Ezetimibe and Simvastatin (ezetimibe 10 mg simvastatin 40 mg) Dailymed
Generic: ezetimibe and simvastatin is used for the treatment of Hypercholesterolemia Hyperlipoproteinemia Type II Hyperlipidemia, Familial Combined Pregnancy Coronary Artery Disease Hyperlipoproteinemias Lactation Hypertriglyceridemia Liver Failure
All Imprints
ezetimibe 10 mg / simvastatin 80 mg oral tablet - aa73 oval white
ezetimibe 10 mg / simvastatin 20 mg oral tablet - aa71 oval white
ezetimibe 10 mg / simvastatin 10 mg oral tablet - aa70 oval white
ezetimibe 10 mg / simvastatin 40 mg oral tablet - aa72 capsule white
ezetimibe 10 mg / simvastatin 40 mg oral tablet - aa72 oval white
Go PRO for all pill images
Recent Major Changes Section
gf
Indications and Usage (1 )                                                               2/2024
Dosage and Administration (2.1 , 2.2 , 2.3 , 2.4 , 2.5 )                        2/2024
Contraindications, Pregnancy and Lactation (4 ) Removed            2/2024
Warnings and Precautions (5.1 ,5.2 ,5.3 )                                        2/2024
1 Indications And Usage
Ezetimibe and Simvastatin Tablets
Ezetimibe and simvastatin tablets are a combination of simvastatin and ezetimibe indicated:
As an adjunct to diet to reduce elevated low density lipoprotein cholesterol (LDL-C):
In adults with primary hyperlipidemia. In adults and pediatric patients aged 10 years and older with heterozygous familial hypercholesterolemia (HeFH). As an adjunct to other LDL-C-lowering therapies to reduce elevated LDL-C in adults with homozygous familial hypercholesterolemia (HoFH).
Simvastatin
 
Simvastatin, when used as a component of ezetimibe and simvastatin tablets, are indicated to reduce the risk of total mortality by reducing risk of coronary heart disease death, non-fatal myocardial infarction and stroke, and the need for coronary and non-coronary revascularization procedures in adults with established coronary heart disease, cerebrovascular disease, peripheral vascular disease, and/or diabetes, who are at high risk of coronary heart disease events.
Ezetimibe and simvastatin tablets are a combination of ezetimibe, a dietary cholesterol absorption inhibitor, and simvastatin, an HMG-CoA reductase inhibitor (statin) indicated: (1 )
- As an adjunct to diet to reduce elevated low density lipoprotein cholesterol (LDL-C):
- In adults with primary hyperlipidemia.
- In adults and pediatric patients aged 10 years and older with heterozygous familial hypercholesterolemia (HeFH).
- As an adjunct to other LDL-C lowering therapies to reduce LDL-C in adults with homozygous familial hypercholesterolemia (HoFH).
Simvastatin
Simvastatin, when used as a component of ezetimibe and simvastatin tablets, is indicated to reduce the risk of total mortality by reducing risk of coronary heart disease death, non-fatal myocardial infarction and stroke, and the need for coronary and non-coronary revascularization procedures in adults with established coronary heart disease, cerebrovascular disease, peripheral vascular disease, and/or diabetes, who are at high risk of coronary heart disease events.
2 Dosage And Administration
- Important Dosage and Administration Information: (
2.1 )
- Take ezetimibe and simvastatin tablets orally once daily in the evening with or without food.
- Maximum recommended dosage is ezetimibe and simvastatin tablet 10/40 mg once daily. Ezetimibe and simvastatin tablets 10/80 mg daily dosage is restricted to patients who have been taking ezetimibe and simvastatin tablets 10/80 mg daily chronically (e.g., for 12 months or more) without evidence of muscle toxicity.
- For patients that require a high-intensity statin or are unable to achieve their LDL-C goal receiving ezetimibe and simvastatin tablets 10/40 mg daily, prescribe alternative LDL-C-lowering treatment.
- If as dose is missed, take the missed dose as soon as possible. Do not double the next dose.
- Assess LDL-C when clinically appropriate, as early as 2 weeks after initiating ezetimibe and simvastatin tablets and adjust the dosage if necessary.
- Adults: Recommended dosage range of 10/10 mg to 10/40 mg once daily. (
2.2 )- See full prescribing information for ezetimibe and simvastatin tablets dosage modifications due to drug interactions. (
2.3 )- Patients with Renal Impairment: Doses exceeding 10/20 mg should be used with caution and close monitoring in patients with moderate to severe renal impairment. (
2.4 )2.1 Important Dosage and Administration Information
Take ezetimibe and simvastatin tablets orally once daily in the evening with or without food. The maximum recommended dosage is ezetimibe and simvastatin tablets 10/40 mg once daily. The ezetimibe and simvastatin tablets 10/80 mg daily dosage is restricted to adult patients who have been taking ezetimibe and simvastatin tablets 10/80 mg daily chronically (e.g., for 12 months or more) without evidence of muscle toxicity [see Warnings and Precautions (5.1)]. For patients that require a high-intensity statin or are unable to achieve their LDL-C goal receiving ezetimibe and simvastatin tablets 10/40 mg daily, prescribe alternative LDL-C-lowering treatment. If as dose is missed, take the missed dose as soon as possible. Do not double the next dose. Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating ezetimibe and simvastatin tablets and adjust the dosage if necessary. 2.2 Recommended Dosage in Adult Patients
The recommended dosage range of ezetimibe and simvastatin tablets 10/10 mg to 10/40 mg once a day.
2.3 Recommended Dosage in Pediatric Patients 10 Years ofAge and Older with HeFH
2.4 Recommended Dosage in Patientswith Renal Impairment
Renal impairment is a risk factor for statin-associated myopathy. Doses of ezetimibe and simvastatin tablets exceeding 10/20 mg should be used with caution and close monitoring in patients with moderate to severe renal impairment [see Warnings and Precautions (5.1) and  Use in Specific Populations (8.6)].
There are no dosage adjustment recommendations for patients with mild renal impairment.
2.5 Dosage Modifications Due to Drug Interactions
Concomitant use of ezetimibe and simvastatin tablets with the following drugs requires dosage modification of ezetimibe and simvastatin tablets [see Warnings and Precautions (5.1) and  Drug Interactions (7.1)].
Patients taking Lomitapide
Reduce the dosage of ezetimibe and simvastatin by 50%. Do not exceed ezetimibe and simvastatin tablets 10/20 mg once daily (or 10/40 mg once daily for patients who have previously taken ezetimibe and simvastatin tablets 10/80 mg daily chronically while taking lomitapide) [see Dosage and Administration (2.1)].
Patients taking Verapamil, Diltiazem, or Dronedarone
Do not exceed ezetimibe and simvastatin tablets 10/10 mg once daily.
Patients taking Amiodarone, Amlodipine, or Ranolazine
Do not exceed ezetimibe and simvastatin tablets 10/20 mg once daily.
Patients taking Bile Acid Sequestrants
In patients taking a bile acid sequestrant, administer ezetimibe and simvastatin tablets at least 2 hours before or 4 hours after the bile acid sequestrant.
3 Dosage Forms And Strengths
- Ezetimibe and simvastatin tablets 10/10 mg, (ezetimibe, USP 10 mg and simvastatin, USP 10 mg tablets) are white to off-white capsule shaped tablets, debossed with ‚ÄúAA 70‚ÄĚ on one side and plain on the other side.
- Ezetimibe and simvastatin tablets 10/20 mg, (ezetimibe, USP 10 mg and simvastatin, USP 20 mg tablets) are white to off-white capsule shaped tablets, debossed with ‚ÄúAA 71‚ÄĚ on one side and plain on the other side.
- Ezetimibe and simvastatin tablets 10/40 mg, (ezetimibe, USP 10 mg and simvastatin, USP 40 mg tablets) are white to off-white capsule shaped tablets, debossed with ‚ÄúAA 72‚ÄĚ on one side and plain on the other side.
- Ezetimibe and simvastatin tablets 10/80 mg, (ezetimibe, USP 10 mg and simvastatin, USP 80 mg tablets) are white to off-white capsule shaped tablets, debossed with ‚ÄúAA 73‚ÄĚ on one side and plain on the other side.
Tablets (ezetimibe mg and simvastatin mg): 10/10, 10/20, 10/40, 10/80 (3 )
4 Contraindications
Ezetimibe and simvastatin tablets are contraindicated in the following conditions:
- Concomitant use of strong CYP3A4 inhibitors (select azole anti-fungals, macrolide antibiotics, anti-viral medications, and nefazodone) [see Drug Interactions (7.1)].
- Concomitant use of cyclosporine, danazol, or danazol [see Drug Interactions (7.1)].
- Acute liver failure or decompensated cirrhosis [see Warnings and Precautions (5.3)].
- Hypersensitivity to simvastatin, ezetimibe, or any excipients in ezetimibe and simvastatin tablets. Hypersensitivity reactions, including anaphylaxis, angioedema, and Stevens-Johnson syndrome, have been reported [see Adverse Reactions (6.2)].
- Concomitant use of strong CYP3A4 inhibitors (select azole anti-fungals, macrolide antibiotics, anti-viral medications and nefazodone). (
4 )- Concomitant use of cyclosporine, danazol or gemfibrozil. (
4 )- Acute liver failure or decompensated cirrhosis. (
4 )- Hypersensitivity to simvastatin, ezetimibe or any excipient of ezetimibe and simvastatin tablets. (
4 )
5 Warnings And Precautions
- Myopathy and Rhabdomyolysis: Risk factors include age 65 years or greater, uncontrolled hypothyroidism, renal impairment, concomitant use with certain other drugs, and higher ezetimibe and simvastatin dosage. Chinese patients may be at higher risk for myopathy. Discontinue ezetimibe and simvastatin tablets if markedly elevated CK levels occur or myopathy is diagnosed or suspected. Temporarily discontinue ezetimibe and simvastatin tablets in patients experiencing an acute or serious condition at high risk of developing renal failure secondary to rhabdomyolysis. Inform patients of the risk of myopathy and rhabdomyolysis when starting or increasing ezetimibe and simvastatin dosage. Instruct patients to promptly report unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever. (
5.1) - Immune-Mediated Necrotizing Myopathy (IMNM): Rare reports of IMNM, an autoimmune myopathy, have been reported. Discontinue ezetimibe and simvastatin tablets if IMNM is suspected. (
5.2 )- Hepatic Dysfunction: Increases in serum transaminases have occurred, some persistent. Rare reports of fatal and non-fatal hepatic failure have occurred. Consider testing liver enzyme before initiating therapy and as clinically indicated thereafter. If serious hepatic injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs, promptly discontinue ezetimibe and simvastatin tablets. (
5.3 )5.1 Myopathy and Rhabdomyolysis
Ezetimibe and simvastatin may cause myopathy and rhabdomyolysis. Acute kidney injury secondary to myoglobinuria and rare fatalities have occurred as a result of rhabdomyolysis in patients treated with statins, including ezetimibe and simvastatin.
In clinical trials of 24,747 simvastatin-treated patients with a median follow-up of 4 years, the incidence of myopathy, defined as unexplained muscle weakness, pain, or tenderness accompanied by creatinine kinase (CK) increases greater than ten times the upper limit of normal (10 X ULN), were approximately 0.03%, 0.08%, and 0.61% in patients treated with simvastatin 20 mg, 40 mg, and 80 mg daily, respectively. In another clinical trial of 12,064 simvastatin-treated patients (with a history of myocardial infarction) with a mean follow-up of 6.7 years, the incidences of myopathy in patients taking simvastatin 20 mg and 80 mg daily were approximately 0.02% and 0.9%, respectively. The incidences of rhabdomyolysis (defined as myopathy with a CK > 40 X ULN) in patients taking simvastatin 20 mg and 80 mg daily were approximately 0% and 0.4%, respectively [see Adverse Reactions (6.1)].
In the Trial of Heart and Renal Protection (SHARP), 9,270 patients with chronic kidney disease were allocated to receive ezetimibe and simvastatin 10/20 mg daily (n = 4650) or placebo (n = 4,620). During a median follow-up period of 4.9 years, the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] > 10 times upper limit of normal [ULN]) was 0.2% for ezetimibe and simvastatin and 0.1% for placebo: the incidence of rhabdomyolysis (defined as myopathy with a CK > 40 times ULN) was 0.09% for ezetimibe and simvastatin and 0.02% for placebo.
In post-marketing experience with ezetimibe, cases of myopathy and rhabdomyolysis have been reported. Most patients who developed rhabdomyolysis were taking a statin prior to initiating ezetimibe. However, rhabdomyolysis has been reported with ezetimibe monotherapy and with the addition of ezetimibe to agents known to be associated with increased risk of rhabdomyolysis, such as fibric acid derivatives. Ezetimibe and simvastatin and a fenofibrate, if taking concomitantly, should both be immediately discontinued if myopathy is diagnosed or suspected.
Risk Factors for Myopathy
Risk factors for myopathy include age 65 years or greater, uncontrolled hypothyroidism, renal impairment, concomitant use with certain other drugs (including other lipid-lowering therapies), and higher ezetimibe and simvastatin dosage; Chinese patients on ezetimibe and simvastatin may be at higher risk for myopathy [see Contraindications (4), Drug Interactions (7.1), and Use in Specific Populations (8.8)]. The risk of myopathy is increased by elevated plasma levels of simvastatin and simvastatin acid. The risk is also greater in patients taking ezetimibe and simvastatin 80 mg daily compared with patients taking lower ezetimibe and simvastatin dosages and compared with patients using other statins with similar or greater LDL-C-lowering efficacy [see Adverse Reactions (6.1)].
Steps to Prevent or Reduce the Risk of Myopathy and Rhabdomyolysis
The concomitant use of strong CYP3A4 inhibitors with ezetimibe and simvastatin are contraindicated. If short-term treatment with strong CYP3A4 inhibitors is required, temporarily suspend ezetimibe and simvastatin during the duration of strong CYP3A4 inhibitor treatment. The concomitant use of ezetimibe and simvastatin with gemfibrozil, cyclosporine, or danazol is also contraindicated [see Contraindications (4) and  Drug Interactions (7.1)].
