRO15 mg(N = 205) Overall [N (%)] 9 (4.3) 15 (7.2) 16 (7.8) Severe [N (%)] 1 (0.5) 1 (0.5) 0 (0.0) Add-on Combination Therapy with Metformin and Sitagliptin (26 weeks) Placebo(N = 153) STEGLATRO5 mg(N = 156) STEGLATRO15 mg(N = 153) Overall [N (%)] 5 (3.3) 7 (4.5) 3 (2.0) Severe [N (%)] 1 (0.7) 1 (0.6) 0 (0.0) In Combination with Insulin and/or an Insulin Secretagogue in Patients with Moderate Renal Impairment (26 weeks) Placebo(N = 133) STEGLATRO5 mg(N = 148) STEGLATRO15 mg(N = 143) Overall [N (%)] 48 (36.1) 53 (35.8) 39 (27.3) Severe [N (%)] 3 (2.3) 5 (3.4) 3 (2.1) Add-on Combination with Insulin with or without Metformin (18 weeks) Placebo(N = 347) STEGLATRO5 mg(N = 348) STEGLATRO15 mg(N = 370) Overall [N (%)] 130 (37.5) 137 (39.4) 144 (38.9) Severe [N (%)] 12 (3.5) 13 (3.7) 19 (5.1) Add-on Combination with a Sulfonylurea (18 weeks) Placebo(N =48) STEGLATRO5 mg(N =55) STEGLATRO15 mg(N =54) Overall [N (%)] 2 (4.2) 4 (7.3) 5 (9.3) Severe [N (%)] 0 (0.0) 0 (0.0) 0 (0.0) Add-on Combination with Metformin and a Sulfonylurea (18 weeks) Placebo(N = 117) STEGLATRO5 mg(N = 100) STEGLATRO15 mg(N = 113) Overall [N (%)] 17 (14.5) 20 (20.0) 30 (26.5) Severe [N (%)] 1 (0.9) 2 (2.0) 2 (1.8)
Genital Mycotic Infections
In the pool of three placebo-controlled clinical trials, the incidence of female genital mycotic infections (e.g., genital candidiasis, genital infection fungal, vaginal infection, vulvitis, vulvovaginal candidiasis, vulvovaginal mycotic infection, vulvovaginitis) occurred in 3%, 9.1%, and 12.2% of females treated with placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively (see Table 1). In females, discontinuation due to genital mycotic infections occurred in 0% and 0.6% of patients treated with placebo and STEGLATRO, respectively.
In the same pool, male genital mycotic infections (e.g., balanitis candida, balanoposthitis, genital infection, genital infection fungal) occurred in 0.4%, 3.7%, and 4.2% of males treated with placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively (see Table 1). Male genital mycotic infections occurred more commonly in uncircumcised males. In males, discontinuations due to genital mycotic infections occurred in 0% and 0.2% of patients treated with placebo and STEGLATRO, respectively. Phimosis was reported in 8 of 1729 (0.5%) male ertugliflozin-treated patients, of which four required circumcision.
Urinary Tract Infections
In VERTIS CV, urinary tract infections (e.g., urinary tract infection, cystitis, dysuria) occurred in 10.2%, 12.2% and 12.0% of patients treated with placebo, STEGLATRO 5 mg and STEGLATRO 15 mg, respectively. The incidences of serious urinary tract infections were 0.8%, 0.9% and 0.4% with placebo, STEGLATRO 5 mg and STEGLATRO 15 mg, respectively.
Laboratory Tests
Changes in Serum Creatinine and eGFR
Initiation of STEGLATRO causes an increase in serum creatinine and decrease in eGFR within weeks of starting therapy and then these changes stabilize. In a study of patients with moderate renal impairment, larger mean changes were observed. In a long-term cardiovascular outcomes trial, an initial increase in serum creatinine and a decrease in eGFR within weeks of starting therapy was observed (at Week 6 eGFR changes of -2.7, -3.8 and -0.4 mL/min/1.73 m2 in the STEGLATRO 5 mg, STEGLATRO 15 mg and placebo arms, respectively). The initial decline was followed by a recovery toward baseline to Week 52 (eGFR change from baseline of - 0.4, - 1.1 and - 0.2 mL/min/1.73 m2 in STEGLATRO 5 mg, STEGLATRO 15 mg, and placebo arms, respectively). Acute hemodynamic changes may play a role in the early renal function changes observed with STEGLATRO since they are reversed after treatment discontinuation.
Increases in Low-Density Lipoprotein Cholesterol (LDL-C)
In the pool of three placebo-controlled trials, dose-related increases in LDL-C were observed in patients treated with STEGLATRO. Mean percent changes from baseline to Week 26 in LDL-C relative to placebo were 2.6% and 5.4% with STEGLATRO 5 mg and STEGLATRO 15 mg, respectively. The range of mean baseline LDL-C was 96.6 to 97.7 mg/dL across treatment groups.
Increases in Hemoglobin
In the pool of three placebo-controlled trials, mean changes (percent changes) from baseline to Week 26 in hemoglobin were -0.21 g/dL (-1.4%) with placebo, 0.46 g/dL (3.5%) with STEGLATRO 5 mg, and 0.48 g/dL (3.5%) with STEGLATRO 15 mg. The range of mean baseline hemoglobin was 13.90 to 14.00 g/dL across treatment groups. At the end of treatment, 0.0%, 0.2%, and 0.4% of patients treated with placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively, had a hemoglobin increase greater than 2 g/dL and above the upper limit of normal.
Increases in Serum Phosphate
In the pool of three placebo-controlled trials, mean changes (percent changes) from baseline in serum phosphate were 0.04 mg/dL (1.9%) with placebo, 0.21 mg/dL (6.8%) with STEGLATRO 5 mg, and 0.26 mg/dL (8.5%) with STEGLATRO 15 mg. The range of mean baseline serum phosphate was 3.53 to 3.54 mg/dL across treatment groups. In a clinical trial of patients with moderate renal impairment, mean changes (percent changes) from baseline at Week 26 in serum phosphate were -0.01 mg/dL (0.8%) with placebo, 0.29 mg/dL (9.7%) with STEGLATRO 5 mg, and 0.24 mg/dL (7.8%) with STEGLATRO 15 mg.
6.2Postmarketing Experience
Additional adverse reactions have been identified during postapproval use. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Necrotizing fasciitis of the perineum (Fournier's Gangrene)
- Angioedema
- Rash
7 Drug Interactions
Table 3: Clinically Significant Drug Interactions with STEGLATRO Insulin and Insulin Secretagogues Clinical Impact: The risk of hypoglycemia when STEGLATRO is used in combination with insulin and/or an insulin secretagogue. Intervention: A lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with STEGLATRO. Lithium Clinical Impact: Concomitant use of an SGLT2 inhibitor with lithium may decrease serum lithium concentrations. Intervention: Monitor serum lithium concentration more frequently during STEGLATRO initiation and dosage changes. Positive Urine Glucose Test Clinical Impact: SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. Intervention: Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. Interference with 1,5-anhydroglucitol (1,5-AG) Assay Clinical Impact: Measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Intervention: Monitoring glycemic control with 1,5-AG assay is not recommended. Use alternative methods to monitor glycemic control.
See full prescribing information for information on drug interactions and interference of STEGLATRO with laboratory tests. (7 )
8 Use In Specific Populations
- Pregnancy: Advise females of the potential risk to a fetus especially during the second and third trimesters. (
8.1 )- Lactation: Breastfeeding not recommended. (
8.2 )- Geriatrics: Higher incidence of adverse reactions related to reduced intravascular volume. (
8.5 )- Renal Impairment: Higher incidence of adverse reactions related to reduced intravascular volume and renal function. (
8.6 )8.1 Pregnancy
Risk Summary
Based on animal data showing adverse renal effects, STEGLATRO is not recommended during the second and third trimesters of pregnancy.
The limited available data with STEGLATRO in pregnant women are not sufficient to determine a drug-associated risk of adverse developmental outcomes. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations).
In animal studies, adverse renal changes were observed in rats when ertugliflozin was administered during a period of renal development corresponding to the late second and third trimesters of human pregnancy. Doses approximately 13 times the maximum clinical dose caused renal pelvic and tubule dilatations and renal mineralization that were not fully reversible. There was no evidence of fetal harm in rats or rabbits at exposures of ertugliflozin approximately 300 times higher than the maximal clinical dose of 15 mg/day when administered during organogenesis (see Data).
