Firocoxib for Dogs Dailymed
Generic: firocoxib
Go PRO for all pill images
Firocoxib Chewable Tablets For Dogs
For oral use in dogs only.
Caution:  Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description:
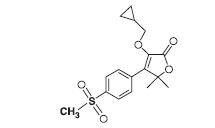
Firocoxib Chewable Tablets forDogs belongs to the coxib class of non-narcotic,non-steroidal anti-inflammatory drugs. Firocoxib is awhite crystalline compound described chemically as3-(cyclopropylmethoxy)-4-(4-(methylsulfonyl)phenyl)-5,5-dimethylfuranone. The empirical formula isC17H20O5S, and the molecular weight is 336.4. Thestructural formula is shown below:
Pharmacokinetics:
The absolute bioavailability of Firocoxib Chewable Tablets for Dogs is approximately 38% when administered as a 5 mg/kg oral dose to fasted adult dogs. Firocoxib is rapidly cleared from the blood via hepatic metabolism and fecal excretion (CLsystemic = ~0.4 L/hr/kg). Despitea high level of plasma protein binding (96%), firocoxib exhibits a large volume of distribution (VdőĽ of total drug = ~4.6 L/kg) and a terminal elimination half life of 7.8 hours (%CV = 30%). The oral drug absorption process is highly variable among subjects. Co-administration of firocoxib with food delays drug absorption (Tmax from 1 to 5 hours) and decreases peak concentrations (Cmax from 1.3 to 0.9 mcg/mL). However, food does not affect the overall oral bioavailability at the recommended dose.
Indications:
Firocoxib Chewable Tablets for Dogs are indicated for the control of pain and inflammation associated with osteoarthritis and for the control ofpost-operative pain and inflammation associated with soft-tissue and orthopedic surgery in dogs.
Dosage And Administration:
Always provide the Client Information Sheet with prescription. Carefully consider the potential benefits and risks of Firocoxib Chewable Tablets for Dogs and other treatment options before deciding to use Firocoxib Chewable Tablets for Dogs. Use the lowest effective dose for the shortest duration consistent with individual response. The recommended dosage of Firocoxib Chewable Tablets for Dogs for oral administration in dogs is 2.27 mg/lb (5.0 mg/kg) body weight once daily as needed for osteoarthritis and for 3 days as needed for postoperative pain and inflammationassociated with soft-tissue and orthopedic surgery. The dogs can be treated with Firocoxib Chewable Tablets for Dogs approximately two hours prior to surgery. The tablets are scored and dosage should be calculated in half tablet increments. Firocoxib Chewable Tablets for Dogs can be administered with or without food.
Contraindications:
Dogs with known hypersensitivity to firocoxib should not receive Firocoxib Chewable Tablets for Dogs.
Warnings:
Not for use in humans. Keep this and all medications out of the reach of children. Consult a physician in case of accidental ingestion by humans.
Keep Firocoxib Chewable Tablets for Dogs in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
For oral use in dogs only. Use of this product at doses above the recommended 2.27 mg/lb (5.0 mg/ kg) in puppies less than seven months of age has been associated with serious adverse reactions, including death (see Animal Safety). Due to tablet sizes and scoring, dogs weighing less than 12.5 lb (5.7 kg) cannot be accurately dosed.
All dogs should undergo a thorough history and physical examination before the initiation of NSAID therapy. Appropriate laboratory testing to establish hematological and serum baseline data is recommended prior to and periodically during administration of any NSAID.
Owners should be advised to observe for signs of potential drug toxicity (see Adverse Reactions and Animal Safety) and be given a Client Information Sheet about Firocoxib Chewable Tablets for Dogs.
Contact Information:
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Pegasus Laboratories, Inc. at 1-800-874-9764 or www.prnpharmacal.com.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
Precautions:
This product cannot be accurately dosed in dogs less than 12.5 pounds in body weight.
Consider appropriate washout times when switchingfrom one NSAID to another or when switching from corticosteroid use to NSAID use.