Ezetimibe and simvastatin dosage modifications are recommended for patients taking lomitapide, verapamil, diltiazem, dronedarone, amiodarone, amlodipine or ranolazine [see Dosage and Administration (2.5)]. Ezetimibe and simvastatin use should be temporarily suspended in patients taking daptomycin. Lipid modifying doses (‚Č• 1 gram/day) of niacin, fibrates, colchicine, and grapefruit juice may also increase the risk of myopathy and rhabdomyolysis [see Drug Interactions (7.1)].
Use the 80 mg daily dosage of ezetimibe and simvastatin only in patients who have been taking simvastatin 80 mg daily chronically without evidence of muscle toxicity [see Dosage and Administration (2.1)]. If patients treated with ezetimibe and simvastatin 80 mg are prescribed an interacting drug that increases the risk for myopathy and rhabdomyolysis, switch to an alternate statin [see Drug Interactions (7.1)].
Discontinue ezetimibe and simvastatin if markedly elevated CK levels occur or if myopathy is either diagnosed or suspected. Muscle symptoms and CK increases may resolve if ezetimibe and simvastatin is discontinued. Temporarily discontinue ezetimibe and simvastatin in patients experiencing an acute or serious condition at high risk of developing renal failure secondary to rhabdomyolysis, e.g., sepsis; shock; severe hypovolemia; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
Inform patients of the risk of myopathy and rhabdomyolysis when starting or increasing the ezetimibe and simvastatin dosage and advise patients receiving ezetimibe and simvastatin 80 mg of the increased risk of myopathy and rhabdomyolysis. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever.
5.2 Immune-Mediated NecrotizingMyopathy
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use, including reports of recurrence when the same or a different statin was administered. IMNM is characterized by proximal muscle weakness and elevated serum creatine kinase that persist despite discontinuation of statin treatment; positive anti-HMG CoA reductase antibody; muscle biopsy showing necrotizing myopathy without significant inflammation; and improvement with immunosuppressive agents. Additional neuromuscular and serologic testing may be necessary. Treatment with immunosuppressive agents may be required. Discontinue ezetimibe and simvastatin if IMNM is suspected.
5.3 Hepatic Dysfunction
Increases in serum transaminases have been reported with use of ezetimibe and simvastatin [see Adverse Reactions (6.1)]. In most cases, these changes appeared soon after initiation, were transient, were not accompanied by symptoms, and resolved or improved on continued therapy or after a brief interruption in therapy. Marked persistent increases of hepatic transaminases have also occurred with ezetimibe and simvastatin. There have been rare post-marketing reports of fatal and non-fatal hepatic failure in patients taking statins, including ezetimibe and simvastatin.
In three placebo-controlled, 12-week trials, the incidence of consecutive elevations (‚Č• 3 X ULN) in serum transaminases was 1.7% overall for patients treated with ezetimibe and simvastatin and appeared to be dose-related with an incidence of 2.6% for patients treated with ezetimibe and simvastatin 10/80. In controlled long-term (48-week) extensions, which included both newly-treated and previously-treated patients, the incidence of consecutive elevations (‚Č• 3 X ULN) in serum transaminases was 1.8% overall and 3.6% for patients treated with ezetimibe and simvastatin 10/80. These elevations in transaminases were generally asymptomatic, not associated with cholestasis, and returned to baseline after discontinuation of therapy or with continued treatment.
In SHARP, 9,270 patients with chronic kidney disease were allocated to receive ezetimibe and simvastatin 10/20 mg daily (n = 4,650), or placebo (n = 4,620). During a median follow-up period of 4.9 years, the incidence of consecutive elevations of transaminases (> 3 X ULN) was 0.7% for ezetimibe and simvastatin and 0.6% for placebo.
Patients who consume substantial quantities of alcohol and/or have a history of liver disease may be at increased risk for hepatic injury.
Consider liver enzyme testing before ezetimibe and simvastatin initiation and when clinically indicated thereafter. Ezetimibe and simvastatin is contraindicated in patients with acute liver failure or decompensated cirrhosis [see Contraindications (4)]. If serious hepatic injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs, promptly discontinue ezetimibe and simvastatin.
5.4 Increases inHBA1c and Fasting Serum Glucose Levels
Increases in HbA1c and fasting serum glucose levels have been reported with statins, including ezetimibe and simvastatin. Optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices.
6 Adverse Reactions
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Myopathy and Rhabdomyolysis [see Warnings and Precautions (5.1)]
- Immune-Mediated Necrotizing Myopathy [see Warnings and Precautions (5.2)]
- Hepatic Dysfunction [see Warnings and Precautions (5.3)]
- Increases in HbA1c and Fasting Serum Glucose Levels [see Warnings and Precautions (5.4)]
- Common (incidence ‚Č• 2% and greater than placebo) adverse reactions in clinical trials: headache, increased ALT, myalgia, upper respiratory tract infection, and diarrhea. (
6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Ezetimibe and Simvastatin
In the ezetimibe and simvastatin placebo-controlled clinical trials database of 1,420 patients (age range 20 to 83 years, 52% female, 87% White, 3% Black or African American, 3% Asians, 5% other races identified as Hispanic or Latino ethnicity) with a median treatment duration of 27 weeks, 5% of patients on ezetimibe and simvastatin and 2.2% of patients on placebo discontinued due to adverse reactions.
The most commonly reported adverse reactions (incidence ‚Č• 2% and greater than placebo) in controlled clinical trials were: headache (5.8%), increased ALT (3.7%), myalgia (3.6%), upper respiratory tract infection (3.6%), and diarrhea (2.8%). The most common adverse reactions in the group treated with ezetimibe and simvastatin that led to treatment discontinuation and occurred at a rate greater than placebo were: increased ALT (0.9%), myalgia (0.6%), increased AST (0.4%), and back pain (0.4%).
Ezetimibe and simvastatin has been evaluated for safety in more than 10,189 patients in clinical trials.
Table 1 summarizes the frequency of clinical adverse reactions reported in ‚Č• 2% of patients treated with ezetimibe and simvastatin (n = 1,420) and at an incidence greater than placebo from four placebo-controlled trials.
Table 1*: Adverse Reactions Reported ‚Č• 2% of Patients Treated with E zetimibe and Simvastatin at an Incidence Greater than Placebo Regardless of Causality
 
% Placebo
N = 371 
% Ezetimibe 10 mg
N = 302  % Simvastatin† N = 1,234 
% Ezetimibe and Simvastatin†
N = 1,420  Headache  5.4  6.0  5.9  5.8  Upper respiratory tract infection  2.7  5.0  5.0  3.6  Myalgia  2.4   2.3   2.6   3.6   Diarrhea  2.2  5.0  3.7  2.8  Pain in extremity  1.3  3.0  2.0  2.3  Influenza  0.8   1.0   1.9  2.3  * Includes two placebo-controlled combination studies in which the active ingredients equivalent to ezetimibe and simvastatin were co-administered and two placebo-controlled studies in which ezetimibe and simvastatin was administered. † All doses.
Study of Heart and Renal Protection
In SHARP, 9,270 patients were allocated to ezetimibe and simvastatin 10/20 mg daily (n = 4,650) or placebo (n = 4,620) for a median follow-up period of 4.9 years. The proportion of patients who permanently discontinued trial treatment as a result of either an adverse event or abnormal safety blood result was 10.4% vs. 9.8% among patients allocated to ezetimibe and simvastatin and placebo, respectively. Comparing those allocated to ezetimibe and simvastatin vs. placebo, the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum CK > 10 times ULN) was 0.2% vs. 0.1% and the incidence of rhabdomyolysis (defined as myopathy with a CK > 40 times ULN) was 0.09% vs. 0.02%, respectively. Consecutive elevations of transaminases (> 3 X ULN) occurred in 0.7% vs. 0.6%, respectively. Patients were asked about the occurrence of unexplained muscle pain or weakness at each trial visit: 21.5% vs. 20.9% patients ever reported muscle symptoms in the ezetimibe and simvastatin and placebo groups, respectively. Cancer was diagnosed during the trial in 9.4% vs. 9.5% of patients assigned to ezetimibe and simvastatin and placebo, respectively.
Ezetimibe
Other adverse reactions reported with ezetimibe in placebo-controlled studies, regardless of causality assessment: Musculoskeletal system disorders: arthralgia; Infections and infestations: sinusitis; Body as a whole ‚Äď general disorders: fatigue.
Simvastatin
In a clinical outcome trial in which 12,064 adult patients with a history of myocardial infarction were treated with simvastatin (mean follow-up 6.7 years), the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] > 10 times (1,200 U/L) upper limit of normal [ULN]) in patients taking simvastatin 20 mg and 80 mg daily was approximately 0.02% and 0.9% respectively. The incidence of rhabdomyolysis (defined as myopathy with a CK > 40 times ULN) in patients taking simvastatin 20 mg and 80 mg daily was approximately 0% and 0.4%. The incidence of myopathy and rhabdomyolysis, was highest during the first year and then notably decreased during the subsequent years of treatment. In this trial, patients were carefully monitored and some interacting medicinal products were excluded. Other adverse reactions reported with simvastatin in placebo-controlled clinical trials: atrial fibrillation; vertigo; abdominal pain, constipation, dyspepsia, flatulence, gastritis; eczema, rash; diabetes mellitus; bronchitis, sinusitis, urinary tract infections; asthenia, edema/swelling; and insomnia.
Laboratory Tests
Marked persistent increases of hepatic serum transaminases have been noted [see Warnings and Precautions (5.3)]. Elevated alkaline phosphatase and ő≥-glutamyl transpeptidase have been reported. About 5% of patients taking simvastatin had elevations of CK levels of 3 or more times the normal value on one or more occasions. This was attributable to the noncardiac fraction of CK [see Warnings and Precautions (5.1)].
6.2 Post-marketing Experience
The following adverse reactions have been identified during post-approval use of ezetimibe and simvastatin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as Whole: fever, chills, malaise, asthenia
Blood and Lymphatic System Disorders: anemia, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR increase, eosinophilia
Gastrointestinal Disorders: pancreatitis, nausea, vomiting
Hepatobiliary Disorders: cholelithiasis, cholecystitis, elevations in liver transaminases including elevations more than 5 X ULN, hepatitis/jaundice, fatal and non-fatal hepatic failure
Immune System Disorders: hypersensitivity syndrome including: anaphylaxis, angioedema, lupus erythematous-like syndrome, dermatomyositis, vasculitis
Musculoskeletal and Connective Tissue Disorders: muscle cramps, immune-mediated necrotizing myopathy, rhabdomyolysis, myalgia, arthralgia, polymyalgia rheumatica, arthritis, elevated creatine phosphokinase
Nervous System Disorders: dizziness, depression, paresthesia, peripheral neuropathy, rare reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. Cognitive impairment was generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks). There have been rare reports of new-onset or exacerbation of myasthenia gravis, including ocular myasthenia, and reports of recurrence when the same or a different statin was administered.
Skin and Subcutaneous Tissue Disorders: rash, pruritus, alopecia, a variety of skin changes (e.g., nodules, discoloration, dryness of skin/mucous membranes, changes to hair/nails), purpura, lichen planus, urticaria, photosensitivity, flushing, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome.
Respiratory and Thoracic: interstitial lung disease, dyspnea
Reproductive System Disorders: erectile dysfunction
7 Drug Interactions
E zetimibe and Simvastatin
- See full prescribing information for details regarding concomitant use of ezetimibe and simvastatin with other drugs or grapefruit juice that increase the risk of myopathy and rhabdomyolysis. (
2.3 ,7.1 )- Cholestyramine: Combination decreases exposure of ezetimibe. (
2.3 ,7.2 )- Coumarin Anticoagulants: Obtain INR before ezetimibe and simvastatin tablets initiation and monitor INR during ezetimibe and simvastatin dosage initiation or adjustment. (
7.3 )- Digoxin: During ezetimibe and simvastatin initiation, monitor digoxin levels. (
7.3 )- Fenofibrates: Combination increases exposure of ezetimibe. If cholelithiasis is suspected in a patient receiving ezetimibe and a fenofibrate, gallbladder studies are indicated and alternative lipid-lowering therapy should be considered. (
7.3 ,12.3 )7.1 Drug Interactions thatIncrease the Risk of Myopathy and Rhabdomyolysis with Ezetimibe and Simvastatin
Ezetimibe and simvastatin is a substrate of CYP3A4 and of the transport protein OATP1B1. Ezetimibe and simvastatin plasma levels can be significantly increased with concomitant administration of inhibitors of CYP3A4 and OATP1B1.
Table 2 includes a ul of drugs that increase the risk of myopathy and rhabdomyolysis when used concomitantly with ezetimibe and simvastatin and instructions for preventing or managing them [see Warnings and Precautions (5.1) and  Clinical Pharmacology (12.3)].
Table 2: Drug Interactions that Increase the Risk of Myopathy and Rhabdomyolysis with Ezetimibe and Simvastatin
Strong CYP3A4 inhibitors
Clinical Impact:
Simvastatin is a substrate of CYP3A4. Concomitant use of strong CYP3A4 inhibitors with ezetimibe and simvastatin increases simvastatin exposure and increases the risk of myopathy and rhabdomyolysis, particularly with higher ezetimibe and simvastatin dosages.
Intervention:
Concomitant use of strong CYP3A4 inhibitors with ezetimibe and simvastatin is contraindicated [see Contraindications (4)]. If treatment with a CYP3A4 inhibitor is unavoidable, suspend ezetimibe and simvastatin during the course of strong CYP3A4 inhibitor treatment.
Examples:
Select azole anti-fungals (e.g., itraconazole, ketoconazole, posaconazole, and voriconazole), select macrolide antibiotics (e.g., erythromycin and clarithromycin, telithromycin), select HIV protease inhibitors (e.g., nelfinavir, ritonavir, and darunavir/ritonavir), select HCV protease inhibitors (e.g., boceprevir and telaprevir), cobicistat-containing products, and nefazodone.