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20-25% in women with HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Poorly-controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Animal Data
When ertugliflozin was orally administered to juvenile rats from PND 21 to PND 90, increased kidney weight, renal tubule and renal pelvis dilatation, and renal mineralization occurred at doses greater than or equal to 5 mg/kg (13-fold human exposures, based on AUC). These effects occurred with drug exposure during periods of renal development in rats that correspond to the late second and third trimester of human renal development, and did not fully reverse within a 1-month recovery period.
In embryo-fetal development studies, ertugliflozin (50, 100 and 250 mg/kg/day) was administered orally to rats on gestation days 6 to 17 and to rabbits on gestation days 7 to 19. Ertugliflozin did not adversely affect developmental outcomes in rats and rabbits at maternal exposures that were approximately 300 times the human exposure at the maximum clinical dose of 15 mg/day, based on AUC. A maternally toxic dose (250 mg/kg/day) in rats (707 times the clinical dose), was associated with reduced fetal viability, and a higher incidence of a visceral malformation (membranous ventricular septal defect). In the pre- and post-natal development study in pregnant rats, ertugliflozin was administered to the dams from gestation day 6 through lactation day 21 (weaning). Decreased post-natal growth (weight gain) was observed at maternal doses ‚Č•100 mg/kg/day (greater than or equal to 331 times the human exposure at the maximum clinical dose of 15 mg/day, based on AUC).
8.2 Lactation
Risk Summary
There is no information regarding the presence of STEGLATRO in human milk, the effects on the breastfed infant, or the effects on milk production. Ertugliflozin is present in the milk of lactating rats (see Data). Since human kidney maturation occurs in utero and during the first 2 years of life when lactational exposure may occur, there may be risk to the developing human kidney. Because of the potential for serious adverse reactions in a breastfed infant, advise women that the use of STEGLATRO is not recommended while breastfeeding.
Data
The lacteal excretion of radiolabeled ertugliflozin in lactating rats was evaluated 10 to 12 days after parturition. Ertugliflozin derived radioactivity exposure in milk and plasma were similar, with a milk/plasma ratio of 1.07, based on AUC. Juvenile rats directly exposed to STEGLATRO during a developmental period corresponding to human kidney maturation were associated with a risk to the developing kidney (persistent increased organ weight, renal mineralization, and renal pelvic and tubular dilatations).
8.4 Pediatric Use
Safety and effectiveness of STEGLATRO in pediatric patients under 18 years of age have not been established.
8.5 Geriatric Use
No dosage adjustment of STEGLATRO is recommended based on age. In STEGLATRO clinical trials, a total of 876 (25.7%) patients treated with STEGLATRO were 65 years and older, and 152 (4.5%) patients treated with STEGLATRO were 75 years and older. Patients 65 years and older had a higher incidence of adverse reactions related to volume depletion compared to younger patients; events were reported in 1.1%, 2.2%, and 2.6% of patients treated with comparator, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
In VERTIS CV, a total of 2780 (50.5%) patients treated with STEGLATRO were 65 years and older, and 595 (10.8%) patients treated with STEGLATRO were 75 years and older. Safety and efficacy were generally similar for patients age 65 years and older compared to patients younger than 65.
8.6Renal Impairment
A 26-week placebo-controlled study of 313 patients with Stage 3 Chronic Kidney Disease (eGFR ‚Č•30 to less than 60 mL/min/1.73 m2) treated with STEGLATRO did not demonstrate improvement in glycemic control.
In the VERTIS CV study, there were 1370 patients (25%) with an eGFR ‚Č•90 mL/min/1.73 m2, 2929 patients (53%) with an eGFR of ‚Č•60 to less than 90 mL/min/1.73 m2, 879 patients (16%) with an eGFR of ‚Č•45 to less than 60 mL/min/1.73 m2, and 299 patients (5%) with eGFR of 30 to <45 mL/min/1.73 m2 treated with STEGLATRO. Similar effects on glycemic control at Week 18 were observed in patients treated with STEGLATRO in each eGFR subgroup and also in the overall patient population.
No dosage adjustment is needed in patients with eGFR ‚Č•45 mL/min/1.73 m2.
8.7Hepatic Impairment
No dosage adjustment of STEGLATRO is necessary in patients with mild or moderate hepatic impairment. Ertugliflozin has not been studied in patients with severe hepatic impairment and is not recommended for use in this patient population [see Clinical Pharmacology (12.3)].
10 Overdosage
In the event of an overdose with STEGLATRO, contact the Poison Control Center. Employ the usual supportive measures as dictated by the patient's clinical status. Removal of ertugliflozin by hemodialysis has not been studied.
11 Description
STEGLATRO (ertugliflozin) tablets for oral use contain ertugliflozin L-pyroglutamic acid, a SGLT2 inhibitor.
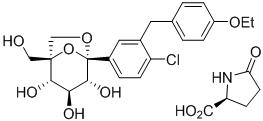
The chemical name of ertugliflozin L-pyroglutamic acid is (1S,2S,3S,4R,5S)-5-(4-chloro-3-(4-ethoxybenzyl)phenyl)-1-(hydroxymethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol, compound with (2S)-5-oxopyrrolidine-2-carboxylic acid. The molecular formula is C27H32ClNO10 and the molecular weight is 566.00.
The chemical structure is:
Ertugliflozin L-pyroglutamic acid is a white to off-white powder that is soluble in ethyl alcohol and acetone, slightly soluble in ethyl acetate and acetonitrile and very slightly soluble in water.
STEGLATRO is supplied as film-coated tablets, containing 6.48 or 19.43 mg of ertugliflozin L-pyroglutamic acid, which is equivalent to 5 and 15 mg of ertugliflozin.
Inactive ingredients are microcrystalline cellulose, lactose monohydrate, sodium starch glycolate, and magnesium stearate.
The film coating contains: hypromellose, lactose monohydrate, macrogol, triacetin, titanium dioxide and iron oxide red.
12 Clinical Pharmacology
12.1Mechanism of Action
SGLT2 is the predominant transporter responsible for reabsorption of glucose from the glomerular filtrate back into the circulation. Ertugliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, ertugliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
12.2 Pharmacodynamics
Urinary Glucose Excretion and Urinary Volume
Dose-dependent increases in the amount of glucose excreted in urine were observed in healthy subjects and in patients with type 2 diabetes mellitus following single- and multiple-dose administration of ertugliflozin. Dose-response modeling indicates that ertugliflozin 5 mg and 15 mg result in near maximal urinary glucose excretion (UGE). Enhanced UGE is maintained after multiple-dose administration. UGE with ertugliflozin also results in increases in urinary volume.
Cardiac Electrophysiology
The effect of STEGLATRO on QTc interval was evaluated in a Phase 1 randomized, placebo- and positive-controlled 3-period crossover study in 42 healthy subjects. At 6.7 times the therapeutic exposures with maximum recommended dose, STEGLATRO does not prolong QTc to any clinically relevant extent.
12.3 Pharmacokinetics
The pharmacokinetics of ertugliflozin are similar in healthy subjects and patients with type 2 diabetes mellitus. The steady state mean plasma AUC and Cmax were 398 ng‚ąôhr/mL and 81.3 ng/mL, respectively, with 5 mg ertugliflozin once-daily treatment, and 1,193 ng‚ąôhr/mL and 268 ng/mL, respectively, with 15 mg ertugliflozin once-daily treatment. Steady-state is reached after 4 to 6 days of once-daily dosing with ertugliflozin. Ertugliflozin does not exhibit time-dependent pharmacokinetics and accumulates in plasma up to 10-40% following multiple dosing.
Absorption
Following single-dose oral administration of 5 mg and 15 mg of ertugliflozin, peak plasma concentrations (median Tmax) of ertugliflozin occur at 1 hour postdose under fasted conditions. Plasma Cmax and AUC of ertugliflozin increase in a dose-proportional manner following single doses from 0.5 mg (0.1 times the lowest recommended dose) to 300 mg (20 times the highest recommended dose) and following multiple doses from 1 mg (0.2 times the lowest recommended dose) to 100 mg (6.7 times the highest recommended dose). The absolute oral bioavailability of ertugliflozin following administration of a 15 mg dose is approximately 100%.
Effect of Food
Administration of STEGLATRO with a high-fat and high-calorie meal decreases ertugliflozin Cmax by 29% and prolongs Tmax by 1 hour, but does not alter AUC as compared with the fasted state. The observed effect of food on ertugliflozin pharmacokinetics is not considered clinically relevant, and ertugliflozin may be administered with or without food. In Phase 3 clinical trials, STEGLATRO was administered without regard to meals.