As a class, cyclooxygenase inhibitory NSAIDsmay be associated with renal, gastrointestinal andhepatic toxicity. Sensitivity to drug-associatedadverse events varies with the individual patient.Dogs that have experienced adverse reactions fromone NSAID may experience adverse reactions fromanother NSAID. Patients at greatest risk for adverseevents are those that are dehydrated, on concomitantdiuretic therapy, or those with existing renal, cardiovascular,and/or hepatic dysfunction. Concurrentadministration of potentially nephrotoxic drugsshould be carefully approached and monitored.NSAIDs may inhibit the prostaglandins that maintainnormal homeostatic function. Such anti-prostaglandineffects may result in clinially significant disease inpatients with underlying or pre-existing disease thathas not been previously diagnosed. Since NSAIDspossess the potential to produce gastrointestinalulceration and/or gastrointestinal perforation,concomitant use of Firocoxib Chewable Tablets forDogs with other anti-inflammatory drugs, such asNSAIDs or corticosteroids, should be avoided.The concomitant use of protein bound drugs withFirocoxib Chewable Tablets for Dogs has not beenstudied in dogs. Commonly used protein-bounddrugs include cardiac, anticonvulsant, and behavioralmedications. The influence of concomitant drugsthat may inhibit the metabolism of FirocoxibChewable Tablets for Dogs has not been evaluated.Drug compatibility should be monitored in patients requiring adjunctive therapy.
If additional pain medication is needed after thedaily dose of Firocoxib Chewable Tablets for Dogs, anon-NSAID class of analgesic may be necessary.
Appropriate monitoring procedures should beemployed during all surgical procedures. Anestheticdrugs may affect renal perfusion, approach concomitantuse of anesthetics and NSAIDs cautiously. Theuse of parenteral fluids during surgery should beconsidered to decrease potential renal complicationswhen using NSAIDs perioperatively.
The safe use of Firocoxib Chewable Tablets for Dogsin pregnant, lactating or breeding dogs has not beenevaluated.
Adverse Reactions:
Osteoarthritis: In controlled field studies, 128dogs (ages 11 months to 15 years) were evaluatedfor safety when given firocoxib chewable tablets ata dose of 2.27 mg/lb (5.0 mg/kg) orally once dailyfor 30 days. The following adverse reactions wereobserved. Dogs may have experienced more thanone of the observed adverse reactions during thestudy.
Adverse Reactions Seen in U.S. Field Studies Adverse Reactions          Firocoxib
           n=128
Active
         Control
         n = 121
Vomiting                5              8 Diarrhea                1             10 Decreased Appetite or Anorexia                3               3  Lethargy                1               3  Pain                2               1  Somnolence                1               1  Hyperactivity                 1               0 
Firocoxib chewable tablets were safely used duringfield studies concomitantly with other therapiesincluding vaccines, anthelmintics, and antibiotics.
Soft-tissue Surgery: In controlled field studiesevaluating soft-tissue postoperative pain and inflam-mation, 258 dogs (ages 10.5 weeks to 16 years)were evaluated for safety when given firocoxibchewable tablets at a dose of 2.27 mg/lb (5.0 mg/kg) orally approximately 2 hours prior to surgeryand once daily thereafter for up to two days. Thefollowing adverse reactions were observed. Dogsmay have experienced more than one of the observed reactions during the study.
Adverse Reactions Seen in the Soft-tissue Surgery Postoperative Pain in Field Studies   Adverse Reactions         Firocoxib
         Group
        n=127
Control
     Group*
     n=131
Vomiting             5         6 Diarrhea             1          1  Bruising At Surgery Site              1          0  Respiratory Arrest             1          0  SQ Crepitusin Rear Leg and Flank             1         0  Swollen Paw             1          0 
*Sham-dosed (pilled)
Orthopedic Surgery: In a controlled field studyevaluating orthopedic pain and inflam-mation, 226 dogs of various breeds, ranging in agefrom 1 to 11.9 years in the firocoxib-treated groupsand 0.7 to 17 years in the control group were evaluatedfor safety. Of the 226 dogs, 118 were givenfirocoxib chewable tablets at a dose of 2.27 mg/lb (5.0 mg/kg) orally approximately 2 hours priorto surgery and once daily thereafter for a total ofthree days. The following adverse reactions wereobserved. Dogs may have experienced more thanone of the observed reactions during the study.
Adverse Reactions Seen in the Orthopedic Surgery Postoperative Pain Field Study   Adverse Reactions           Firocoxib
           Group
           n=118
       Control
       Group*
       n=108
Vomiting                1           0 Diarrhea                2**           1  Bruising at Surgery Site                2            3  Inappetance/Decreased Appetite                1            2  Prexia                 0            1  Incision Swelling, Redness                 9            5  Oozing Incision                 2            0 
A case may be represented in more than one category
*Sham-dosed (pilled).