Cyclosporine, Danazol, or Gemfibrozil
Clinical Impact:
The risk of myopathy and rhabdomyolysis is increased with concomitant use of cyclosporine, danazol, or gemfibrozil with ezetimibe and simvastatin. Gemfibrozil may cause myopathy when given alone.
Intervention:
Concomitant use of cyclosporine, danazol, or gemfibrozil with ezetimibe and simvastatin is contraindicated [see Contraindications (4)].
Amiodarone, Dronedarone, Ranolazine, or Calcium Channel Blockers
Clinical Impact:
The risk of myopathy and rhabdomyolysis is increased by concomitant use of amiodarone, dronedarone, ranolazine, or calcium channel blockers with ezetimibe and simvastatin.
Intervention:
For patients taking verapamil, diltiazem, or dronedarone, do not exceed ezetimibe and simvastatin 10/10 mg daily. For patients taking amiodarone, amlodipine, or ranolazine, do not exceed ezetimibe and simvastatin 10/20 mg daily [see Dosage and Administration (2.3)].
Lomitapide
Clinical Impact:
Simvastatin exposure is approximately doubled with concomitant use of lomitapide and the risk of myopathy and rhabdomyolysis is increased.
Intervention:
Reduce the dose of ezetimibe and simvastatin by 50% if initiating lomitapide. Do not exceed ezetimibe and simvastatin 10/20 mg daily (or ezetimibe and simvastatin 10/40 mg daily for patients who have previously taken ezetimibe and simvastatin 10/80 mg daily chronically) while taking lomitapide [see Dosage and Administration (2.1, 2.3)].
Daptomycin
Clinical Impact:
Cases of rhabdomyolysis have been reported with simvastatin administered with daptomycin. Both ezetimibe and simvastatin and daptomycin can cause myopathy and rhabdomyolysis when given alone and the risk of myopathy and rhabdomyolysis may be increased by co-administration.
Intervention:
If treatment with daptomycin is required, consider temporarily suspending ezetimibe and simvastatin during the course of daptomycin treatment.
Niacin
Clinical Impact:
Cases of myopathy and rhabdomyolysis have been observed with concomitant use of lipid modifying dosages of niacin-containing products (‚Č• 1 gram/day niacin) with ezetimibe and simvastatin. The risk of myopathy is greater in Chinese patients. In a clinical trial (median follow-up 3.9 years) of patients at high risk of CVD and with well-controlled LDL-C levels on simvastatin 40 mg/day with or without ezetimibe 10 mg/day, there was no incremental benefit on cardiovascular outcomes with the addition of lipid-modifying doses of niacin
Intervention:
Concomitant use of ezetimibe and simvastatin with lipid-modifying dosages of niacin is not recommended in Chinese patients [see Use in Specific Populations (8.8)]. For non-Chinese patients, consider if the benefit of using lipid-modifying doses of niacin concomitantly with ezetimibe and simvastatin outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy, particularly during initiation of therapy and during upward dose titration of either drug.
Fibrates (other than Gemfibrozil)
Clinical Impact:
Fibrates may cause myopathy when given alone. The risk of myopathy and rhabdomyolysis is increased with concomitant use of fibrates with ezetimibe and simvastatin.
Intervention:
Consider if the benefit of using fibrates concomitantly with ezetimibe and simvastatin outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy, particularly during initiation of therapy and during upward dose titration of either drug.
Colchicine
Clinical Impact:
Cases of myopathy and rhabdomyolysis have been reported with concomitant use of colchicine with ezetimibe and simvastatin.
Intervention:
Consider if the benefit of using colchicine concomitantly with ezetimibe and simvastatin outweighs the increased risk of myopathy and rhabdomyolysis. If concomitant use is decided, monitor patients for signs and symptoms of myopathy, particularly during initiation of therapy and during upward dose titration of either drug.
Grapefruit Juice
Clinical Impact:
Grapefruit juice can raise the plasma levels of simvastatin and may increase the risk of myopathy and rhabdomyolysis.
Intervention:
Avoid grapefruit juice when taking ezetimibe and simvastatin.
7.2 Drug Interactions thatDecrease the Efficacy of Ezetimibe and Simvastatin
Table 3 presents drug interactions that may decrease the efficacy of ezetimibe and simvastatin and instructions for preventing or managing them.
 
Table 3: Drug Interactions that Decrease the Efficacy of Ezetimibe and Simvastatin
 
Bile Acid Sequestrants
Clinical Impact:
Concomitant cholestyramine administration decreased the mean exposure of total ezetimibe approximately 55%. The incremental LDL-C reduction due to adding ezetimibe and simvastatin to cholestyramine may be reduced by this interaction [see Clinical Pharmacology (12.3)].
Intervention:
In patients taking a bile acid sequestrant, administer ezetimibe and simvastatin at least 2 hours before or at least 4 hours after cholestyramine [see Dosage and Administration (2.3)].
7.3 Ezetimibe and Simvastatin Tablets Effect onOther Drugs
Table 4 presents ezetimibe and simvastatin tablets effect on other drugs and instructions for preventing or managing them.
Table 4: E zetimibe and Simvastatin Effects on Other Drugs
Coumarin Anticoagulants
Clinical Impact:
Ezetimibe and simvastatin may potentiate the effect of coumarin anticoagulants and increase the INR. The concomitant use of simvastatin (20 mg to 40 mg) and coumarin anticoagulants increased the INR from a baseline of 1.7 to 1.8 in healthy subjects and from 2.6 to 3.4 in patients with hyperlipidemia. There are post-marketing reports of clinically evident bleeding and/or increased INR in patients taking concomitant statins (with or without ezetimibe) and coumarin anticoagulants.
Intervention:
In patients taking coumarin anticoagulants, obtain an INR before starting ezetimibe and simvastatin and frequently enough after initiation, dose titration, or discontinuation to ensure that no significant alteration in INR occurs. Once the INR is stable, monitor INR at regularly recommended intervals.
Digoxin
Clinical Impact:
Concomitant use of digoxin with ezetimibe and simvastatin may result in elevated plasma digoxin concentrations [see Clinical Pharmacology (12.3)].
Intervention:
Monitor digoxin levels in patients taking digoxin when ezetimibe and simvastatin is initiated.
Fenofibrates
Clinical Impact:
Both ezetimibe and fenofibrates may increase cholesterol excretion into the bile, leading to cholelithiasis.
Intervention:
If cholelithiasis is suspected in a patient receiving ezetimibe and simvastatin and a fenofibrate, gallbladder studies are indicated and alternative lipid-lowering therapy should be considered [see the product labeling for fenofibrate and fenofibric acid].
8 Use In Specific Populations
- Pregnancy: May cause fetal harm (
8.1 )- Lactation: Breastfeeding not recommended during treatment with ezetimibe and simvastatin tablets. (
8.2 )8.1 Pregnancy
Ezetimibe and Simvastatin
Risk Summary
Discontinue ezetimibe and simvastatin when pregnancy is recognized. Alternatively, consider the ongoing therapeutic needs of the individual patient.
Ezetimibe and simvastatin decrease synthesis of cholesterol and possibly other biologically active substances derived from cholesterol; therefore, ezetimibe and simvastatin may cause fetal harm when administered to pregnant patients based on the mechanism of action [see Clinical Pharmacology (12.1)]. In addition, treatment of hyperlipidemia is not generally necessary during pregnancy. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hyperlipidemia for most patients.
Available data from case series and prospective and retrospective observational cohort studies over decades of use with statins in pregnant women have not identified a drug-associated risk of major congenital malformations. Published data from prospective and retrospective observational cohort studies with ezetimibe and simvastatin use in pregnant women are insufficient to determine if there is a drug-associated risk of miscarriage (see Data).
In animal reproduction studies, no adverse developmental effects were observed in pregnant rats or rabbits orally administered simvastatin during the period of organogenesis at doses that resulted in 2.5 and 2 times, respectively, the human exposure at the maximum recommended human dosage of 80 mg/day, based on body surface area (mg/m2). In animal reproduction studies, no adverse developmental effects were observed in pregnant rats and rabbits orally administered ezetimibe during the period of organogenesis at doses that resulted in up to 10 and 150 times, respectively, the human exposure at the MRHD, based on AUC (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Ezetimibe
There are insufficient data on ezetimibe use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
Simvastatin
A Medicaid cohort linkage trial of 1,152 statin-exposed pregnant women compared to 8,86,996 controls did not find a significant teratogenic effect from maternal use of statins in the first trimester of pregnancy, after adjusting for potential confounders ‚Äď including maternal age, diabetes mellitus, hypertension, obesity, and alcohol and tobacco use ‚Äď using propensity score-based methods. The relative risk of congenital malformations between the group with statin use and the group with no statin use in the first trimester was 1.07 (95% confidence interval 0.85 to 1.37) after controlling for confounders, particularly pre-existing diabetes mellitus. There were also no statistically significant increases in any of the organ-specific malformations assessed after accounting for confounders. In the majority of pregnancies, statin treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified. Trial limitations include reliance on physician coding to define the presence of a malformation, lack of control for certain confounders such as body mass index, use of prescription dispensing as verification for the use of a statin, and lack of information on non-live births.
Animal Data
Ezetimibe
In oral (gavage) embryo-fetal development studies of ezetimibe conducted in rats (gestation days 6 to 15) and rabbits (gestation days 7 to 19), there was no evidence of maternal toxicity or embryolethality at any dose tested (250, 500, 1,000 mg/kg/day) at exposure equivalent to 10 to 150 times the clinical exposure, based on AUC, in rats and rabbits. In rats, increased incidences of common fetal skeletal findings (extra pair of thoracic ribs, unossified cervical vertebral centra, shortened ribs) were observed at 1,000 mg/kg/day (~10 times the human exposure at 10 mg daily based on AUC0-24hr for total ezetimibe). In rabbits treated with ezetimibe, an increased incidence of extra thoracic ribs was observed at 1,000 mg/kg/day (150 times the human exposure at 10 mg daily based on AUC0-24hr for total ezetimibe).
The animal-to-human exposure multiple for total ezetimibe at the no-observed effect level was 6 times for rat and 134 times for rabbit. Fetal exposure to ezetimibe (conjugated and unconjugated) was confirmed in subsequent placental transfer studies conducted using a maternal dose of 1,000 mg/kg/day. The fetal maternal plasma exposure ratio (total ezetimibe) was 1.5 for rats on gestation day 20 and 0.03 for rabbits on gestation day 22.
The effect of ezetimibe on prenatal and postnatal development and maternal function was evaluated in pregnant rats at doses of 100, 300 or 1,000 mg/kg/day (gestation day 6 through lactation day 21). No maternal toxicity or adverse developmental outcomes were observed up to and including the highest dose tested (17 times the human exposure at 10 mg daily based on AUC0-24hr for total ezetimibe).
Multiple-dose studies of ezetimibe given in combination with statins in rats and rabbits during organogenesis resulted in higher ezetimibe and statin exposures. Reproductive findings occurred at lower doses in combination therapy compared to monotherapy.
Simvastatin
Simvastatin was given to pregnant rats at doses of 6.25, 12.5 and 25 mg/kg/day (0.6 times, 1.3 times, and 2.5 times, respectively, the maximum recommended dosage of 80 mg/day when normalized to body surface area) from gestation days 6 to 17 and to pregnant rabbits from gestation days 6 to 18 at doses of 2.5, 5, and 10 mg/kg/day (0.5 times, 1 times, and 2 times, respectively, the maximum recommended dosage of 80 mg/day when normalized to body surface area). For both species, there was no evidence of maternal toxicity, or embryolethality. In rats, mean fetal body weights in the 25 mg/kg/day group were decreased 5.4%. Similar fetal body weight effects were not observed in rabbits.
Simvastatin doses of 6.25, 12.5 and 25 mg/kg/day (0.6 times, 1.3 times, and 2.5 times, respectively, the maximum recommended dosage of 80 mg/day when normalized to body surface area) were given to pregnant rats from gestation day 15 to lactation day 21. Slight decreases in maternal body weight gain and pup postnatal day 0 weight were observed in the 25 mg/kg/day dose group. Mean body weight gain of pups during lactation was slightly decreased at doses ‚Č• 12.5 mg/kg/day. Post weaning weight, behavior, reproductive performance and fertility of the offspring were not affected at any dose tested.
Placental transfer of simvastatin was not evaluated in rats or rabbits. However, it has been shown that other drugs in this class cross the placenta.
8.2Lactation
Risk Summary
There is no information about the presence of ezetimibe or simvastatin in human breast milk, the effects of the drug on the breastfed infant or the effect of the drug on milk production. However, it has been shown that other statins pass into human milk. Statins, including ezetimibe and simvastatin, decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol and may cause harm to the breast fed infant.
Because of the potential for serious adverse reactions in a breastfed infant, based on the mechanism of action, advise patients that breastfeeding is not recommended during treatment with ezetimibe and simvastatin [see Use in Specific Populations (8.2) and  Clinical Pharmacology (12.1)].
Data
Animal Data
Ezetimibe was present in the milk of lactating rats. The pup to maternal plasma ratio for total ezetimibe was 0.5 on lactation day 12.
8.4 Pediatric Use
The safety and effectiveness of ezetimibe in combination with a statin as an adjunct to diet to reduce LDL-C have been established in pediatric patients 10 years of age and older with HeFH. Use of ezetimibe and simvastatin for this indication is based on a double-blind, placebo-controlled clinical trial in 248 pediatric patients (142 males and 106 postmenarchal females) 10 years of age and older with HeFH [see Clinical Studies (14)]. In this limited controlled trial, there was no significant effect on growth or sexual maturation in the adolescent males or females, or on menstrual cycle length in females.
The safety and effectiveness of ezetimibe and simvastatin have not been established in pediatric patients younger than 10 years of age with HeFH, or in pediatric patients with other types of hyperlipidemia.