Distribution
The mean steady-state volume of distribution of ertugliflozin following an intravenous dose is 85.5 L. Plasma protein binding of ertugliflozin is 93.6% and is independent of ertugliflozin plasma concentrations. Plasma protein binding is not meaningfully altered in patients with renal or hepatic impairment. The blood-to-plasma concentration ratio of ertugliflozin is 0.66.
Elimination
Metabolism
Metabolism is the primary clearance mechanism for ertugliflozin. The major metabolic pathway for ertugliflozin is UGT1A9 and UGT2B7-mediated O-glucuronidation to two glucuronides that are pharmacologically inactive at clinically relevant concentrations. CYP-mediated (oxidative) metabolism of ertugliflozin is minimal (12%).
Excretion
The mean systemic plasma clearance following an intravenous 100 ¬Ķg dose was 11.2 L/hr. The mean elimination half-life in type 2 diabetic patients with normal renal function was estimated to be 16.6 hours based on the population pharmacokinetic analysis. Following administration of an oral [14C]-ertugliflozin solution to healthy subjects, approximately 40.9% and 50.2% of the drug-related radioactivity was eliminated in feces and urine, respectively. Only 1.5% of the administered dose was excreted as unchanged ertugliflozin in urine and 33.8% as unchanged ertugliflozin in feces, which is likely due to biliary excretion of glucuronide metabolites and subsequent hydrolysis to parent.
Specific Populations
Patients with Renal Impairment
In a clinical pharmacology study in patients with type 2 diabetes mellitus and mild, moderate, or severe renal impairment (as determined by eGFR), following a single-dose administration of 15 mg STEGLATRO, the mean increases in AUC of ertugliflozin were 1.6-, 1.7-, and 1.6-fold, respectively, for mild, moderate and severe renally impaired patients, compared to subjects with normal renal function. These increases in ertugliflozin AUC are not considered clinically meaningful. The 24-hour urinary glucose excretion declined with increasing severity of renal impairment [see Warnings and Precautions (5.3) and Use in Specific Populations (8.6)]. The plasma protein binding of ertugliflozin was unaffected in patients with renal impairment.
Patients with Hepatic Impairment
Moderate hepatic impairment (based on the Child-Pugh classification) did not result in an increase in exposure of ertugliflozin. The AUC of ertugliflozin decreased by approximately 13%, and Cmax decreased by approximately 21% compared to subjects with normal hepatic function. This decrease in ertugliflozin exposure is not considered clinically meaningful. There is no clinical experience in patients with Child-Pugh class C (severe) hepatic impairment. The plasma protein binding of ertugliflozin was unaffected in patients with moderate hepatic impairment [see Use in Specific Populations (8.7)].
Effects of Age, Body Weight, Gender, and Race
Based on a population pharmacokinetic analysis, age, body weight, gender, and race do not have a clinically meaningful effect on the pharmacokinetics of ertugliflozin.
Drug Interaction Studies
In Vitro Assessment of Drug Interactions
In in vitro studies, ertugliflozin and ertugliflozin glucuronides did not inhibit CYP450 isoenzymes (CYPs) 1A2, 2C9, 2C19, 2C8, 2B6, 2D6, or 3A4, and did not induce CYPs 1A2, 2B6, or 3A4. Ertugliflozin was not a time-dependent inhibitor of CYP3A in vitro. Ertugliflozin did not inhibit UGT1A6, 1A9, or 2B7 in vitro and was a weak inhibitor (IC50 >39 ¬ĶM) of UGT1A1 and 1A4. Ertugliflozin glucuronides did not inhibit UGT1A1, 1A4, 1A6, 1A9, or 2B7 in vitro. Overall, ertugliflozin is unlikely to affect the pharmacokinetics of drugs eliminated by these enzymes. Ertugliflozin is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) transporters and is not a substrate of organic anion transporters (OAT1, OAT3), organic cation transporters (OCT1, OCT2), or organic anion transporting polypeptides (OATP1B1, OATP1B3). Ertugliflozin or ertugliflozin glucuronides do not meaningfully inhibit P-gp, OCT2, OAT1, or OAT3 transporters, or transporting polypeptides OATP1B1 and OATP1B3, at clinically relevant concentrations. Overall, ertugliflozin is unlikely to affect the pharmacokinetics of concurrently administered medications that are substrates of these transporters.
In Vivo Assessment of Drug Interactions
No dose adjustment of STEGLATRO is recommended when coadministered with commonly prescribed medicinal products. Ertugliflozin pharmacokinetics were similar with and without coadministration of metformin, glimepiride, sitagliptin, and simvastatin in healthy subjects (see Figure 1). Coadministration of ertugliflozin with multiple doses of 600 mg once-daily rifampin (an inducer of UGT and CYP enzymes) resulted in approximately 39% and 15% mean reductions in ertugliflozin AUC and Cmax, respectively, relative to ertugliflozin administered alone. These changes in exposure are not considered clinically relevant. Ertugliflozin had no clinically relevant effect on the pharmacokinetics of metformin, glimepiride, sitagliptin, and simvastatin when coadministered in healthy subjects (see Figure 2). Physiologically-based PK (PBPK) modeling suggests that coadministration of mefenamic acid (UGT inhibitor) may increase the AUC and Cmax of ertugliflozin by 1.51- and 1.19-fold, respectively. These predicted changes in exposure are not considered clinically relevant.
Figure 1: Effects of Other Drugs on the Pharmacokinetics of Ertugliflozin
Figure 2: Effects of Ertugliflozin on the Pharmacokinetics of Other Drugs
13 Nonclinical Toxicology
13.1Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity was evaluated in CD-1 mice and Sprague-Dawley rats. In the mouse study, ertugliflozin was administered by oral gavage at doses of 5, 15, and 40 mg/kg/day for up to 97 weeks in males and 102 weeks in females. There were no ertugliflozin-related neoplastic findings at doses up to 40 mg/kg/day (approximately 50 times human exposure at the maximum recommended human dose [MRHD] of 15 mg/day based on AUC). In the rat study, ertugliflozin was administered by oral gavage at doses of 1.5, 5, and 15 mg/kg/day for up to 92 weeks in females and 104 weeks in males. Ertugliflozin-related neoplastic findings included an increased incidence of adrenal medullary pheochromocytoma (PCC) in male rats at 15 mg/kg/day. Although the molecular mechanism remains unknown, this finding may be related to carbohydrate malabsorption leading to altered calcium homeostasis, which has been associated with PCC development in rats and has unclear relevancy to human risk. The no-observed-effect level (NOEL) for neoplasia was 5 mg/kg/day (approximately 16 times human exposure at the MRHD of 15 mg/day, based on AUC).
Mutagenesis
Ertugliflozin was not mutagenic or clastogenic with or without metabolic activation in the microbial reverse mutation, in vitro cytogenetic (human lymphocytes), and in vivo rat micronucleus assays.
Impairment of Fertility
In the rat fertility and embryonic development study, male and female rats were administered ertugliflozin at 5, 25, and 250 mg/kg/day. No effects on fertility were observed at 250 mg/kg/day (approximately 480 and 570 times male and female human exposures, respectively, at the MRHD of 15 mg/day based on AUC comparison).
14 Clinical Studies
14.1 Glycemic Control Trials in Patients with Type 2 Diabetes Mellitus
STEGLATRO has been studied as monotherapy and in combination with metformin, sitagliptin, a sulfonylurea, insulin (with or without metformin), metformin plus sitagliptin, metformin plus a sulfonylurea and compared to a sulfonylurea (glimepiride). STEGLATRO has also been studied in patients with type 2 diabetes mellitus and moderate renal impairment.
In patients with type 2 diabetes mellitus treatment with STEGLATRO reduced hemoglobin A1c (HbA1c) compared to placebo. Reduction in HbA1c was generally similar across subgroups defined by age, sex, race, geographic region, baseline body mass index (BMI), and duration of type 2 diabetes mellitus.
Monotherapy
A total of 461 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) on diet and exercise participated in a randomized, double-blind, multi-center, 26-week, placebo-controlled study (NCT01958671) to evaluate the efficacy and safety of STEGLATRO monotherapy. These patients, who were either treatment na√Įve or not receiving any background antihyperglycemic treatment ‚Č•8 weeks, entered a 2-week, single-blind, placebo run-in period and were randomized to placebo, STEGLATRO 5 mg, or STEGLATRO 15 mg, administered once daily.
