Exelderm Dailymed
Generic: sulconazole nitrate is used for the treatment of Candidiasis, Cutaneous Hypersensitivity Tinea Pedis Tinea Versicolor
Go PRO for all pill images
(sulconazole nitrate, USP) Solution, 1.0%
Rx only
For topical use only. Not for ophthalmic use.
Description
EXELDERM (sulconazole nitrate, USP) SOLUTION, 1.0% is a broad-spectrum antifungal agent intended for topical application. Sulconazole nitrate, USP, the active ingredient in EXELDERM SOLUTION, is an imidazole derivative with antifungal and antiyeast activity. Its chemical name is (±)-1-[2,4-dichloro-β-[(p-chlorobenzyl)-thio]-phenethyl] imidazole mononitrate and it has the following chemical structure:
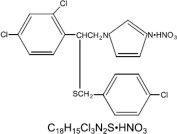
Sulconazole nitrate, USP is a white to off-white crystalline powder with a molecular weight of 460.77. It is freely soluble in pyridine; slightly soluble in ethanol, acetone, and chloroform; and very slightly soluble in water. It has a melting point of about 130°C.
EXELDERM SOLUTION contains sulconazole nitrate, USP 10 mg/mL in a solution of propylene glycol, poloxamer 407, polysorbate 20, butylated hydroxyanisole, and purified water, with sodium hydroxide and, if necessary, nitric acid added to adjust the pH.
Clinical Pharmacology
Sulconazole nitrate is an imidazole derivative that inhibits the growth of the common pathogenic dermatophytes including Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis. It also inhibits the organism responsible for tinea versicolor, Malassezia furfur, and certain gram-positive bacteria.
A maximization test with sulconazole nitrate solution showed no evidence of irritation or contact sensitization.
Indications And Usage
EXELDERM (sulconazole nitrate, USP) SOLUTION, 1.0% is a broad-spectrum antifungal agent indicated for the treatment of tinea cruris and tinea corporis caused by Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; and for the treatment of tinea versicolor. Effectiveness has not been proven in tinea pedis (athlete's foot).
Symptomatic relief usually occurs within a few days after starting EXELDERM SOLUTION and clinical improvement usually occurs within one week.
Contraindications
EXELDERM (sulconazole nitrate, USP) SOLUTION, 1.0% is contraindicated in patients who have a history of hypersensitivity to any of the ingredients.
Precautions
General
EXELDERM (sulconazole nitrate, USP) SOLUTION, 1.0% is for external use only. Avoid contact with the eyes. If irritation develops, the solution should be discontinued and appropriate therapy instituted.
Information for Patients
Patients should be told to use EXELDERM SOLUTION as directed by the physician, to use it externally only, and to avoid contact with the eyes.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to determine carcinogenic potential have not been performed. In vitro studies have shown no mutagenic activity.
Pregnancy
Sulconazole nitrate has been shown to be embryotoxic in rats when given in doses 125 times the human dose (in mg/kg). The drug at this dose given orally to rats also resulted in prolonged gestation and dystocia. Several females died during the perinatal period, most likely due to labor complications. Sulconazole nitrate was not teratogenic in rats or rabbits at oral doses of 50 mg/kg/day.
There are no adequate and well-controlled studies in pregnant women. Sulconazole nitrate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when sulconazole nitrate is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children have not been established.
Geriatric Use
Clinical studies of EXELDERM SOLUTION, 1.0%, did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients.
Adverse Reactions
There were no systemic effects and only infrequent cutaneous adverse reactions in 370 patients treated with sulconazole nitrate solution in controlled clinical trials. Approximately 1% of these patients reported itching and 1% burning or stinging. These complaints did not usually interfere with treatment.
Dosage And Administration
A small amount of solution should be gently massaged into the affected and surrounding skin areas once or twice daily.
Symptomatic relief usually occurs within a few days after starting EXELDERM (sulconazole nitrate) SOLUTION, 1.0%, and clinical improvement usually occurs within 1 week. To reduce the possibility of recurrence, tinea cruris, tinea corporis, and tinea versicolor should be treated for 3 weeks.
If significant clinical improvement is not seen after 4 weeks of treatment, an alternate diagnosis should be considered.
How Supplied
EXELDERM (sulconazole nitrate, USP) SOLUTION, 1.0% is a clear, slightly viscous, colorless to slightly yellow liquid with a slight characteristic odor. It is supplied as follows:
30 mL plastic bottle – NDC 69489-721-30
Avoid excessive heat, above 40° C (104° F), and protect from light.
To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
JOURNEY® MEDICAL CORPORATION
Manufactured for:Journey Medical Corp. Scottsdale, AZ 85258 www.JMCderm.com
141112 Revised March 2021
Principal Display Panel 30 Ml Bottle Label
NDC 69489-721-30
Exelderm ®
(sulconazolenitrate, USP) Solution, 1.0%
30 mL
Rx only
For topical use only.
Not for ophthalmic use.
JOURNEY
MEDICAL CORPORATION

Principal Display Panel 30 Ml Bottle Label
Exelderm ® NDC 69489-721-30
(sulconazole nitrate, USP) Solution, 1.0%
Rx only
For topical use only.
Not for ophthalmic use.
30 mL

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site