8.5 Geriatric Use
Advanced age (‚Č• 65 years) is a risk factor for ezetimibe and simvastatin-associated myopathy and rhabdomyolysis. Dose selection for an elderly patient should be cautious, recognizing the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy and the higher risk of myopathy. Monitor geriatric patients receiving ezetimibe and simvastatin for the increased risk of myopathy [see Warnings and Precautions (5.1)].
Of the 10,189 patients who received ezetimibe and simvastatin in clinical studies, 3,242 (32%) were 65 and older (this included 844 (8%) who were 75 and older). No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Renal impairment is a risk factor for myopathy and rhabdomyolysis. Monitor all patients with renal impairment for development of myopathy. Doses of ezetimibe and simvastatin exceeding 10/20 mg should be used with caution and close monitoring in patients with moderate to severe renal impairment [see Dosage and Administration (2.4) and  Warnings and Precautions (5.1)].
In the SHARP trial of 9,270 patients with moderate to severe renal impairment (6,247 non-dialysis patients with median serum creatinine 2.5 mg/dL and median estimated glomerular filtration rate 25.6 mL/min/1.73 m2, and 3,023 dialysis patients), the incidence of serious adverse events, adverse events leading to discontinuation of trial treatment, or adverse events of special interest (musculoskeletal adverse events, liver enzyme abnormalities, incident cancer) was similar between patients ever assigned to ezetimibe and simvastatin 10/20 mg (n = 4,650) or placebo (n = 4,620) during a median follow-up of 4.9 years.
8.7 Hepatic Impairment
Ezetimibe and simvastatin is contraindicated in patients with acute liver failure or decompensated cirrhosis. [see Contraindications (4) and  Warnings and Precautions (5.3)].
8.8 ChinesePatients
In a clinical trial in which patients at high risk of CVD were treated with simvastatin 40 mg/day (median follow-up 3.9 years), the incidence of myopathy was approximately 0.05% for non-Chinese patients (n = 7,367) compared with 0.24% for Chinese patients (n = 5,468). In this trial the incidence of myopathy for Chinese patients on simvastatin 40 mg/day or ezetimibe and simvastatin 10/40 mg/day co-administered with extended-release niacin 2 g/day was 1.24%.
Chinese patients may be at higher risk for myopathy, monitor these patients appropriately. Co-administration of ezetimibe and simvastatin with lipid-modifying doses of niacin-containing products (‚Č• 1 g/day niacin) is not recommended in Chinese patients [see Warnings and Precautions (5.1)¬†and¬† Drug Interactions (7.1)].
10 Overdosage
No specific antidotes for ezetimibe and simvastatin are known. In the event of an overdose with ezetimibe and simvastatin, consider contacting the Poison Help Line (1-800-222-1222) or a medical toxicologist for overdosage management recommendations.
11 Description
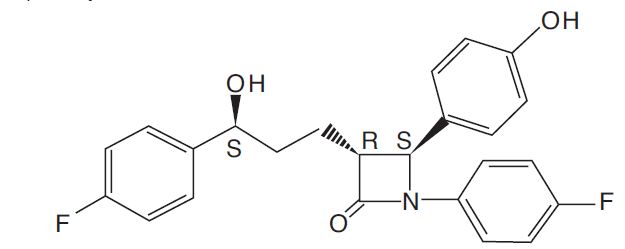
Ezetimibe and simvastatin tablets contain ezetimibe USP, a dietary cholesterol absorption inhibitor, and simvastatin USP, an HMG-CoA reductase inhibitor. The chemical name of ezetimibe is 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)(4-hydroxyphenyl)-2-azetidinone. The molecular formula is C24H21F2NO3 and its molecular weight is 409.4 g/mol.
Ezetimibe, USP is a white, crystalline powder that is freely to very soluble in ethanol, methanol, and acetone and practically insoluble in water. Its structural formula is:
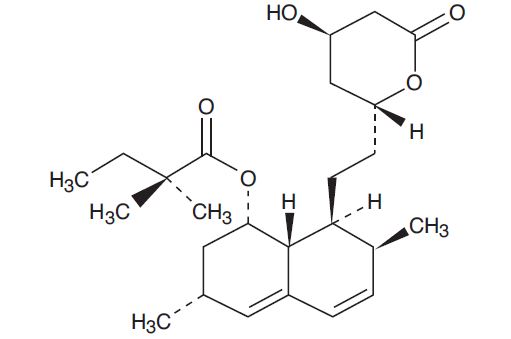
Simvastatin, USP an inactive lactone, is hydrolyzed to the corresponding ő≤-hydroxyacid form, which is an inhibitor of HMG-CoA reductase. Simvastatin, USP is butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthalenyl ester, [1S-[1őĪ,3őĪ,7ő≤,8ő≤(2S *,4S *),-8aő≤]]. The molecular formula of simvastatin is C25H38O5 and its molecular weight is 418.57 g/mol.
Simvastatin, USP is a white to off-white, nonhygroscopic, crystalline powder that is practically insoluble in water and freely soluble in chloroform, methanol and ethanol. Its structural formula is:
Ezetimibe and simvastatin is available for oral use as tablets containing 10 mg of ezetimibe, USP and 10 mg of simvastatin, USP (ezetimibe and simvastatin 10/10), 20 mg of simvastatin, USP (ezetimibe and simvastatin 10/20), 40 mg of simvastatin, USP (ezetimibe and simvastatin 10/40), or 80 mg of simvastatin, USP (ezetimibe and simvastatin 10/80). Each tablet contains the following inactive ingredients: butylated hydroxyanisole, citric acid monohydrate, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and propyl gallate.
12 Clinical Pharmacology
12.1 Mechanism of Action
Ezetimibe and simvastatin
Plasma cholesterol is derived from intestinal absorption and endogenous synthesis. Ezetimibe and simvastatin tablets contain ezetimibe and simvastatin, two lipid-lowering compounds with complementary mechanisms of action.
Ezetimibe
Ezetimibe reduces blood cholesterol by inhibiting the absorption of cholesterol by the small intestine. The molecular target of ezetimibe has been shown to be the sterol transporter, Niemann-Pick C1-Like 1 (NPC1L1), which is involved in the intestinal uptake of cholesterol and phytosterols.
Ezetimibe localizes at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood.
Simvastatin
Simvastatin is a prodrug and is hydrolyzed to its active ő≤-hydroxyacid form, simvastatin acid, after administration. Simvastatin acid and its metabolites are inhibitors of HMG-CoA reductase, the rate-limiting enzyme converts HMG-CoA to mevalonate, a precursor of cholesterol.
12.2 Pharmacodynamics
Ezetimibe and simvastatin reduce total cholesterol (total-C), LDL-C, apolipoprotein (Apo) B, and non-high-density lipoprotein cholesterol (non-HDL-C) in patients with hyperlipidemia.
Ezetimibe
In a 2-week clinical trial in 18 hypercholesterolemic patients, ezetimibe inhibited intestinal cholesterol absorption by 54%, compared with placebo. Ezetimibe had no clinically meaningful effect on the plasma concentrations of the fat-soluble vitamins A, D, and E and did not impair adrenocortical steroid hormone production.
Simvastatin
Inhibition of HMG-CoA reductase by simvastatin acid accelerates the expression of LDL-receptors, followed by the uptake of LDL-C from blood to the liver, leading to a decrease in plasma LDL-C and total cholesterol. Sustained inhibition of cholesterol synthesis in the liver also decreases levels of very-low density lipoproteins. The maximum LDL-C reduction of ZOCOR is usually achieved by 4 weeks and is maintained after that.
12.3 Pharmacokinetics
The results of a bioequivalence trial in healthy subjects demonstrated that the ezetimibe and simvastatin 10 mg/10 mg to 10 mg/80 mg combination tablets are bioequivalent to co-administration of corresponding doses of ezetimibe (ZETIA¬ģ) and simvastatin (ZOCOR¬ģ) as individual tablets.
Absorption
Ezetimibe
After oral administration, ezetimibe is absorbed and extensively conjugated to a pharmacologically active phenolic glucuronide (ezetimibe-glucuronide). Mean maximum plasma concentrations (Cmax) occur within 1 to 2 hours for ezetimibe-glucuronide and 4 to 12 hours for ezetimibe. The absolute bioavailability of ezetimibe cannot be determined as the compound is virtually insoluble in aqueous media suitable for injection.
Simvastatin
The availability of the ő≤-hydroxyacid to the systemic circulation following an oral dose of simvastatin was found to be less than 5% of the dose, consistent with extensive hepatic first-pass extraction.
Effect of Food on Oral Absorption
Ezetimibe
Concomitant food administration (high-fat or non-fat meals) had no effect on the extent of absorption of ezetimibe when administered as 10-mg tablets. The Cmax value of ezetimibe was increased by 38% with consumption of high-fat meals.
Simvastatin
Relative to the fasting state, the plasma profiles of both active and total inhibitors of HMG-CoA reductase were not affected when simvastatin was administered immediately before an American Heart Association recommended low-fat meal.
Distribution
Ezetimibe
Ezetimibe and ezetimibe-glucuronide are highly bound (> 90%) to human plasma proteins.
Simvastatin
Both simvastatin and its ő≤-hydroxyacid metabolite are highly bound (approximately 95%) to human plasma proteins. When radiolabeled simvastatin was administered to rats, simvastatin-derived radioactivity crossed the blood-brain barrier.
Elimination
Metabolism
Ezetimibe
Ezetimibe is primarily metabolized in the small intestine and liver via glucuronide conjugation with subsequent biliary and renal excretion. Minimal oxidative metabolism has been observed in all species evaluated.
In humans, ezetimibe is rapidly metabolized to ezetimibe-glucuronide. Ezetimibe and ezetimibe-glucuronide are the major drug-derived compounds detected in plasma, constituting approximately 10% to 20% and 80% to 90% of the total drug in plasma, respectively. Both ezetimibe and ezetimibe-glucuronide are eliminated from plasma with a half-life of approximately 22 hours for both ezetimibe and ezetimibe-glucuronide. Plasma concentration-time profiles exhibit multiple peaks, suggesting enterohepatic recycling.
Simvastatin
Simvastatin is a lactone that is readily hydrolyzed in vivo to the corresponding ő≤-hydroxyacid, a potent inhibitor of HMG-CoA reductase. Inhibition of HMG-CoA reductase is a basis for an assay in pharmacokinetic studies of the ő≤-hydroxyacid metabolites (active inhibitors) and, following base hydrolysis, active plus latent inhibitors (total inhibitors) in plasma following administration of simvastatin. The major active metabolites of simvastatin present in human plasma are the ő≤-hydroxyacid of simvastatin and its 6‚Äô-hydroxy, 6‚Äô-hydroxymethyl, and 6‚Äô-exomethylene derivatives.
Excretion
Ezetimibe
Following oral administration of 14C-ezetimibe (20 mg) to human subjects, total ezetimibe (ezetimibe + ezetimibe-glucuronide) accounted for approximately 93% of the total radioactivity in plasma. After 48 hours, there were no detectable levels of radioactivity in the plasma.
Approximately 78% and 11% of the administered radioactivity were recovered in the feces and urine, respectively, over a 10-day collection period. Ezetimibe was the major component in feces and accounted for 69% of the administered dose, while ezetimibe-glucuronide was the major component in urine and accounted for 9% of the administered dose.
Simvastatin
Following an oral dose of 14C-labeled simvastatin in man, 13% of the dose was excreted in urine and 60% in feces. Plasma concentrations of total radioactivity (simvastatin plus 14C-metabolites) peaked at 4 hours and declined rapidly to about 10% of peak by 12 hours post-dose.
Specific Populations
Geriatric Patients
Ezetimibe
In a multiple-dose trial with ezetimibe given 10 mg once daily for 10 days, plasma concentrations for total ezetimibe were about 2-fold higher in older (‚Č• 65 years) healthy subjects compared to younger subjects.
Simvastatin
In a trial including 16 geriatric patients between 70 and 78 years of age who received simvastatin 40 mg/day, the mean plasma level of total inhibitors activity was increased approximately 45% compared with 18 patients between 18 to 30 years of age. [see Use in Specific Populations (8.5)].
Gender
Ezetimibe
In a multiple-dose trial with ezetimibe given 10 mg once daily for 10 days, plasma concentrations for total ezetimibe were slightly higher (< 20%) in females than in males.
Race
Ezetimibe
Based on a meta-analysis of multiple-dose pharmacokinetic studies, there were no pharmacokinetic differences between Black or African American and White subjects. Studies in Asian subjects indicated that the pharmacokinetics of ezetimibe was similar to those seen in White subjects.
Hepatic Impairment
Ezetimibe
After a single 10-mg dose of ezetimibe, the mean exposure (based on area under the curve [AUC]) to total ezetimibe was increased approximately 1.7-fold in patients with mild hepatic impairment (Child-Pugh score 5 to 6), compared to healthy subjects. The mean AUC values for total ezetimibe and ezetimibe increased approximately 3- to 4-fold and 5- to 6-fold, respectively, in patients with moderate (Child-Pugh score 7 to 9) or severe hepatic impairment (Child-Pugh score 10 to 15). In a 14-day, multiple-dose trial (10 mg daily) in patients with moderate hepatic impairment, the mean AUC for total ezetimibe and ezetimibe increased approximately 4-fold compared to healthy subjects.
Renal Impairment
Ezetimibe
After a single 10-mg dose of ezetimibe in patients with severe renal disease (n = 8; mean CrCl ‚ȧ 30 mL/min/1.73 m2), the mean AUC for total ezetimibe and ezetimibe increased approximately 1.5-fold, compared to healthy subjects (n = 9).
Simvastatin
Pharmacokinetic studies with another statin having a similar principal route of elimination to that of simvastatin have suggested that for a given dose level higher systemic exposure may be achieved in patients with severe renal impairment (as measured by creatinine clearance).