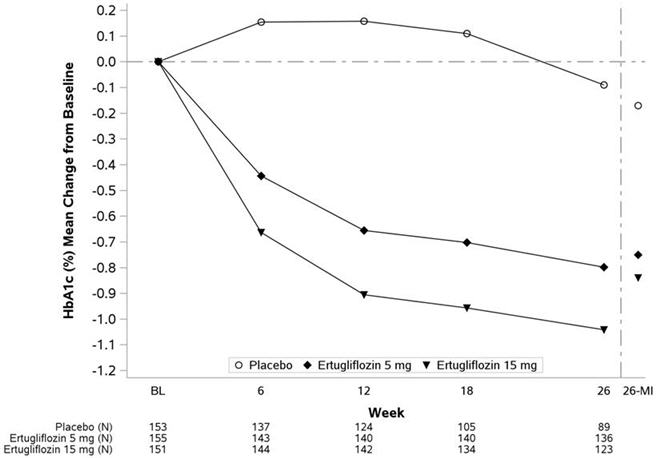
At Week 26, treatment with STEGLATRO at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c compared to placebo. STEGLATRO also resulted in a greater proportion of patients achieving an HbA1c <7% compared with placebo (see Table 4 and Figure 3).
Table 4: Results at Week 26 from a Placebo-Controlled Monotherapy Study of STEGLATRO in Patients with Type 2 Diabetes Mellitus N includes all randomized and treated patients with a baseline measurement of the outcome variable. At Week 26, the primary HbA1c endpoint was missing for 23%, 11%, and 16% of patients, and during the trial, rescue medication was initiated by 25%, 2%, and 3% of patients randomized to placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Missing Week 26 measurements were imputed using multiple imputation with a mean equal to the baseline value of the patient. Results include measurements collected after initiation of rescue medication. For those patients who did not receive rescue medication and had values measured at 26 weeks, the mean changes from baseline for HbA1c were -0.1%, -0.8%, and -1.0% for placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Placebo STEGLATRO 5 mg STEGLATRO 15 mg HbA1c (%) N = 153 N = 155 N = 151   Baseline (mean) 8.1 8.2 8.4   Change from baseline (LS mean Intent-to-treat analysis using ANCOVA adjusted for baseline value, prior antihyperglycemic medication, and baseline eGFR. )-0.2 -0.7 -0.8   Difference from placebo (LS mean , 95% CI) -0.6 p<0.001 compared to placebo. (-0.8, -0.4)-0.7 (-0.9, -0.4) Patients [N (%)] with HbA1c <7% 26 (16.9) 47 (30.1) 59 (38.8) FPG (mg/dL) N = 150 N = 151 N = 149   Baseline (mean) 180.2 180.9 179.1   Change from baseline (LS mean ) -11.6 -31.0 -36.4   Difference from placebo (LS mean , 95% CI) -19.4 (-27.6, -11.2) -24.8 (-33.2, -16.4)
The mean baseline body weight was 94.2 kg, 94.0 kg, and 90.6 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.0 kg, -3.0 kg, and -3.1 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from placebo (95% CI) for STEGLATRO 5 mg was -2.0 kg (-2.8, -1.2) and for STEGLATRO 15 mg was -2.1 kg (-2.9, -1.3).
Figure 3: HbA1c (%) Change Over Time in a 26-Week Placebo-Controlled Monotherapy Study of STEGLATRO in Patients with Type 2 Diabetes Mellitus Data to the left of the vertical line are observed means (non-model-based) excluding values occurring post glycemic rescue. Data to the right of the vertical line represent the final Week 26 data, including all values regardless of use of glycemic rescue medication and use of study drug, with missing Week 26 values imputed using multiple imputation (26-MI) with a mean equal to the baseline value of the patient (see Table 4).
Combination Therapy
Add-on Combination Therapy with Metformin
A total of 621 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) on metformin monotherapy (‚Č•1,500 mg/day for ‚Č•8 weeks) participated in a randomized, double-blind, multi-center, 26-week, placebo-controlled study (NCT02033889) to evaluate the efficacy and safety of STEGLATRO in combination with metformin. Patients entered a 2-week, single-blind, placebo run-in, and were randomized to placebo, STEGLATRO 5 mg, or STEGLATRO 15 mg administered once daily in addition to continuation of background metformin therapy.
At Week 26, treatment with STEGLATRO at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c compared to placebo. STEGLATRO also resulted in a greater proportion of patients achieving an HbA1c <7% compared to placebo (see Table 5).
Table 5: Results at Week 26 from a Placebo-Controlled Study for STEGLATRO Used in Combination with Metformin in Patients with Type 2 Diabetes Mellitus N includes all randomized and treated patients with a baseline measurement of the outcome variable. At Week 26, the primary HbA1c endpoint was missing for 12%, 6%, and 9% of patients, and during the trial, rescue medication was initiated by 18%, 3%, and 1% of patients randomized to placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Missing Week 26 measurements were imputed using multiple imputation with a mean equal to the baseline value of the patient. Results include measurements collected after initiation of rescue medication. For those patients who did not receive rescue medication and had values measured at 26 weeks, the mean changes from baseline for HbA1c were -0.2%, -0.7%, and -1.0% for placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Placebo STEGLATRO 5 mg STEGLATRO 15 mg HbA1c (%) N = 207 N = 205 N = 201   Baseline (mean) 8.2 8.1 8.1   Change from baseline (LS mean Intent-to-treat analysis using ANCOVA adjusted for baseline value, prior antihyperglycemic medication, menopausal status and baseline eGFR. )-0.2 -0.7 -0.9   Difference from placebo (LS mean , 95% CI) -0.5 p<0.001 compared to placebo. (-0.7, -0.4)-0.7 (-0.9, -0.5) Patients [N (%)] with HbA1c <7% 38 (18.4) 74 (36.3) 87 (43.3) FPG (mg/dL) N = 202 N = 199 N = 201   Baseline (mean) 169.1 168.1 167.9   Change from baseline (LS mean ) -8.7 -30.3 -40.9   Difference from placebo (LS mean , 95% CI) -21.6 (-27.8, -15.5) -32.3 (-38.5, -26.0)
The mean baseline body weight was 84.5 kg, 84.9 kg, and 85.3 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.4 kg, -3.2 kg, and -3.0 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from placebo (95% CI) for STEGLATRO 5 mg was -1.8 kg (-2.4, -1.2) and for STEGLATRO 15 mg was -1.7 kg (-2.2, -1.1).
The mean baseline systolic blood pressure was 129.3 mmHg, 130.5 mmHg, and 130.2 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.8 mmHg, -5.1 mmHg, and -5.7 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from placebo (95% CI) for STEGLATRO 5 mg was -3.3 mmHg (-5.6, -1.1) and for STEGLATRO 15 mg was -3.8 mmHg (-6.1, -1.5).
Active Controlled Study versus Glimepiride as Add-on Combination Therapy with Metformin
A total of 1,326 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 9%) on metformin monotherapy participated in a randomized, double-blind, multi-center, 52-week, active comparator controlled study (NCT01999218) to evaluate the efficacy and safety of STEGLATRO in combination with metformin. These patients, who were receiving metformin monotherapy (‚Č•1,500 mg/day for ‚Č•8 weeks), entered a 2-week, single-blind, placebo run-in period and were randomized to glimepiride, STEGLATRO 5 mg, or STEGLATRO 15 mg administered once daily in addition to continuation of background metformin therapy. Glimepiride was initiated at 1 mg/day and titrated up to a maximum dose of 6 or 8 mg/day (depending on maximum approved dose in each country) or a maximum tolerated dose or down-titrated to avoid or manage hypoglycemia. The mean daily dose of glimepiride was 3.0 mg.
STEGLATRO 15 mg was non-inferior to glimepiride after 52 weeks of treatment. (See Table 6.)
Table 6: Results at Week 52 from an Active-Controlled Study Comparing STEGLATRO to Glimepiride as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on Metformin N includes all randomized and treated patients with a baseline measurement of the outcome variable. At Week 52, the primary HbA1c endpoint was missing for 15%, 20%, and 16% of patients and during the trial, rescue medication was initiated by 3%, 6%, and 4% of patients randomized to glimepiride, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Missing Week 52 measurements were imputed using multiple imputation with a mean equal to the baseline value of the patient. Results include measurements collected after initiation of rescue medication. For those patients who did not receive rescue medication and had values measured at 52 weeks, the mean changes from baseline for HbA1c were -0.8%, -0.6%, and -0.7% for glimepiride, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Glimepiride STEGLATRO 5 mg STEGLATRO 15 mg HbA1c (%) N = 437 N = 447 N = 440   Baseline (mean) 7.8 7.8 7.8   Change from baseline (LS mean Intent-to-treat analysis using ANCOVA adjusted for baseline value, prior antihyperglycemic medication and baseline eGFR. )-0.6 -0.5 -0.5   Difference from glimepiride (LS mean , 95% CI) 0.2 Non-inferiority is declared when the upper bound of the two-sided 95% confidence interval (CI) for the mean difference is less than 0.3%. (0.0, 0.3)0.1 (-0.0, 0.2) Patients [N (%)] with HbA1c <7% 208 (47.7) 177 (39.5) 186 (42.2)
The mean baseline body weight was 86.8 kg, 87.9 kg, and 85.6 kg in the glimepiride, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 52 were 0.6 kg, -2.6 kg, and -3.0 kg in the glimepiride, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from glimepiride (95% CI) for STEGLATRO 5 mg was -3.2 kg (-3.7, -2.7) and for STEGLATRO 15 mg was -3.6 kg (-4.1, -3.1).
In Combination with Sitagliptin versus STEGLATRO Alone and Sitagliptin Alone, as Add-on to Metformin
A total of 1,233 patients with type 2 diabetes mellitus with inadequate glycemic control (HbA1c between 7.5% and 11%) on metformin monotherapy (‚Č•1,500 mg/day for ‚Č•8 weeks) participated in a randomized, double-blind, 26-week, active controlled study (NCT02099110) to evaluate the efficacy and safety of STEGLATRO 5 mg or 15 mg in combination with sitagliptin 100 mg compared to the individual components. Patients were randomized to one of five treatment arms: STEGLATRO 5 mg, STEGLATRO 15 mg, sitagliptin 100 mg, STEGLATRO 5 mg + sitagliptin 100 mg, or STEGLATRO 15 mg + sitagliptin 100 mg.
At Week 26, STEGLATRO 5 mg or 15 mg + sitagliptin 100 mg provided statistically significantly greater reductions in HbA1c compared to STEGLATRO (5 mg or 15 mg) alone or sitagliptin 100 mg alone. The mean change from baseline in HbA1c was -1.4% for STEGLATRO 5 mg or 15 mg + sitagliptin 100 mg versus -1.0%, for STEGLATRO 5 mg, STEGLATRO 15 mg, or sitagliptin 100 mg, respectively. More patients receiving STEGLATRO 5 mg or 15 mg + sitagliptin 100 mg achieved an HbA1c <7% (53.3% and 50.9%, for STEGLATRO 5 mg or 15 mg, respectively, + sitagliptin 100 mg) compared to the individual components (29.3%, 33.7%, and 38.5% for STEGLATRO 5 mg, STEGLATRO 15 mg, or sitagliptin 100 mg, respectively).
Add-on Combination Therapy with Metformin and Sitagliptin
A total of 463 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) on metformin (‚Č•1,500 mg/day for ‚Č•8 weeks) and sitagliptin 100 mg once daily participated in a randomized, double-blind, multi-center, 26-week, placebo-controlled study (NCT02036515) to evaluate the efficacy and safety of STEGLATRO. Patients entered a 2-week, single-blind, placebo run-in period and were randomized to placebo, STEGLATRO 5 mg, or STEGLATRO 15 mg.
At Week 26, treatment with STEGLATRO at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c. STEGLATRO also resulted in a higher proportion of patients achieving an HbA1c <7% compared to placebo (see Table 7).
Table 7: Results at Week 26 from an Add-on Study of STEGLATRO in Combination with Metformin and Sitagliptin in Patients with Type 2 Diabetes Mellitus N includes all randomized and treated patients with a baseline measurement of the outcome variable. At Week 26, the primary HbA1c endpoint was missing for 10%, 11%, and 7% of patients and during the trial, rescue medication was initiated by 16%, 1%, and 2% of patients randomized to placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Missing Week 26 measurements were imputed using multiple imputation with a mean equal to the baseline value of the patient. Results include measurements collected after initiation of rescue medication. For those patients who did not receive rescue medication and had values measured at 26 weeks, the mean changes from baseline for HbA1c were -0.2%, -0.8%, and -0.9% for placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Placebo STEGLATRO 5 mg STEGLATRO 15 mg HbA1c (%) N = 152 N = 155 N = 152   Baseline (mean) 8.0 8.1 8.0   Change from baseline (LS mean Intent-to-treat analysis using ANCOVA adjusted for baseline value, prior antihyperglycemic medication and baseline eGFR. )-0.2 -0.7 -0.8   Difference from placebo (LS mean , 95% CI) -0.5 p<0.001 compared to placebo. (-0.7, -0.3)-0.6 (-0.8, -0.4) Patients [N (%)] with HbA1c <7% 31 (20.2) 54 (34.6) 64 (42.3) FPG (mg/dL) N = 152 N = 156 N = 152   Baseline (mean) 169.6 167.7 171.7   Change from baseline (LS mean ) -6.5 -25.7 -32.1   Difference from placebo (LS mean , 95% CI) -19.2 (-26.8, -11.6) -25.6 (-33.2, -18.0)
The mean baseline body weight was 86.5 kg, 87.6 kg, and 86.6 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.0 kg, -3.0 kg, and -2.8 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from placebo (95% CI) for STEGLATRO 5 mg was -1.9 kg (-2.6, -1.3) and for STEGLATRO 15 mg was -1.8 kg (-2.4, -1.2).
The mean baseline systolic blood pressure was 130.2 mmHg, 132.1 mmHg, and 131.6 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 26 were -0.2 mmHg, -3.8 mmHg, and -4.5 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from placebo (95% CI) for STEGLATRO 5 mg was -3.7 mmHg (-6.1, -1.2) and for STEGLATRO 15 mg was -4.3 mmHg (-6.7, -1.9).
Initial Combination Therapy with Sitagliptin
A total of 291 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 8% and 10.5%) on diet and exercise participated in a randomized, double-blind, multi-center, placebo-controlled 26-week study (NCT02226003) to evaluate the efficacy and safety of STEGLATRO in combination with sitagliptin. These patients, who were not receiving any background antihyperglycemic treatment for ‚Č•8 weeks, entered a 2-week, single-blind, placebo run-in period and were randomized to placebo, STEGLATRO 5 mg or STEGLATRO 15 mg in combination with sitagliptin (100 mg) once daily.
At Week 26, treatment with STEGLATRO 5 mg and 15 mg in combination with sitagliptin at 100 mg daily provided statistically significant reductions in HbA1c compared to placebo. STEGLATRO 5 mg and 15 mg in combination with sitagliptin at 100 mg daily also resulted in a higher proportion of patients achieving an HbA1c <7% and greater reductions in FPG compared with placebo.
Add-on Combination Therapy with Insulin (with or without Metformin)
In an 18-week randomized, double-blind, multi-center, placebo-controlled, glycemic sub-study of VERTIS CV (eValuation of ERTugliflozin efficacy and Safety CardioVascular, NCT01986881, study details see 14.2), a total of 1065 patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease with inadequate glycemic control (HbA1c between 7% and 10.5%) on background therapy of insulin ‚Č•20 units/day (59% also on metformin ‚Č•1,500 mg/day) were randomized to placebo, STEGLATRO 5 mg or STEGLATRO 15 mg once daily treatment.
At Week 18, treatment with STEGLATRO at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c compared to placebo (see Table 8).
Table 8: Results at Week 18 from an Add-on Study of STEGLATRO in Combination with Insulin (with or without Metformin) in Patients with Type 2 Diabetes Mellitus N includes all randomized and treated patients with a baseline measurement of the outcome variable. At Week 18, the primary HbA1c endpoint was missing for 10%, 9%, and 12% of patients and during the trial, rescue medication was initiated by 12%, 7%, and 6% of patients randomized to placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Results include measurements collected after initiation of rescue medication. Prior to Week 18, background antidiabetic medication was held stable. Missing Week 18 measurements were imputed using multiple imputation with a mean equal to the baseline value of the patient (Return to Baseline analysis). Placebo STEGLATRO 5 mg STEGLATRO 15 mg SE: standard error. HbA1c (%) N = 346 N = 346 N = 367   Baseline (mean) 8.4 8.4 8.4   Change from baseline (LS mean Intent-to-treat analysis using ANCOVA adjusted for baseline value, insulin stratum, and baseline eGFR. , SE)-0.2 (0.05) -0.7 (0.05) -0.7 (0.05)   Difference from placebo (LS mean , 95% CI) -0.5 p<0.001 compared to placebo. (-0.6, -0.4)-0.5 (-0.7, -0.4) Patients [N (%)] with HbA1c <7% Missing values imputed as not meeting the <7% criterion. 37 (10.7) 79 (22.8) 81 (22.1) FPG (mg/dL) N = 343 N = 346 N = 368   Baseline (mean) 167.4 173.8 175.4   Change from baseline (LS mean , SE) -6.3 (2.91) -25.6 (2.90) -29.8 (2.86)   Difference from placebo (LS mean , 95% CI) -19.2 (-26.8, -11.6) -23.4 (-30.9, -16.0)
The mean baseline body weights were 93.3 kg, 93.8 kg, and 92.1 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 18 were - 0.2 kg, -1.6 kg, and -1.9 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The differences from placebo (95% CI) for STEGLATRO 5 mg were - 1.4 kg (- 1.9, - 0.9) and for STEGLATRO 15 mg was -1.6 kg (-2.1, -1.1).