Drug Interactions [see also Drug Interactions (7)]
No clinically significant pharmacokinetic interaction was seen when ezetimibe was co-administered with simvastatin. No specific pharmacokinetic drug interaction studies with ezetimibe and simvastatin have been conducted other than the following trial with NIASPAN (Niacin extended-release tablets).
Niacin: The effect of ezetimibe and simvastatin (10/20 mg daily for 7 days) on the pharmacokinetics of NIASPAN extended-release tablets (1,000 mg for 2 days and 2,000 mg for 5 days following a low-fat breakfast) was studied in healthy subjects. The mean Cmax and AUC of niacin increased 9% and 22%, respectively. The mean Cmax and AUC of nicotinuric acid increased 10% and 19%, respectively (N = 13). In the same trial, the effect of NIASPAN on the pharmacokinetics of ezetimibe and simvastatin was evaluated (N = 15). While concomitant NIASPAN decreased the mean Cmax of total ezetimibe (1%), and simvastatin (2%), it increased the mean Cmax of simvastatin acid (18%). In addition, concomitant NIASPAN increased the mean AUC of total ezetimibe (26%), simvastatin (20%), and simvastatin acid (35%).
Cases of myopathy/rhabdomyolysis have been observed with simvastatin co-administered with lipid-modifying doses (‚Č• 1 g/day niacin) of niacin-containing products. [see Warnings and Precautions (5.1)¬†and¬† Drug Interactions (7.1)].
Cytochrome P450: Ezetimibe had no significant effect on a series of probe drugs (caffeine, dextromethorphan, tolbutamide, and intravenous midazolam) known to be metabolized by cytochrome P450 (1A2, 2D6, 2C8/9 and 3A4) in a ‚Äúcocktail‚ÄĚ trial of twelve healthy adult males. This indicates that ezetimibe is neither an inhibitor nor an inducer of these cytochrome P450 isozymes, and it is unlikely that ezetimibe will affect the metabolism of drugs that are metabolized by these enzymes.
In a trial of 12 healthy volunteers, simvastatin at the 80-mg dose had no effect on the metabolism of the probe cytochrome P450 isoform 3A4 (CYP3A4) substrates midazolam and erythromycin. This indicates that simvastatin is not an inhibitor of CYP3A4 and, therefore, is not expected to affect the plasma levels of other drugs metabolized by CYP3A4.
Simvastatin acid is a substrate of the transport protein OATP1B1. Concomitant administration of medicinal products that are inhibitors of the transport protein OATP1B1 may lead to increased plasma concentrations of simvastatin acid and an increased risk of myopathy. For example, cyclosporine has been shown to increase the AUC of statins; although the mechanism is not fully understood, the increase in AUC for simvastatin acid is presumably due, in part, to inhibition of CYP3A4 and/or OATP1B1 [see Drug Interactions (7)].
Simvastatin is a substrate for CYP3A4. Inhibitors of CYP3A4 can raise the plasma levels of HMG-CoA reductase inhibitory activity and increase the risk of myopathy. [see Warnings and Precautions (5.1) and  Drug Interactions (7.1)].
Ezetimibe
Table 5 displays the effect of co-administered drugs on total ezetimibe.
Table 5: Effect of Co-administered Drugs on Total Ezetimibe
Co-administered Drug and Dosing Regimen
Total Ezetimibe*
Change in AUC
Change in Cmax
Cyclosporine-stable dose required (75 mg to 150 mg BID)†,‡
‚ÜĎ240%
‚ÜĎ290%
Fenofibrate, 200 mg QD, 14 days‡
‚ÜĎ48%
‚ÜĎ64%
Gemfibrozil, 600 mg BID, 7 days‡
‚ÜĎ64%
‚ÜĎ91%
Cholestyramine, 4 g BID, 14 days‡
‚Üď55%
‚Üď4%
Aluminum & magnesium hydroxide combination antacid, single-dose§
‚Üď4%
‚Üď30%
Cimetidine, 400 mg BID, 7 days
‚ÜĎ6%
‚ÜĎ22%
Glipizide, 10 mg, single-dose
‚ÜĎ4%
‚Üď8%
Statins
Lovastatin 20 mg QD, 7 days
‚ÜĎ9%
‚ÜĎ3%
Pravastatin 20 mg QD, 14 days
‚ÜĎ7%
‚ÜĎ23%
Atorvastatin 10 mg QD, 14 days
‚Üď2%
‚ÜĎ12%
Rosuvastatin 10 mg QD, 14 days
‚ÜĎ13%
‚ÜĎ18%
Fluvastatin 20 mg QD, 14 days
‚Üď19%
‚ÜĎ7%
* Based on 10 mg-dose of ezetimibe.
† Post-renal transplant patients with mild impaired or normal renal function. In a different trial, a renal transplant patient with severe renal insufficiency (creatinine clearance of 13.2 mL/min/1.73 m2) who was receiving multiple medications, including cyclosporine, demonstrated a 12-fold greater exposure to total ezetimibe compared to healthy subjects.
‡ See 7. Drug Interactions.
§ Supralox, 20 mL.
Table 6 displays the effects of ezetimibe co-administration on systemic exposure to other drugs.
Table 6: Effect of Ezetimibe Co-administration on Systemic Exposure to Other Drugs
Co-administered Drug and its Dosage Regimen
Ezetimibe Dosage Regimen
Change in AUC of Co-administered Drug
Change in Cmax of Co-administered Drug
Warfarin, 25 mg single-dose on Day 7
10 mg QD, 11 days
‚Üď2% (R-warfarin) ‚Üď4% (S-warfarin)
‚ÜĎ3% (R-warfarin) ‚ÜĎ1% (S-warfarin)
Digoxin, 0.5 mg single-dose
10 mg QD, 8 days
‚ÜĎ2%
‚Üď7%
Gemfibrozil, 600 mg BID, 7 days*
10 mg QD, 7 days
‚Üď1%
‚Üď11%
Ethinyl estradiol & Levonorgestrel, QD, 21 days
10 mg QD, Days 8 to 14 of 21 day oral contraceptive cycle
Ethinyl estradiol 0% Levonorgestrel 0%
Ethinyl estradiol ‚Üď9% Levonorgestrel ‚Üď5%
Glipizide, 10 mg on Days 1 and 9
10 mg QD, Days 2 to 9
‚Üď3%
‚Üď5%
Fenofibrate, 200 mg QD, 14 days*
10 mg QD, 14 days
‚ÜĎ11%
‚ÜĎ7%
Cyclosporine, 100 mg single-dose Day 7*
20 mg QD, 8 days
‚ÜĎ15%
‚ÜĎ10%
Statins
Lovastatin 20 mg QD, 7 days
10 mg QD, 7 days
‚ÜĎ19%
‚ÜĎ3%
Pravastatin 20 mg QD, 14 days
10 mg QD, 14 days
‚Üď20%
‚Üď24%
Atorvastatin 10 mg QD, 14 days
10 mg QD, 14 days
‚Üď4%
‚ÜĎ7%
Rosuvastatin 10 mg QD, 14 days
10 mg QD, 14 days
‚ÜĎ19%
‚ÜĎ17%
Fluvastatin 20 mg QD, 14 days
10 mg QD, 14 days
‚Üď39%
‚Üď27%
* See 7. Drug Interactions.
Simvastatin
Table 7 displays the effects of co-administration drugs or grapefruit juice on simvastatin systemic exposure [see Drug Interactions (7)].
Table 7: Effect of Co-administered Drugs or Grapefruit Juice on Simvastatin Systemic Exposure
Co-administered Drug or Grapefruit Juice
Dosing of Co-administered Drug or Grapefruit Juice
Dosing of Simvastatin
Geometric Mean Ratio (Ratio*with / without co-administered drug) No Effect = 1.00
AUC
Cmax
Telithromycin†
200 mg QD for 4 days
80 mg
simvastatin acid‡
12
15
simvastatin
8.9
5.3
Nelfinavir†
1,250 mg BID for 14 days
20 mg QD for 28 days
simvastatin acid‡
simvastatin
6
6.2
Itraconazole†
200 mg QD for 4 days
80 mg
simvastatin acid‡
13.1
simvastatin
13.1
Posaconazole
100 mg (oral suspension) QD for 13 days
40 mg
simvastatin acid‡
7.3
9.2
simvastatin
10.3
9.4
200 mg (oral suspension) QD for 13 days
40 mg
simvastatin acid‡
8.5
9.5
simvastatin
10.6
11.4
Gemfibrozil
600 mg BID for 3 days
40 mg
simvastatin acid‡
2.85
2.18
simvastatin
1.35
0.91
Grapefruit Juice§ (high dose)
200 mL of double-strength TID¶
60 mg single-dose
simvastatin acid
7
simvastatin
16
Grapefruit Juice§ (low dose)
8 oz (about 237 mL) of single-strength#
20 mg single-dose
simvastatin acid
1.3
simvastatin
1.9
Verapamil SR
240 mg QD Days 1 to 7 then 240 mg BID on Days 8 to 10
80 mg on Day 10
simvastatin acid
2.3
2.4
simvastatin
2.5
2.1
Diltiazem
120 mg BID for 10 days
80 mg on Day 10
simvastatin acid
2.69
2.69
simvastatin
3.10
2.88
Diltiazem
120 mg BID for 14 days
20 mg on Day 14
simvastatin
4.6
3.6
Dronedarone
400 mg BID for 14 days
40 mg QD for 14 days
simvastatin acid
1.96
2.14
simvastatin
3.90
3.75
Amiodarone
400 mg QD for 3 days
40 mg on Day 3
simvastatin acid
1.75
1.72
simvastatin
1.76
1.79
Amlodipine
10 mg QD for 10 days
80 mg on Day 10
simvastatin acid
1.58
1.56
simvastatin
1.77
1.47
Ranolazine SR
1,000 mg BID for 7 days
80 mg on Day 1 and Days 6 to 9
simvastatin acid
2.26
2.28
simvastatin
1.86
1.75
Lomitapide
60 mg QD for 7 days
40 mg single-dose
simvastatin acid
1.7
1.6
simvastatin
2
2
Lomitapide
10 mg QD for 7 days
20 mg single-dose
simvastatin acid
1.4
1.4
simvastatin
1.6
1.7
Fenofibrate
160 mg QD for 14 days
80 mg QD on Days 8 to 14
simvastatin acid
0.64
0.89
simvastatin
0.89
0.83
Propranolol
80 mg single-dose
80 mg single-dose
total inhibitor
0.79
‚Üď from 33.6 to 21.1 ng‚ąôeq/mL
active inhibitor
0.79
‚Üď from 7.0 to 4.7 ng‚ąôeq/mL
* Results based on a chemical assay except results with propranolol as indicated.
† Results could be representative of the following CYP3A4 inhibitors: ketoconazole, erythromycin, clarithromycin, HIV protease inhibitors, and nefazodone.
‚Ä° Simvastatin acid refers to the ő≤-hydroxyacid of simvastatin.
§ The effect of amounts of grapefruit juice between those used in these two studies on simvastatin pharmacokinetics has not been studied.
¶ Double-strength: one can of frozen concentrate diluted with one can of water. Grapefruit juice was administered TID for 2 days, and 200 mL together with single-dose simvastatin and 30 and 90 minutes following single-dose simvastatin on Day 3.
# Single-strength: one can of frozen concentrate diluted with 3 cans of water. Grapefruit juice was administered with breakfast for 3 days, and simvastatin was administered in the evening on Day 3.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ezetimibe and Simvastatin
No animal carcinogenicity or fertility studies have been conducted with the combination of ezetimibe and simvastatin. The combination of ezetimibe with simvastatin did not show evidence of mutagenicity in vitro in a microbial mutagenicity (Ames) test with Salmonella typhimurium and Escherichia coli with or without metabolic activation. No evidence of clastogenicity was observed in vitro in a chromosomal aberration assay in human peripheral blood lymphocytes with ezetimibe and simvastatin with or without metabolic activation. There was no evidence of genotoxicity at doses up to 600 mg/kg with the combination of ezetimibe and simvastatin (1:1) in the in vivo mouse micronucleus test.
Ezetimibe
A 104-week dietary carcinogenicity trial with ezetimibe was conducted in rats at doses up to 1,500 mg/kg/day (males) and 500 mg/kg/day (females) (~20 times the human exposure at 10 mg daily based on AUC0-24hr for total ezetimibe). A 104-week dietary carcinogenicity trial with ezetimibe was also conducted in mice at doses up to 500 mg/kg/day (> 150 times the human exposure at 10 mg daily based on AUC0-24hr for total ezetimibe). There were no statistically significant increases in tumor incidences in drug-treated rats or mice.
No evidence of mutagenicity was observed in vitro in a microbial mutagenicity (Ames) test with Salmonella typhimurium and Escherichia coli with or without metabolic activation. No evidence of clastogenicity was observed in vitro in a chromosomal aberration assay in human peripheral blood lymphocytes with or without metabolic activation. In addition, there was no evidence of genotoxicity in the in vivo mouse micronucleus test.
In oral (gavage) fertility studies of ezetimibe conducted in rats, there was no evidence of reproductive toxicity at doses up to 1,000 mg/kg/day in male or female rats (~7 times the human exposure at 10 mg daily based on AUC0-24hr for total ezetimibe).
Simvastatin
In a 72-week carcinogenicity trial, mice were administered daily doses of simvastatin of 25 mg/kg, 100 mg/kg, and 400 mg/kg body weight, which resulted in mean plasma drug levels approximately 1, 4, and 8 times higher than the mean human plasma drug level, respectively, (as total inhibitory activity based on AUC) after an 80-mg oral dose. Liver carcinomas were significantly increased in high-dose females and mid- and high-dose males with a maximum incidence of 90% in males. The incidence of adenomas of the liver was significantly increased in mid- and high-dose females. Drug treatment also significantly increased the incidence of lung adenomas in mid- and high-dose males and females. Adenomas of the Harderian gland (a gland of the eye of rodents) were significantly higher in high-dose mice than in controls. No evidence of a tumorigenic effect was observed at 25 mg/kg/day.