The mean baseline systolic blood pressures were 134.0 mmHg, 135.6 mmHg, and 133.7 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 18 were 0.7 mmHg, -2.2 mmHg, and -1.7 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The differences from placebo (95% CI) for STEGLATRO 5 mg was ‚Äď 2.9 mmHg (-4.9, -1.0) and for STEGLATRO 15 mg were -2.5 mmHg (- 4.4, - 0.5).
Add-on Combination Therapy with Metformin and Sulfonylurea
In an 18-week randomized, double-blind, multi-center, placebo-controlled, glycemic sub-study of VERTIS CV (NCT01986881, study details see 14.2), a total of 330 patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease with inadequate glycemic control (HbA1c between 7% and 10.5%) with background therapy of metformin ‚Č•1,500 mg/day and a sulfonylurea (SU) were randomized to placebo, STEGLATRO 5 mg or STEGLATRO 15 mg once daily treatment.
At Week 18, treatment with STEGLATRO at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c compared to placebo (see Table 9).
Table 9: Results at Week 18 from an Add-on Study of STEGLATRO in Combination with Metformin and a SU in Patients with Type 2 Diabetes Mellitus N includes all randomized and treated patients with a baseline measurement of the outcome variable. At Week 18, the primary HbA1c endpoint was missing for 9%, 8%, and 6% of patients and during the trial, rescue medication was initiated by 10%, 7%, and 3% of patients randomized to placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively. Results include measurements collected after initiation of rescue medication. Missing Week 18 measurements were imputed using multiple imputation with a mean equal to the baseline value of the patient (Return to Baseline analysis). Placebo STEGLATRO 5 mg STEGLATRO 15 mg SE: standard error HbA1c (%) N = 116 N = 99 N = 113   Baseline (mean) 8.3 8.4 8.3   Change from baseline (LS mean Intent-to-treat analysis using ANCOVA adjusted for baseline value and baseline eGFR. , SE)-0.3 (0.08) -0.8 (0.09) -0.9 (0.08)   Difference from placebo (LS mean , 95% CI) -0.6 p<0.001 compared to placebo. (-0.8, -0.3)-0.7 (-0.9, -0.4) Patients [N (%)] with HbA1c <7% Missing values imputed as not meeting the <7% criterion. 17 (14.7) 39 (39.4) 38 (33.6) FPG (mg/dL) N = 117 N = 99 N = 113   Baseline (mean) 177.3 183.5 174.0   Change from baseline (LS mean , SE) -3.5 (3.65) -31.3 (3.87) -33.0 (3.67)   Difference from placebo (LS mean , 95% CI) -27.9 (-37.8, -17.9) -29.5 (-39.0, -19.9)
The mean baseline body weights were 90.5 kg, 92.1 kg, and 92.9 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 18 were - 0.6 kg, -2.0 kg, and - 2.2 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The differences from placebo (95% CI) for STEGLATRO 5 mg were - 1.4 kg (- 2.2, - 0.7) and for STEGLATRO 15 mg was - 1.6 kg (- 2.3, - 0.9).
Use in Patients with Type 2 Diabetes Mellitus and Moderate Renal Impairment
26-Week Placebo-Controlled Study
The efficacy of STEGLATRO was assessed in a multicenter, randomized, double-blind, placebo-controlled study (NCT01986855) of patients with type 2 diabetes mellitus and moderate renal impairment (468 patients with eGFR ‚Č•30 to <60 mL/min/1.73 m2). In this study, 202 patients exposed to STEGLATRO (5 mg or 15 mg) had an eGFR between 45 and 60 mL/min/1.73 m2 and 111 patients exposed to STEGLATRO (5 mg or 15 mg) had an eGFR between 30 and 45 mL/min/1.73 m2. The mean duration of diabetes for the study population was approximately 14 years, and the majority of patients were receiving background insulin (55.9%) and/or sulfonylurea (40.3%) therapy. Approximately 50% had a history of cardiovascular disease or heart failure.
STEGLATRO did not show efficacy in this study. The HbA1c reductions from baseline to Week 26 were not significantly different between placebo and STEGLATRO 5 mg or 15 mg [see Use in Specific Populations (8.6)].
14.2 Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus and Established Cardiovascular Disease
The effect of STEGLATRO on cardiovascular risk in adult patients with type 2 diabetes and established atherosclerotic cardiovascular disease was evaluated in the VERTIS CV study (NCT01986881), a multicenter, multi-national, randomized, double-blind, placebo-controlled, event-driven trial. The study compared the risk of experiencing a major adverse cardiovascular event (MACE) between STEGLATRO and placebo when these were added to and used concomitantly with standard of care treatments for diabetes and atherosclerotic cardiovascular disease.
A total of 8246 patients were randomized (placebo N=2747, STEGLATRO 5 mg N=2752, STEGLATRO 15 mg N=2747) and followed for a median of 3 years. Approximately 88% of the study population was Caucasian, 6% Asian, and 3% Black. The mean age was 64 years and approximately 70% were male.
All patients in the study had inadequately controlled type 2 diabetes mellitus at baseline (HbA1c greater than or equal to 7%). The mean duration of type 2 diabetes mellitus was 13 years, the mean HbA1c at baseline was 8.2% and the mean eGFR was 76 mL/min/1.73 m2. At baseline, patients were treated with one (32%) or more (67%) antidiabetic medications including biguanides (metformin) (76%), insulin (47%), sulfonylureas (41%) DPP-4 inhibitors (11%) and GLP-1 receptor agonists (3%).
Almost all patients (99%) had established atherosclerotic cardiovascular disease at baseline including: a documented history of coronary artery disease (76%), cerebrovascular disease (23%) or peripheral artery disease (19%). Approximately 24% patients had a history of heart failure (HF). At baseline, the mean systolic blood pressure was 133 mmHg, the mean diastolic blood pressure was 77 mmHg, the mean LDL was 89 mg/dL, and the mean HDL was 44 mg/dL. At baseline, approximately 81% of patients were treated with renin angiotensin system inhibitors, 69% with beta-blockers, 43% with diuretics, 82% with statins, 4% with ezetimibe, and 89% with antiplatelet agents.
The primary endpoint in VERTIS CV was the time to first occurrence of MACE. A major adverse cardiovascular event was defined as occurrence of either a cardiovascular death or a nonfatal myocardial infarction (MI) or a nonfatal stroke. The statistical analysis plan pre-specified that the 5 and 15 mg doses would be combined for the analysis. A Cox proportional hazards model was used to test for non-inferiority against the pre-specified risk margin of 1.3 for the hazard ratio of MACE. Type-1 error was controlled across multiple tests using a hierarchical testing strategy.
The incidence rate of MACE was similar between the STEGLATRO-treated and placebo-treated patients. The estimated hazard ratio of MACE associated with STEGLATRO relative to placebo was 0.97 with 95.6% confidence interval (0.85, 1.11). The upper bound of this confidence interval excluded a risk larger than 1.3 (Table 10). Results for the individual 5 mg and 15 mg doses were consistent with results for the combined dose group.
Table 10: Analysis of MACE and its Components from the VERTIS-CV Study Intent-to-treat analysis set. Endpoint MACE was evaluated in subjects who took at least one dose of study medication and, for subjects who discontinued study medication prior to the end of the study, censored events that occurred more than 365 days after the last dose of study medication. Other endpoints were evaluated using all randomized subjects and events that occurred any time after the first dose of study medication until the last contact date. The total number of first events was analyzed for each endpoint. Placebo (N=2747) STEGLATRO (N=5499) Hazard Ratio vs Placebo (CI) HR and CI are based on Cox proportional hazards regression model, stratified by cohorts. For MACE a 95.6% CI is presented, for other endpoints a 95% CI is presented. N (%) Event Rate (per 100 person-years) N (%) Event Rate (per 100 person-years) N=Number of patients, CI=Confidence interval, CV=Cardiovascular, MI=Myocardial infarction. MACE (CV death, non-fatal MI, or non-fatal stroke) Composite 327 (11.9) 4.0 653 (11.9) 3.9 0.97(0.85, 1.11) Components of Composite Endpoint Non-fatal MI 148 (5.4) 1.6 310 (5.6) 1.7 1.04(0.86, 1.27) Non-fatal Stroke 78 (2.8) 0.8 157 (2.9) 0.8 1.00(0.76, 1.32) CV death 184 (6.7) 1.9 341 (6.2) 1.8 0.92(0.77, 1.11)
16 How Supplied/storage And Handling
STEGLATRO (ertugliflozin) tablets are available as follows:
Strength Description How Supplied NDC 5 mg tablets pink, triangular-shaped, biconvex tablets, with ‚Äú701‚ÄĚ debossed on one side and plain on the other side unit-of-use bottles of 30 0006-5363-03 unit-of-use bottles of 90 0006-5363-06 15 mg tablets red, triangular-shaped, biconvex tablets, with ‚Äú702‚ÄĚ debossed on one side and plain on the other side unit-of-use bottles of 30 0006-5364-03 unit-of-use bottles of 90 0006-5364-06 STORAGE AND HANDLING SECTION
Store at 20¬įC -25¬įC (68¬įF -77¬įF), excursions permitted between 15¬įC -30¬įC (between 59¬įF -86¬įF) [see USP Controlled Room Temperature]. Protect from moisture. Store in a dry place.
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
Inform patients that STEGLATRO can cause potentially fatal ketoacidosis and that type 2 diabetes mellitus and pancreatic disorders (e.g., history of pancreatitis or pancreatic surgery) are risk factors.
Educate all patients on precipitating factors (such as insulin dose reduction or missed insulin doses, infection, reduced caloric intake, ketogenic diet, surgery, dehydration, and alcohol abuse) and symptoms of ketoacidosis (including nausea, vomiting, abdominal pain, tiredness, and labored breathing). Inform patients that blood glucose may be normal even in the presence of ketoacidosis.
Advise patients that they may be asked to monitor ketones. If symptoms of ketoacidosis occur, instruct patients to discontinue STEGLATRO and seek medical attention immediately [see Warnings and Precautions (5.1)].
Amputation
Inform patients of the potential for an increased risk of amputations. Counsel patients about the importance of routine preventative foot care. Instruct patients to monitor for new pain or tenderness, sores or ulcers, or infections involving the leg or foot and to seek medical advice immediately if such signs or symptoms develop [see Warnings and Precautions (5.2)].
Volume Depletion
Inform patients that symptomatic hypotension may occur with STEGLATRO and advise them to contact their doctor if they experience such symptoms [see Warnings and Precautions (5.3)]. Inform patients that dehydration may increase the risk for hypotension, and to have adequate fluid intake.
Serious Urinary Tract Infections
Inform patients of the potential for urinary tract infections, which may be serious. Provide them with information on the symptoms of urinary tract infections. Advise them to seek medical advice if such symptoms occur [see Warnings and Precautions (5.4)].
Hypoglycemia with Concomitant Use of Insulin and/or Insulin Secretagogue
Inform patients that the incidence of hypoglycemia may increase when STEGLATRO is added to insulin and/or an insulin secretagogue. Educate patients or caregivers on the signs and symptoms of hypoglycemia [see Warnings and Precautions (5.5)].
Necrotizing Fasciitis of the Perineum (Fournier's Gangrene)
Inform patients that necrotizing infections of the perineum (Fournier's Gangrene) have occurred with SGLT2 inhibitors. Counsel patients to promptly seek medical attention if they develop pain or tenderness, redness, or swelling of the genitals or the area from the genitals back to the rectum, along with a fever above 100.4¬įF or malaise [see Warnings and Precautions (5.6)].
Genital Mycotic Infections in Females (e.g., Vulvovaginitis)
Inform female patients that vaginal yeast infections may occur and provide them with information on the signs and symptoms of vaginal yeast infection. Advise them of treatment options and when to seek medical advice [see Warnings and Precautions (5.7)].
Genital Mycotic Infections in Males (e.g., Balanitis or Balanoposthitis)
Inform male patients that yeast infections of the penis (e.g., balanitis or balanoposthitis) may occur, especially in uncircumcised males. Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). Advise them of treatment options and when to seek medical advice [see Warnings and Precautions (5.7)].
Fetal Toxicity
Advise pregnant patients of the potential risk to a fetus with treatment with STEGLATRO. Instruct patients to immediately inform their healthcare provider if pregnant or planning to become pregnant [see Use in Specific Populations (8.1)].
Lactation
Advise patients that use of STEGLATRO is not recommended while breastfeeding [see Use in Specific Populations (8.2)].
Laboratory Tests
Due to its mechanism of action, inform patients that their urine will test positive for glucose while taking STEGLATRO.
Missed Dose
Instruct patients to take STEGLATRO only as prescribed. If a dose is missed, it should be taken as soon as the patient remembers. Advise patients not to double their next dose.
Manufactured for: Merck Sharp & Dohme LLC Rahway, NJ 07065, USA
For patent information: www.msd.com/research/patent
Copyright © 2017-2024 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates. All rights reserved.
uspi-mk8835-t-2406r009
Spl Medguide Section
Medication GuideSTEGLATRO¬ģ [steh-GLA-troh](ertugliflozin)tablets, for oral use This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 09/2023 Read this Medication Guide carefully before you start taking STEGLATRO and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. What is the most important information I should know about STEGLATRO? STEGLATRO may cause serious side effects, including:
- Diabetic ketoacidosis (increased ketones in your blood or urine) in people with type 1 diabetes and other ketoacidosis. STEGLATRO can cause ketoacidosis that can be life-threatening and may lead to death. Ketoacidosis is a serious condition which needs to be treated in a hospital. People with type 1 diabetes have a high risk of getting ketoacidosis. People with type 2 diabetes or pancreas problems also have an increased risk of getting ketoacidosis. Ketoacidosis can also happen in people who: are sick, cannot eat or drink as usual, skip meals, are on a diet high in fat and low in carbohydrates (ketogenic diet), take less than the usual amount of insulin or miss insulin doses, drink too much alcohol, have a loss of too much fluid from the body (volume depletion), or who have surgery. Ketoacidosis can happen even if your blood sugar is less than 250 mg/dL. Your healthcare provider may ask you to periodically check ketones in your urine or blood. Stop taking STEGLATRO and call your healthcare provider or get medical help right away if you get any of the following. If possible, check for ketones in your urine or blood, even if your blood sugar is less than 250 mg/dL:
- nausea
- vomiting
- stomach-area (abdominal) pain
- tiredness
- trouble breathing
- ketones in your urine or blood
Call your healthcare provider right away if you have new pain or tenderness, any sores, ulcers, or infections in your leg or foot. Your healthcare provider may decide to stop your STEGLATRO for a while if you have any of these signs or symptoms. Talk to your healthcare provider about proper foot care.
- Amputations. STEGLATRO may increase your risk of lower limb amputations. You may be at a higher risk of lower limb amputation if you:
- have a history of amputation
- have had blocked or narrowed blood vessels, usually in your leg
- have damage to the nerves (neuropathy) in your leg
- have had diabetic foot ulcers or sores
You may be at risk of dehydration if you:
- Dehydration. STEGLATRO can cause some people to become dehydrated (the loss of body water and salt). Dehydration may cause you to feel dizzy, faint, lightheaded, or weak, especially when you stand up (orthostatic hypotension). There have been reports of sudden worsening of kidney function in people who are taking STEGLATRO.
Talk to your healthcare provider about what you can do to prevent dehydration including how much fluid you should drink on a daily basis. Call your healthcare provider right away if you reduce the amount of food or liquid you drink, for example if you are sick or cannot eat, or you start to lose liquids from your body, for example from vomiting, diarrhea or being in the sun too long.