In a separate 92-week carcinogenicity trial in mice at doses up to 25 mg/kg/day, no evidence of a tumorigenic effect was observed (mean plasma drug levels were 1 times higher than humans given 80 mg simvastatin as measured by AUC).
In a two-year trial in rats at 25 mg/kg/day, there was a statistically significant increase in the incidence of thyroid follicular adenomas in female rats exposed to approximately 11 times higher levels of simvastatin than in humans given 80 mg simvastatin (as measured by AUC).
A second two-year rat carcinogenicity trial with doses of 50 and 100 mg/kg/day produced hepatocellular adenomas and carcinomas (in female rats at both doses and in males at 100 mg/kg/day). Thyroid follicular cell adenomas were increased in males and females at both doses; thyroid follicular cell carcinomas were increased in females at 100 mg/kg/day. The increased incidence of thyroid neoplasms appears to be consistent with findings from other statins. These treatment levels represented plasma drug levels (AUC) of approximately 7 and 15 times (males) and 22 and 25 times (females) the mean human plasma drug exposure after an 80-mg daily dose.
No evidence of mutagenicity was observed in a microbial mutagenicity (Ames) test with or without rat or mouse liver metabolic activation. In addition, no evidence of damage to genetic material was noted in an in vitro alkaline elution assay using rat hepatocytes, a V-79 mammalian cell forward mutation trial, an in vitro chromosome aberration trial in CHO cells, or an in vivo chromosomal aberration assay in mouse bone marrow.
There was decreased fertility in male rats treated with simvastatin for 34 weeks at 25 mg/kg body weight (4 times the maximum human exposure level, based on AUC, in patients receiving 80 mg/day); however, this effect was not observed during a subsequent fertility trial in which simvastatin was administered at this same dose level to male rats for 11 weeks (the entire cycle of spermatogenesis including epididymal maturation). No microscopic changes were observed in the testes of rats from either trial. At 180 mg/kg/day (which produces exposure levels 22 times higher than those in humans taking 80 mg/day based on surface area, mg/m2), seminiferous tubule degeneration (necrosis and loss of spermatogenic epithelium) was observed. In dogs, there was drug-related testicular atrophy, decreased spermatogenesis, spermatocytic degeneration and giant cell formation at 10 mg/kg/day (approximately 2 times the human exposure, based on AUC, at 80 mg/day). The clinical significance of these findings is unclear.
14 Clinical Studies
Primary Hyperlipidemia in Adults
Ezetimibe and Simvastatin
Ezetimibe and simvastatin reduce LDL-C in adult patients with primary hyperlipidemia. Maximal to near maximal response is generally achieved within 2 weeks and maintained during chronic therapy.
Ezetimibe and simvastatin is effective in males and females with primary hyperlipidemia. There were insufficient numbers of patients who self-identified as Black or African American, Asian, or other races to determine if these patients responded differently than White patients.
Five multicenter, double-blind trials conducted with either ezetimibe and simvastatin or co-administered ezetimibe and simvastatin equivalent to ezetimibe and simvastatin in patients with primary hyperlipidemia are reported: two were comparisons with simvastatin, two were comparisons with atorvastatin, and one was a comparison with rosuvastatin.
In a multicenter, double-blind, placebo-controlled, 12-week trial, 1,528 patients with primary hyperlipidemia were randomized to one of ten treatment groups: placebo, ezetimibe (10 mg), simvastatin (10 mg, 20 mg, 40 mg, or 80 mg), or ezetimibe and simvastatin (10/10, 10/20, 10/40, or 10/80).
When patients receiving ezetimibe and simvastatin were compared to those receiving all doses of simvastatin, ezetimibe and simvastatin significantly lowered total-C, LDL-C, Apo B, TG, and non-HDL-C. The effects of ezetimibe and simvastatin on HDL-C were similar to the effects seen with simvastatin. Further analysis showed ezetimibe and simvastatin significantly increased HDL-C compared with placebo. (see Table 8.) The lipid response to ezetimibe and simvastatin was similar in patients with TG levels greater than or less than 200 mg/dL.
Table 8: Response to Ezetimibe and Simvastatin in Patients with Primary Hyperlipidemia (Mean* % Change from Untreated Baseline†)
Treatment
(Daily Dose)
N
Total-C
LDL-C
Apo B
HDL-C
TG*
Non-HDL-C
Pooled data (All ezetimibe and simvastatin doses)‡
609
-38
-53
-42
+7
-24
-49
Pooled data (All simvastatin doses)‡
622
-28
-39
-32
+7
-21
-36
Ezetimibe 10 mg
149
-13
-19
-15
+5
-11
-18
Placebo
148
-1
-2
0
0
-2
-2
Ezetimibe and simvastatin by dose
10/10
152
-31
-45
-35
+8
-23
-41
10/20
156
-36
-52
-41
+10
-24
-47
10/40
147
-39
-55
-44
+6
-23
-51
10/80
154
-43
-60
-49
+6
-31
-56
Simvastatin by dose
10 mg
158
-23
-33
-26
+5
-17
-30
20 mg
150
-24
-34
-28
+7
-18
-32
40 mg
156
-29
-41
-33
+8
-21
-38
80 mg
158
-35
-49
-39
+7
-27
-45
* For triglycerides, median % change from baseline.
† Baseline - on no lipid-lowering drug.
‡ Ezetimibe and simvastatin doses pooled (10/10 to 10/80) significantly reduced total-C, LDL-C, Apo B, TG, and non-HDL-C compared to simvastatin and significantly increased HDL-C compared to placebo.
In a multicenter, double-blind, controlled, 23-week trial, 710 patients with known CHD or CHD risk equivalents, as defined by the NCEP ATP III guidelines, and an LDL-C ‚Č• 130 mg/dL were randomized to one of four treatment groups: co-administered ezetimibe and simvastatin equivalent to ezetimibe and simvastatin (10/10, 10/20, and 10/40) or simvastatin 20 mg. Patients not reaching an LDL-C < 100 mg/dL had their simvastatin dose titrated at 6-week intervals to a maximal dose of 80 mg. At Week 5, the LDL-C reductions with ezetimibe and simvastatin 10/10, 10/20, or 10/40 were significantly larger than with simvastatin 20 mg (see Table 9).
Table 9: Response to Ezetimibe and Simvastatin after 5 Weeks in Patients with CHD or CHD Risk Equivalents and an LDL-C ‚Č• 130 mg/dL
 
Simvastatin
20 mg
Ezetimibe and Simvastatin
10/10
Ezetimibe and Simvastatin
10/20
Ezetimibe and Simvastatin
10/40
N
253
251
109
97
Mean baseline LDL-C
174
165
167
171
Percent change LDL-C
-38
-47
-53
-59
In a multicenter, double-blind, 6-week trial, 1,902 patients with primary hyperlipidemia were randomized to one of eight treatment groups: ezetimibe and simvastatin (10/10, 10/20, 10/40, or 10/80) or atorvastatin (10 mg, 20 mg, 40 mg, or 80 mg).
Across the dosage range, when patients receiving ezetimibe and simvastatin were compared to those receiving milligram-equivalent statin doses of atorvastatin, ezetimibe and simvastatin lowered total-C, LDL-C, Apo B, and non-HDL-C significantly more than atorvastatin. Only the 10/40 mg and 10/80 mg ezetimibe and simvastatin doses increased HDL-C significantly more than the corresponding milligram-equivalent statin dose of atorvastatin. The effects of ezetimibe and simvastatin on TG were similar to the effects seen with atorvastatin. (see Table 10.)
Table 10: Response to Ezetimibe and Simvastatin and Atorvastatin in Patients with Primary Hyperlipidemia (Mean* % Change from Untreated Baseline†)
Treatment
(Daily Dose)
N
Total-C‡
LDL-C‡
Apo B‡
HDL-C
TG*
Non-HDL-
C‡
Ezetimibe and simvastatin by dose
10/10
230
-34§
-47§
-37§
+8
-26
-43§
10/20
233
-37§
-51§
-40§
+7
-25
-46§
10/40
236
-41§
-57§
-46§
+9§
-27
-52§
10/80
224
-43§
-59§
-48§
+8§
-31
-54§
Atorvastatin by dose
10 mg
235
-27
-36
-31
+7
-21
-34
20 mg
230
-32
-44
-37
+5
-25
-41
40 mg
232
-36
-48
-40
+4
-24
-45
80 mg
230
-40
-53
-44
+1
-32
-50
* For triglycerides, median % change from baseline.
† Baseline - on no lipid-lowering drug.
‡ Ezetimibe and simvastatin doses pooled (10/10 to 10/80) provided significantly greater reductions in total-C, LDL-C, Apo B, and non-HDL-C compared to atorvastatin doses pooled (10 to 80).
§ p < 0.05 for difference with atorvastatin at equal mg doses of the simvastatin component.
In a multicenter, double-blind, 24-week, forced-titration trial, 788 patients with primary hyperlipidemia were randomized to receive co-administered ezetimibe and simvastatin equivalent to ezetimibe and simvastatin (10/10 and 10/20) or atorvastatin 10 mg. For all three treatment groups, the dose of the statin was titrated at 6-week intervals to 80 mg. At each pre-specified dose comparison, ezetimibe and simvastatin tablets lowered LDL-C to a greater degree than atorvastatin (see Table 11).
Table 11: Response to Ezetimibe and Simvastatin and Atorvastatin in Patients with Primary Hyperlipidemia (Mean* % Change from Untreated Baseline†)
Treatment
N
Total-C
LDL-C
Apo B
HDL-C
TG*
Non-HDL-
C
Week 6
Atorvastatin 10 mg‡
262
-28
-37
-32
+5
-23
-35
Ezetimibe and simvastatin 10/10§
263
-34¶
-46¶
-38¶
+8¶
-26
-43¶
Ezetimibe and simvastatin 10/20#
263
-36¶
-50¶
-41¶
+10¶
-25
-46¶
Week 12
Atorvastatin 20 mg
246
-33
-44
-38
+7
-28
-42
Ezetimibe and simvastatin 10/20
250
-37¶
-50¶
-41¶
+9
-28
-46¶
Ezetimibe and simvastatin 10/40
252
-39¶
-54¶
-45¶
+12¶
-31
-50¶
Week 18
Atorvastatin 40 mg
237
-37
-49
-42
+8
-31
-47
Ezetimibe and simvastatin 10/40√ě
482
-40¶
-56¶
-45¶
+11¶
-32
-52¶
Week 24
Atorvastatin 80 mg
228
-40
-53
-45
+6
-35
-50
Ezetimibe and simvastatin 10/80√ě
459
-43¶
-59¶
-49¶
+12¶
-35
-55¶
* For triglycerides, median % change from baseline.
† Baseline - on no lipid-lowering drug.
‡ Atorvastatin: 10 mg start dose titrated to 20 mg, 40 mg, and 80 mg through Weeks 6, 12, 18, and 24.
§ Ezetimibe and simvastatin: 10/10 start dose titrated to 10/20, 10/40, and 10/80 through Weeks 6, 12, 18, and 24.
¬∂¬†p‚ȧ0.05 for difference with atorvastatin in the specified week.
# Ezetimibe and simvastatin: 10/20 start dose titrated to 10/40, 10/40, and 10/80 through Weeks 6, 12, 18, and 24.
√쬆Data pooled for common doses of ezetimibe and simvastatin at Weeks 18 and 24.
In a multicenter, double-blind, 6-week trial, 2,959 patients with primary hyperlipidemia, were randomized to one of six treatment groups: ezetimibe and simvastatin (10/20, 10/40, or 10/80) or rosuvastatin (10 mg, 20 mg, or 40 mg).
The effects of ezetimibe and simvastatin and rosuvastatin on total-C, LDL-C, Apo B, TG, non-HDL-C and HDL-C are shown in Table 12.
Table 12: Response to Ezetimibe and Simvastatin and Rosuvastatin in Patients with Primary Hyperlipidemia (Mean* % Change from Untreated Baseline†)
Treatment
(Daily Dose)
N
Total-C‡
LDL-C‡
Apo B‡
HDL-C
TG*
Non-HDL-
C‡
Ezetimibe and simvastatin by dose
10/20
476
-37§
-52§
-42§
+7
-23§
-47§
10/40
477
-39¶
-55¶
-44¶
+8
-27
-50¶
10/80
474
-44#
-61#
-50#
+8
-30#
-56#
Rosuvastatin by dose
10 mg
475
-32
-46
-37
+7
-20
-42
20 mg
478
-37
-52
-43
+8
-26
-48
40 mg
475
-41
-57
-47
+8
-28
-52
* For triglycerides, median % change from baseline.
† Baseline - on no lipid-lowering drug.
‡ Ezetimibe and simvastatin doses pooled (10/20 to 10/80) provided significantly greater reductions in total-C, LDL-C, Apo B, and non-HDL-C compared to rosuvastatin doses pooled (10-40 mg).
§ p<0.05 vs. rosuvastatin 10 mg.
¶ p<0.05 vs. rosuvastatin 20 mg.
# p<0.05 vs. rosuvastatin 40 mg.
In a multicenter, double-blind, 24-week trial, 214 patients with type 2 diabetes mellitus treated with thiazolidinediones (rosiglitazone or pioglitazone) for a minimum of 3 months and simvastatin 20 mg for a minimum of 6 weeks were randomized to receive either simvastatin 40 mg or the co-administered active ingredients equivalent to ezetimibe and simvastatin 10/20. The median LDL-C and HbA1c levels at baseline were 89 mg/dL
and 7.1%, respectively.
Ezetimibe and simvastatin 10/20 was significantly more effective than doubling the dose of simvastatin to 40 mg. The median percent changes from baseline for ezetimibe and simvastatin vs. simvastatin were: LDL-C -25% and -5%; total-C -16% and -5%; Apo B -19% and -5%; and non-HDL-C -23% and -5%. Results for HDL-C and TG between the two treatment groups were not significantly different.
Ezetimibe
In two multicenter, double-blind, placebo-controlled, 12-week trials in 1,719 patients with primary hyperlipidemia, ezetimibe significantly lowered total-C (-13%), LDL-C (-19%), Apo B (-14%), and TG (-8%), and increased HDL-C (+3%) compared to placebo. Reduction in LDL-C was consistent across age, sex, and baseline LDL-C.
Simvastatin
In two large, placebo-controlled clinical trials, the Scandinavian Simvastatin Survival Trial (N = 4,444 patients) and the Heart Protection Trial (N = 20,536 patients), the effects of treatment with simvastatin were assessed in patients at high risk of coronary events because of existing coronary heart disease, diabetes, peripheral vessel disease, history of stroke or other cerebrovascular disease. Simvastatin was proven to reduce: the risk of total mortality by reducing CHD deaths; the risk of non-fatal myocardial infarction and stroke; and the need for coronary and non-coronary revascularization procedures.
No incremental benefit of ezetimibe and simvastatin on cardiovascular morbidity and mortality over and above that demonstrated for simvastatin has been established.
Heterozygous Familial Hypercholesterolemia (HeFH) in Pediatric Patients
The effects of ezetimibe co-administered with simvastatin (n = 126) compared to simvastatin monotherapy (n = 122) have been evaluated in males and females with HeFH. In a multicenter, double-blind, controlled trial followed by an open-label phase, 142 males and 106 postmenarchal females, 10 to 17 years of age (mean age 14.2 years, 43% females, 82% White, 4% Asian, 2% Black or African American, 13% multi-racial; 14% identified as Hispanic or Latino ethnicity) with HeFH were randomized to receive either ezetimibe co-administered with simvastatin or simvastatin monotherapy. Inclusion in the trial required 1) a baseline LDL-C level between 160 mg/dL and 400 mg/dL and 2) a medical history and clinical presentation consistent with HeFH. The mean baseline LDL-C value was 225 mg/dL (range: 161 mg/dL to 351 mg/dL) in the ezetimibe co-administered with simvastatin group compared to 219 mg/dL (range: 149 mg/dL to 336 mg/dL) in the simvastatin monotherapy group. The patients received co-administered ezetimibe and simvastatin (10 mg, 20 mg, or 40 mg) or simvastatin monotherapy (10 mg, 20 mg, or 40 mg) for 6 weeks, co-administered ezetimibe and 40-mg simvastatin or 40-mg simvastatin monotherapy for the next 27 weeks, and open-label co-administered ezetimibe and simvastatin (10 mg, 20 mg, or 40 mg) for 20 weeks thereafter.
The results of the trial at Week 6 are summarized in Table 13. Results at Week 33 were consistent with those at Week 6.
Table 13: Mean Percent Difference at Week 6 Between the Pooled ZETIA Co-administered with Simvastatin Group and the Pooled Simvastatin Monotherapy Group in Adolescent Patients with HeFH
Total-C
LDL-C
Apo B
Non-HDL-C
Mean percent difference between treatment groups
-12%
-15%
-12%
-14%
95% Confidence Interval
(-15%, -9%)
(-18%, -12%)
(-15%, -9%)
(-17%, -11%)
 
Homozygous Familial Hypercholesterolemia (HoFH) in Adults
A double-blind, randomized, 12-week trial was performed in patients with a clinical and/or genotypic diagnosis of HoFH. Data were analyzed from a subgroup of patients (n = 14) receiving simvastatin 40 mg at baseline. Increasing the dose of simvastatin from 40 mg to 80 mg (n = 5) produced a reduction of LDL-C of 13% from baseline on simvastatin 40 mg. Co-administered ezetimibe and simvastatin equivalent to  (10/40 and 10/80 pooled, n = 9), produced a reduction of LDL-C of 23% from baseline on simvastatin 40 mg. In those patients co-administered ezetimibe and simvastatin equivalent to ezetimibe and simvastatin (10/80, n = 5), a reduction of LDL-C of 29% from baseline on simvastatin 40 mg was produced.
Chronic Kidney Disease (CKD) in Adults
The Trial of Heart and Renal Protection (SHARP) was a multinational, randomized, placebo-controlled, double-blind trial that investigated the effect of ezetimibe and simvastatin on the time to a first major vascular event (MVE) among 9,438 patients with moderate to severe chronic kidney disease (approximately one-third on dialysis at baseline) who did not have a history of myocardial infarction or coronary revascularization. An MVE was defined as nonfatal MI, cardiac death, stroke, or any revascularization procedure. Patients were allocated to treatment using a method that took into account the distribution of 8 important baseline characteristics of patients already enrolled and minimized the imbalance of those characteristics across the groups.
For the first year, 9,438 patients were allocated 4:4:1, to ezetimibe and simvastatin 10/20, placebo, or simvastatin 20 mg daily, respectively. The 1-year simvastatin arm enabled the comparison of ezetimibe and simvastatin to simvastatin with regard to safety and effect on lipid levels. At 1 year the simvastatin-only arm was re-allocated 1:1 to ezetimibe and simvastatin 10/20 or placebo. A total of 9,270 patients were ever allocated to ezetimibe and simvastatin 10/20 (n = 4,650) or placebo (n = 4,620) during the trial. The median follow-up duration was 4.9 years. Patients had a mean age of 61 years; 63% were male, 72% were White, and 23% were diabetic; and, for those not on dialysis at baseline, the median serum creatinine was 2.5 mg/dL and the median estimated glomerular filtration rate (eGFR) was 25.6 mL/min/1.73 m2, with 94% of patients having an eGFR < 45 mL/min/1.73m2. Eligibility did not depend on lipid levels. Mean LDL-C at baseline was 108 mg/dL. At 1 year, the mean LDL-C was 26% lower in the simvastatin arm and 38% lower in the ezetimibe and simvastatin arm relative to placebo. At the midpoint of the trial (2.5 years), the mean LDL-C was 32% lower for ezetimibe and simvastatin relative to placebo. Patients no longer taking trial medication were included in all lipid measurements.
In the primary intent-to-treat analysis, 639 (15.2%) of 4,193 patients initially allocated to ezetimibe and simvastatin and 749 (17.9%) of 4,191 patients initially allocated to placebo experienced an MVE. This corresponded to a relative risk reduction of 16% (p = 0.001) (see Figure 1). Similarly, 526 (11.3%) of 4,650 patients ever allocated to ezetimibe and simvastatin and 619 (13.4%) of 4,620 patients ever allocated to placebo experienced a major atherosclerotic event (MAE; a subset of the MVE composite that excluded non-coronary cardiac deaths and hemorrhagic stroke), corresponding to a relative risk reduction of 17% (p = 0.002). The trial demonstrated that treatment with ezetimibe and simvastatin 10/20 mg versus placebo reduced the risk for MVE and MAE in this CKD population. The trial design precluded drawing conclusions regarding the independent contribution of either ezetimibe or simvastatin to the observed effect.
The treatment effect of ezetimibe and simvastatin on MVE was attenuated among patients on dialysis at baseline compared with those not on dialysis at baseline. Among 3,023 patients on dialysis at baseline, ezetimibe and simvastatin reduced the risk of MVE by 6% (RR 0.94: 95% CI 0.80 to 1.09) compared with 22% (RR 0.78: 95% CI 0.69 to 0.89) among 6,247 patients not on dialysis at baseline (interaction P = 0.08).
Figure 1: Effect of Ezetimibe and Simvastatin on the Primary Endpoint of Risk of Major Vascular Events
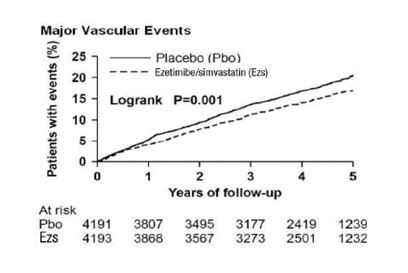
The individual components of MVE in all patients ever allocated to ezetimibe and simvastatin or placebo are presented in Table 14.
Table 14: Number of First Events for Each Component of the Major Vascular Event Composite Endpoint in SHARP*
 
Outcome
Ezetimibe and Simvastatin 10/20
(N = 4,650)
Placebo
(N = 4,620)
Risk Ratio
(95% CI)
P-value
Major Vascular Events
701 (15.1%)
814 (17.6%)
0.85 (0.77 to 0.94)
0.001
Nonfatal MI
134 (2.9%)
159 (3.4%)
0.84 (0.66 to 1.05)
0.12
Cardiac Death
253 (5.4%)
272 (5.9%)
0.93 (0.78 to 1.10)
0.38
Any Stroke
171 (3.7%)
210 (4.5%)
0.81 (0.66 to 0.99)
0.038
Non-hemorrhagic Stroke
131 (2.8%)
174 (3.8%)
0.75 (0.60 to 0.94)
0.011
Hemorrhagic Stroke
45 (1.0%)
37 (0.8%)
1.21 (0.78 to 1.86)
0.40
Any Revascularization
284 (6.1%)
352 (7.6%)
0.79 (0.68 to 0.93)
0.004
* Intention-to-treat analysis on all SHARP patients ever allocated to ezetimibe and simvastatin or placebo.
Among patients not on dialysis at baseline, ezetimibe and simvastatin did not reduce the risk of progressing to end-stage renal disease compared with placebo (RR 0.97: 95% CI 0.89 to 1.05).
Simvastatin Cardiovascular Outcome Trials in Adults at High Risk of Coronary Heart Disease Events
In a randomized, double-blind, placebo-controlled, multi-centered trial [the Scandinavian Simvastatin Survival Trial (Trial 4S)], the effect of therapy with simvastatin on total mortality was assessed in 4,444 adult patients with CHD (history of angina and/or a previous myocardial infarction) and baseline total cholesterol (total-C) between 212 mg/dL and 309 mg/dL who were on a lipid-lowering diet. In Trial 4S, patients were treated with standard care, including lipid-lowering diet, and randomized to either simvastatin 20 mg/day to 40 mg/day (n = 2,221) or placebo (n = 2,223) for a median duration of 5.4 years.
- Simvastatin significantly reduced the risk of mortality by 30% (p = 0.0003, 182 deaths in the simvastatin group vs 256 deaths in the placebo group). The risk of CHD mortality was significantly reduced by 42% (p = 0.00001, 111 deaths in the simvastatin group vs 189 deaths in the placebo group). There was no statistically significant difference between groups in non-cardiovascular mortality.
- Simvastatin significantly reduced the risk for the secondary composite endpoint (time to first occurrence of CHD death, definite or probable hospital verified non-fatal MI, silent MI verified by ECG, or resuscitated cardiac arrest) by 34% (p < 0.00001, 431 vs 622 patients with one or more events). Simvastatin reduced the risk of major coronary events to a similar extent across the range of baseline total and LDL cholesterol levels. The risk of having a hospital-verified non-fatal MI was reduced by 37%.
- Simvastatin significantly reduced the risk for undergoing myocardial revascularization procedures (coronary artery bypass grafting or percutaneous transluminal coronary angioplasty) by 37% (p < 0.00001, 252 vs 383 patients).
- Simvastatin significantly reduced the risk of fatal plus non-fatal cerebrovascular events (combined stroke and transient ischemic attacks) by 28% (p = 0.033, 75 vs 102 patients).
- Over the course of the trial, treatment with simvastatin led to mean reductions in total-C, LDL-C and triglycerides (TG) of 25%, 35%, and 10%, respectively, and a mean increase in high-density lipoprotein cholesterol (HDL-C) of 8%. In contrast, treatment with placebo led to increases in total-C, LDL-C and TG of 1%, 1%, and 7%, respectively.
- Because there were only 53 female deaths (approximately 18% of the trial population was female), the effect of simvastatin on mortality in females could not be adequately assessed. However, simvastatin significantly reduced the risk of having major coronary events in females by 34% (60 vs 91 women with one or more event).
- Simvastatin resulted in similar decreases in relative risk for total mortality, CHD mortality, and major coronary events in geriatric patients (‚Č• 65 years) compared with younger adults.
The Heart Protection Trial (Trial HPS) was a randomized, placebo-controlled, double-blind, multi-centered trial with a mean duration of 5 years conducted in 10,269 patients on simvastatin 40 mg and 10,267 on placebo. Patients had a mean age of 64 years (range 40 to 80 years old), 97% were White, and were at high risk of developing a major coronary event because of existing CHD (65%), diabetes (Type 2, 26%; Type 1, 3%), history of stroke or other cerebrovascular disease (16%), peripheral vascular disease (33%), or they were males ‚Č• 65 years with hypertension in (6%). At baseline:
- 3,421 patients (17%) had LDL-C levels below 100 mg/dL, including 953 (5%) below 80 mg/dL; and
- 10,047 patients (49%) had levels greater than 130 mg/dL.
Patients were randomized to simvastatin or placebo using a covariate adaptive method which considered the distribution of 10 important baseline characteristics of patients already enrolled.
The Trial HPS results showed that simvastatin 40 mg/day significantly reduced: total and CHD mortality; and non-fatal MI, stroke, and revascularization procedures (coronary and non-coronary) (see Table 15).
Table 15: CHD Mortality and Cardiovascular Events in Adult Patients with High Risk of Developing a Major Coronary Event in Trial HPS
Endpoint
Simvastatin
(N = 10,269)
n (%)*
Placebo
(N = 10,267)
n (%)*
Risk Reduction (%) (95% CI)
p-Value
Primary
Mortality
CHD mortality
1,328 (12.9%)
587 (5.7%)
1,507 (14.7%)
707 (6.9%)
13% (6% to 19%)
18% (8% to 26%
p = 0.0003
p = 0.0005
Secondary
Non-fatal MI
Stroke
357 (3.5%)
444 (4.3%)
574 (5.6%)
585 (5.7%)
38% (30% to 46%)
25% (15% to 34%)
p < 0.0001
p < 0.0001
Tertiary
Coronary revascularization
Peripheral and other non-coronary revascularization
513 (5%)
450 (4.4%)
725 (7.1%)
532 (5.2%)
30% (22% to 38%)
16% (5% to 26%)
p < 0.0001
p = 0.006
* n = number of patients with indicated event
Two composite endpoints were defined to have enough events to assess relative risk reductions across a range of baseline characteristics:
- Major coronary events (MCE) was comprised of CHD mortality and non-fatal MI. Analyzed by time-to-first event; 898 patients (8.7%) treated with simvastatin had events and 1,212 patients (11.8%) treated with placebo had events.