- take medicines to lower your blood pressure, including water pills (diuretics)
- are on a low sodium (salt) diet
- have kidney problems
- are 65 years of age or older
- Vaginal yeast infection. Symptoms of a vaginal yeast infection include:
- vaginal odor
- white or yellowish vaginal discharge (discharge may be lumpy or look like cottage cheese)
- vaginal itching
- Yeast infection of the penis (balanitis or balanoposthitis). Swelling of an uncircumcised penis may develop that makes it difficult to pull back the skin around the tip of the penis. Other symptoms of yeast infection of the penis include:
- redness, itching, or swelling of the penis
- foul smelling discharge from the penis
- rash of the penis
- pain in the skin around your penis
Talk to your healthcare provider about what to do if you get symptoms of a yeast infection of the vagina or penis. Your healthcare provider may suggest you use an over-the-counter antifungal medicine. Talk to your healthcare provider right away if you use an over-the-counter antifungal medicine and your symptoms do not go away. What is STEGLATRO?
- STEGLATRO is a prescription medicine used in adults with type 2 diabetes to improve blood sugar (glucose) control along with diet and exercise.
- STEGLATRO is not recommended to decrease blood sugar (glucose) in people with type 1 diabetes.
- It is not known if STEGLATRO is safe and effective in children under 18 years of age.
Who should not take STEGLATRO?Do not take STEGLATRO if you:
If you have any of these symptoms, stop taking STEGLATRO and call your healthcare provider right away or go to the nearest hospital emergency room.
- are allergic to ertugliflozin or any of the ingredients in STEGLATRO. See the end of this Medication Guide for a ul of ingredients in STEGLATRO. Symptoms of a serious allergic reaction to STEGLATRO may include:
- skin rash
- raised red patches on your skin (hives)
- swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing
Before you take STEGLATRO, tell your healthcare provider about all of your medical conditions, including if you: Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. STEGLATRO may affect the way other medicines work, and other medicines may affect how STEGLATRO works. Know the medicines you take. Keep a ul of them to show your healthcare provider and pharmacist when you get a new medicine.
- have type 1 diabetes or have had diabetic ketoacidosis.
- have a decrease in your insulin dose.
- have a serious infection.
- have a history of infection of the vagina or penis.
- have a history of amputation.
- have had blocked or narrowed blood vessels, usually in your leg.
- have damage to the nerves (neuropathy) in your leg.
- have had diabetic foot ulcers or sores.
- have kidney problems.
- have liver problems.
- have a history of urinary tract infections or problems with urination.
- are on a low sodium (salt) diet. Your healthcare provider may change your diet or your dose.
- are going to have surgery. Your healthcare provider may stop your STEGLATRO before you have surgery. Talk to your healthcare provider if you are having surgery about when to stop taking STEGLATRO and when to start it again.
- are eating less or there is a change in your diet.
- are dehydrated.
- have or have had problems with your pancreas, including pancreatitis or surgery on your pancreas.
- drink alcohol very often or drink a lot of alcohol in the short term (‚Äúbinge‚ÄĚ drinking).
- have ever had an allergic reaction to STEGLATRO.
- are pregnant or plan to become pregnant. STEGLATRO may harm your unborn baby. If you become pregnant while taking STEGLATRO, your healthcare provider may switch you to a different medicine to control your blood sugar. Talk to your healthcare provider about the best way to control your blood sugar if you plan to become pregnant or while you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if STEGLATRO passes into your breast milk. You should not breastfeed if you take STEGLATRO.
How should I take STEGLATRO?
- Take STEGLATRO by mouth 1 time in the morning each day, with or without food, exactly as your healthcare provider tells you to take it.
- Your healthcare provider may tell you to take STEGLATRO along with other diabetes medicines. Low blood sugar can happen more often when STEGLATRO is taken with certain other diabetes medicines. See ‚Äú What are the possible side effects of STEGLATRO ?‚ÄĚ.
- If you miss a dose, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose and take the medicine at the next regularly scheduled time. Do not take 2 doses of STEGLATRO at the same time. Talk with your healthcare provider if you have questions about a missed dose.
- If you take too much STEGLATRO, call your healthcare provider or go to the nearest hospital emergency room right away.
- When your body is under some types of stress, such as fever, trauma (such as a car accident), infection, or surgery, the amount of diabetes medicine you need may change. Tell your healthcare provider right away if you have any of these conditions and follow your healthcare provider’s instructions.
- STEGLATRO will cause your urine to test positive for glucose.
- Your healthcare provider may do certain blood tests before you start STEGLATRO and during treatment as needed. Your healthcare provider may change your dose of STEGLATRO based on the results of your blood tests.
What are the possible side effects of STEGLATRO?STEGLATRO may cause serious side effects, including: See " What is the most important information I should know about STEGLATRO? "
- Serious urinary tract infections. Serious urinary tract infections that may lead to hospitalization have happened in people who are taking STEGLATRO. Tell your healthcare provider if you have any signs or symptoms of a urinary tract infection such as a burning feeling when passing urine, a need to urinate often, the need to urinate right away, pain in the lower part of your stomach (pelvis), or blood in the urine. Sometimes people may also have a fever, back pain, nausea, or vomiting.
- Low blood sugar (hypoglycemia). If you take STEGLATRO with another medicine that can cause low blood sugar such as a sulfonylurea or insulin, your risk of getting low blood sugar is higher. The dose of your sulfonylurea or insulin may need to be lowered while you take STEGLATRO. Signs and symptoms of low blood sugar may include:
- headache
- confusion
- hunger
- shaking or feeling jittery
- drowsiness
- dizziness
- fast heartbeat
- weakness
- sweating
- irritability
- A rare but serious bacterial infection that causes damage to the tissue under the skin (necrotizing fasciitis) in the area between and around the anus and genitals (perineum). Necrotizing fasciitis of the perineum has happened in women and men who take medicines that lower blood sugar in the same way as STEGLATRO. Necrotizing fasciitis of the perineum may lead to hospitalization, may require multiple surgeries, and may lead to death. Seek medical attention immediately if you have fever above 100.4¬įF or you are feeling very weak, tired or uncomfortable (malaise) and you develop any of the following symptoms in the area between and around your anus and genitals:
- pain or tenderness
- swelling
- redness of skin (erythema)
- Serious allergic reaction. If you have any symptoms of a serious allergic reaction, stop taking STEGLATRO and call your healthcare provider right away or go to the nearest hospital emergency room. See ‚Äú Who should not take STEGLATRO? ‚ÄĚ. Your healthcare provider may give you a medicine for your allergic reaction and prescribe a different medicine for your diabetes.
The most common side effects of STEGLATRO include: These are not all the possible side effects of STEGLATRO.Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
- vaginal yeast infections and yeast infections of the penis (See "What is the most important information I should know about STEGLATRO?")
- changes in urination, including urgent need to urinate more often, in larger amounts, or at night
How should I store STEGLATRO? Keep STEGLATRO and all medicines out of the reach of children.
- Store STEGLATRO at room temperature between 68¬įF to 77¬įF (20¬įC to 25¬įC).
- Keep STEGLATRO dry.
General information about the safe and effective use of STEGLATRO. Medicines are sometimes prescribed for purposes other than those uled in a Medication Guide. Do not use STEGLATRO for a condition for which it was not prescribed. Do not give STEGLATRO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about STEGLATRO that is written for health professionals. For more information about STEGLATRO, go to www.steglatro.com or call 1-800-622-4477. What are the ingredients in STEGLATRO?Active ingredient: ertugliflozin. Inactive ingredients: microcrystalline cellulose, lactose monohydrate, sodium starch glycolate, and magnesium stearate. The tablet film coating contains the following inactive ingredients: hypromellose, lactose monohydrate, macrogol, triacetin, titanium dioxide, and iron oxide red. Manufactured for: Merck Sharp & Dohme LLC Rahway, NJ 07065, USA For patent information, go to: www.msd.com/research/patent Copyright © 2017-2023 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates. All rights reserved.usmg-mk8835-t-2309r006
Principal Display Panel - 5 Mg Tablet Bottle Label
NDC 0006-5363-03
Steglatro¬ģ (ertugliflozin) tablets
5 mg
Dispense the accompanying Medication Guideto each patient.
Each tablet contains 6.48 mg ertugliflozin L-pyroglutamicacid (equivalent to 5 mg ertugliflozin).
Rx only
30 Tablets
Principal Display Panel - 15 Mg Tablet Bottle Label
NDC 0006-5364-03
Steglatro¬ģ (ertugliflozin) tablets
15 mg
Dispense the accompanying Medication Guideto each patient.
Each tablet contains 19.43 mg ertugliflozin L-pyroglutamicacid (equivalent to 15 mg ertugliflozin).
Rx only
30 Tablets
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