- Major vascular events (MVE) was comprised of MCE, stroke, and revascularization procedures including coronary, peripheral and other non-coronary procedures. Analyzed by time-to-first event; 2,033 patients (19.8%) treated with simvastatin had events and 2,585 patients (25.2%) on placebo had events.
Simvastatin use led to significant relative risk reductions for both composite endpoints (27% for MCE and 24% for MVE, p < 0.0001) and for all components of the composite endpoints. The risk reductions produced by simvastatin in both MCE and MVE were evident and consistent regardless of cardiovascular disease related medical history at trial entry (i.e., CHD alone; or peripheral vascular disease, cerebrovascular disease, diabetes or treated hypertension, with or without CHD), gender, age, baseline levels of LDL-C, baseline concomitant cardiovascular medications (i.e., aspirin, beta blockers, or calcium channel blockers), smoking status, or obesity. Patients with diabetes showed risk reductions for MCE and MVE due to simvastatin treatment regardless of baseline HbA1c levels or obesity.
16 How Supplied/storage And Handling
Ezetimibe and simvastatin tablets, 10/10 mg, are supplied as white to off-white, capsule shaped tablets, debossed with ‚ÄúAA 70‚ÄĚ on one side and plain on the other side.
They are available as follows:
Bottles of 30:                          NDC 69238-1155-3
Bottles of 90:                          NDC 69238-1155-9
Ezetimibe and simvastatin tablets, 10/20 mg, are supplied as white to off-white, capsule shaped tablets, debossed with ‚ÄúAA 71‚ÄĚ on one side and plain on the other side.
They are available as follows:
Bottles of 30:                          NDC 69238-1156-3
Bottles of 90:                          NDC 69238-1156-9
Ezetimibe and simvastatin tablets, 10/40 mg, are supplied as white to off-white, capsule shaped tablets, debossed with ‚ÄúAA 72‚ÄĚ on one side and plain on the other side.
They are available as follows:
Bottles of 30:                          NDC 69238-1157-3
Bottles of 90:                          NDC 69238-1157-9
Ezetimibe and simvastatin tablets, 10/80 mg, are supplied as white to off-white, capsule shaped tablets, debossed with ‚ÄúAA 73‚ÄĚ on one side and plain on the other side.
They are available as follows:
Bottles of 30:                          NDC 69238-1158-3
Bottles of 90:                          NDC 69238-1158-9
Storage
Store at 20¬į to 25¬įC (68¬į to 77¬įF) [see USP Controlled Room Temperature]. Keep container tightly closed.
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myopathy and Rhabdomyolysis
Advise patients that ezetimibe and simvastatin may cause myopathy and rhabdomyolysis. Inform patients taking the 80 mg daily dose of simvastatin that they are at an increased risk. Inform patients that the risk is also increased when taking certain types of medication or consuming grapefruit juice and they should discuss all medication, both prescription and over the counter, with their healthcare provider. Instruct patients to inform other healthcare providers prescribing a new medication or increasing the dose of an existing medication that they are taking ezetimibe and simvastatin tablets. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever [see Contraindications (4), Warnings and Precautions (5.1), and  Drug Interactions (7.1)].
Hepatic Dysfunction
Inform patients that ezetimibe and simvastatin may cause liver enzyme elevations and possibly liver failure. Advise patients to promptly report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice [see Warnings and Precautions (5.3)].
Increases in HbA1c and Fasting Serum Glucose Levels
Inform patients that increases in HbA1c and fasting serum glucose levels may occur with ezetimibe and simvastatin. Encourage patients to optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices [see Warnings and Precautions (5.4)].
Pregnancy
Advise pregnant patients and patients who can become pregnant of the potential risk to a fetus. Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if ezetimibe and simvastatin should be discontinued [see Use in Specific Populations (8.1)].
Lactation
Advise patients that breastfeeding is not recommended during treatment with ezetimibe and simvastatin tablets [see Use in Specific Populations (8.2)].
Missed Dose
Instruct patients to take ezetimibe and simvastatin tablets only as prescribed. If a dose is missed, it should be taken as soon as possible. Advise patients not to double their next dose.
Manufactured by: Amneal Pharmaceuticals Pvt. Ltd. Oral Solid Dosage Unit Ahmedabad 382213, INDIA
Distributed by: Amneal Pharmaceuticals LLC Bridgewater, NJ 08807
Rev. 04-2024-08
Patient Information
Ezetimibe and Simvastatin (e zet‚Äô i mibe and sim‚ÄĚ va stat‚Äô in) Tablets, for oral use
Read this Information carefully before you start taking ezetimibe and simvastatin tablets and each time you get a ezetimibe and simvastatin tablets. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. If you have any questions about ezetimibe and simvastatin tablets, ask your healthcare provider. Only your healthcare provider can determine if ezetimibe and simvastatin tablets are right for you.
What are ezetimibe and simvastatin tablets?
Ezetimibe and simvastatin tablets are a prescription medicine that contains the cholesterol lowering medicines, simvastatin and ezetimibe:
- Ezetimibe and simvastatin tablets are used along with diet to lower elevated low-density lipoprotein cholesterol (LDL-C) or bad cholesterol in:
- adults with primary hyperlipidemia (high level of fats in your blood).
- adults and children 10 years of age and older with heterozygous familial hypercholesterolemia (HeFH). HeFH is an inherited condition that causes high levels of bad cholesterol.
- Ezetimibe and simvastatin tablets are also used with other cholesterol lowering treatments to lower elevated LDL-C levels in adults with homozygous familial hypercholesterolemia (HoFH). HoFH is an inherited condition that causes high levels of bad cholesterol.
- Simvastatin when used as a component of ezetimibe and simvastatin tablets is used to lower:
- the risk of death by lowering the risk of heart disease death.
- the risk of heart attacks and strokes.
- the need for certain types of heart and blood vessel procedures to improve blood flow called arterial revascularization in people with known heart, cerebrovascular disease (conditions that affect blood flow and the blood vessels in the brain), peripheral vascular disease (a blood circulation disorder that causes the blood vessels outside of your heart and brain to narrow, block, or spasm), and diabetes, who are at high risk for heart disease problems.
The safety and effectiveness of ezetimibe and simvastatin tablets have not been established in children younger than 10 years of age with inherited heterozygous familial hypercholesterolemia (HeFH) or other types of hyperlipidemia.
Do not take ezetimibe and simvastatin tablets if you:
- take certain medicines called CYP3A4 inhibitors such as:
- certain antifungal medicines (such as itraconazole, ketoconazole, posaconazole, voriconazole).
- certain antibiotics (including erythromycin, clarithromycin, telithromycin).
- HIV protease inhibitors (such as indinavir, nelfinavir, ritonavir, saquinavir, tipranavir, or atazanavir and cobicistat-containing products such as (elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate).
- certain hepatitis C virus protease inhibitors (such as boceprevir or telaprevir).
- the antidepressant nefazodone.
- take medicines called cyclosporine, danazol, or gemfibrozil.
- have liver problems.
- are allergic to simvastatin, ezetimibe, or any of the ingredients in ezetimibe and simvastatin tablets. See the end of this Patient Information leaflet for a complete ul of ingredients in ezetimibe and simvastatin tablets.
Ask your healthcare provider or pharmacist if you are not sure if your medicine is uled above.
Before you take ezetimibe and simvastatin tablets, tell your healthcare provider about all of your medical conditions, including if you:
- have unexplained muscle aches or weakness.
- have or have had myasthenia gravis (a disease causing general muscle weakness including in some cases muscles used for breathing), ocular myasthenia (a disease causing eye muscle weakness).
- have kidney problems.
- have liver problems or drink more than 2 glasses of alcohol daily.
- have thyroid problems.
- are 65 years of age or older.
- are of Chinese descent.
- are pregnant or plan to become pregnant. If you become pregnant while taking ezetimibe and simvastatin tablets, call your healthcare provider right away to discuss stopping ezetimibe and simvastatin tablets.
- are breastfeeding or plan to breastfeed. It is not known if ezetimibe and simvastatin passes into your breast milk. Do not breastfeed while taking ezetimibe and simvastatin tablets.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Talk to your healthcare provider before you start taking any new medicines.
Tell your healthcare provider who prescribes ezetimibe and simvastatin tablets if another healthcare provider increases the dose of another medicine you are taking.
Ezetimibe and simvastatin tablets may affect the way other medicines work, and other medicines may affect how ezetimibe and simvastatin tablets work. Especially tell your healthcare provider if you take:
- digoxin (a drug used to treat irregular heartbeat).
- coumarin anticoagulants (drugs that prevent blood clots, such as warfarin).
- fibric acid derivatives (such as fenofibrate).
Taking ezetimibe and simvastatin tablets with certain substances can also increase the risk of muscle problems. Especially tell your healthcare provider if you take:
- amiodarone or dronedarone (medicines used to treat an irregular heartbeat).
- verapamil, diltiazem, amlodipine, or ranolazine (medicines used to treat high blood pressure, chest pain associated with heart disease, or other heart conditions).
- lomitapide (a medicine used to treat a serious and rare genetic cholesterol condition).
- daptomycin (a drug used to treat complicated skin and bloodstream infections).
- large doses of niacin or nicotinic acid, especially if you are of Chinese descent.
- colchicine (a medicine used to treat gout).
- grapefruit juice.
Ask your healthcare provider or pharmacist for a ul of medicines if you are not sure. Know the medicines you take. Keep a ul of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take ezetimibe and simvastatin tablets?
- Take ezetimibe and simvastatin tablets exactly as your healthcare provider tells you to take it.
- Do not change your dose or stop taking ezetimibe and simvastatin tablets without talking to your healthcare provider.
- Take ezetimibe and simvastatin tablets 1 time each day in the evening.
- If you miss a dose, take it as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule. Do not take 2 doses of ezetimibe and simvastatin tablets at the same time. Talk with your healthcare provider if you have questions about a missed dose.
- While taking ezetimibe and simvastatin tablets, continue to follow your cholesterol-lowering diet and to exercise as your healthcare provider told you to.
- Your healthcare provider may do blood tests to check your cholesterol while you take ezetimibe and simvastatin tablets. Your healthcare provider may change your dose of ezetimibe and simvastatin tablets if needed.
If you take too much ezetimibe and simvastatin tablets, call your healthcare provider or Poison Help Line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What are the possible side effects of ezetimibe and simvastatin tablets?
Ezetimibe and simvastatin tablets may cause serious side effects including:
- Muscle pain, tenderness, and weakness (myopathy). Muscle problems, including muscle breakdown, can be serious in some people and rarely cause kidney damage that can lead to death.
Tell your healthcare provider right away if:
- you have unexplained muscle pain, tenderness, or weakness, especially if you have a fever or feel more tired than usual, while you take ezetimibe and simvastatin tablets.
- you have muscle problems that do not go away even after your healthcare provider has advised you to stop taking ezetimibe and simvastatin tablets. Your healthcare provider may do further tests to diagnose the cause of your muscle problems.
Your chances of getting muscle problems are higher if you:
- are taking certain other medicines while you take ezetimibe and simvastatin tablets.
- are 65 years of age or older.
- are female.
- have thyroid problems (hypothyroidism) that are not controlled.
- have kidney problems.
- are taking higher doses of ezetimibe and simvastatin tablets.
- are Chinese.
- Liver problems. Your healthcare provider should do blood tests to check your liver before you start taking ezetimibe and simvastatin tablets and if you have any symptoms of liver problems while you take ezetimibe and simvastatin tablets. Call your healthcare provider right away if you have the following symptoms of liver problems:
- feeling tired or weak
- loss of appetite
- right-sided upper belly pain
- dark urine
- yellowing of your skin or the whites of your eyes
- increase in blood sugar (glucose) levels). Ezetimibe and simvastatin tablets may cause an increase in your blood sugar levels.
The most common side effects of ezetimibe and simvastatin tablets include:
- headache
- increased liver enzyme levels
- muscle pain
- upper respiratory infection
- diarrhea
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of ezetimibe and simvastatin tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ezetimibe and simvastatin tablets?
- Store ezetimibe and simvastatin tablets at room temperature between 20¬į to 25¬įC (68¬į to 77¬įF).
- Keep ezetimibe and simvastatin tablets in its original container until you use it.
- Keep ezetimibe and simvastatin tablets in a tightly closed container.
Keep ezetimibe and simvastatin tablets and all medicines out of the reach of children.
General information about safe and effective use of ezetimibe and simvastatin tablets.
Medicines are sometimes prescribed for purposes other than those uled in a Patient Information leaflet. Do not use ezetimibe and simvastatin tablets for a condition for which it was not prescribed. Do not give ezetimibe and simvastatin tablets to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about ezetimibe and simvastatin tablets that is written for health professionals.
What are the ingredients in ezetimibe and simvastatin tablets?
Active Ingredients: ezetimibe, USP and simvastatin, USP.
Inactive Ingredients: butylated hydroxyanisole, citric acid monohydrate, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and propyl gallate.
This Patient Information has been approved by the U.S. Food and Drug Administration.
*All trademarks are the property of their respective owners.
For more information, go to www.amneal.com, or call 1-877-835-5472.
Manufactured by: Amneal Pharmaceuticals Pvt. Ltd. Oral Solid Dosage Unit Ahmedabad 382213, INDIA
Distributed by: Amneal Pharmaceuticals LLC Bridgewater, NJ 08807
Rev. 04-2024-05
Dispense with Patient Information available at:
documents.amneal.com/mg/ppi-ezetimibe-simvastatin-tab.pdf
Principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site