**One dog had hemorrhagic gastroenteritis
Post-Approval Experience (Rev. 2009): Thefollowing adverse reactions are based on postapprovaladverse drug event reporting. Thecategories are uled in decreasing order offrequency by body system:
Gastrointestinal: vomiting, anorexia, diarrhea,melena, gastrointestinal perforation, hematemesis,hematachezia, weight loss, gastrointestinal ulceration,peritonitis, abdominal pain, hypersalivation,nausea
Urinary: elevated BUN, elevated creatinine, polydypsia,polyuria, hematuria, urinary incontinence,proteinuria, kidney failure, azotemia, urinary tractinfection
Neurological/Behavioral/Special Sense: depression/lethargy, ataxia, seizures, nervousness, confusion,weakness, hyperactivity, tremor, paresis, head tilt,nystagmus, mydriasis, aggression, uveitis
Hepatic: elevated ALP, elevated ALT, elevatedbilirubin, decreased albumin, elevated AST, icterus,decreased or increased total protein and globulin,pancreatitis, ascites, liver failure, decreased BUN
Hematological: anemia, neutrophilia, thrombocytopenia,neutropenia
Cardiovascular/Respiratory: tachypnea, dyspnea,tachycardia
Dermatologic/Immunologic: pruritis, fever, alopecia,moist dermatitis, autoimmune hemolytic anemia,facial/muzzle edema, urticaria
In some cases, death has been reported as anoutcome of the adverse events uled above.
Contact Information:
To report suspected adversedrug events, for technical assistance or to obtaina copy of the Safety Data Sheet (SDS), contactPegasus Laboratories, Inc. at 1-800-874-9764 orwww.prnpharmacal.com.
For additional information about adverse drugexperience reporting for animal drugs, contactFDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
Information For Dog Owners:
Firocoxib ChewableTablets for Dogs, like other drugs of its class, isnot free from adverse reactions. Owners should beadvised of the potential for adverse reactions and beinformed of the clinical signs associated with drugintolerance. Adverse reactions may include vomiting,diarrhea, decreased appetite, dark or tarry stools,increased water consumption, increased urination,pale gums due to anemia, yellowing of gums,skin or white of the eye due to jaundice, lethargy,incoordination, seizure, or behavioral changes.
Serious adverse reactions associated with thisdrug class can occur without warning and in raresituations result in death (see Adverse Reactions).Owners should be advised to discontinue FirocoxibChewable Tablets for Dogs and contact their veterinarianimmediately if signs of intolerance areobserved. The vast majority of patients with drugrelated adverse reactions have recovered when thesigns are recognized, the drug is withdrawn, andveterinary care, if appropriate, is initiated. Ownersshould be advised of the importance of periodicfollow up for all dogs during administration ofany NSAID.
Clinical Pharmacology:
Mode of action: FirocoxibChewable Tablets for Dogs is a cyclooxygenaseinhibiting(coxib) class, non-narcotic, non-ste-roidal anti-inflammatory drug (NSAID) with anti-inflammatory and analgesic properties. There are twomain cyclooxygenase enzymes, COX-1 and COX-2,and a newly discovered third enzyme, COX-3, whichhas yet to be fully characterized.1 Cyclooxygenase-1(COX-1) is the enzyme responsible for facilitatingconstitutive physiologic processes, e.g., plateletaggregation, gastric mucosal protection, and renalperfusion.2 It also is constitutively expressed inthe brain, spinal cord, and reproductive tract.3Cyclooxygenase-2 (COX-2) is responsible for thesynthesis of inflammatory mediators, but it is alsoconstitutively expressed in the brain, spinal cordand kidneys.4, 5, 6 Cyclooxygenase-3 (COX-3) is alsoconstitutively expressed in the canine and humanbrain and also the human heart.7 Results from in vitro
studies showed firocoxib to be highly selective forthe COX-2 enzyme when canine blood was exposedto drug concentrations comparable to those observedfollowing a once daily 5 mg/kg oral dose in dogs.8However, the clinical significance of these findingshas not been established.
Effectiveness:
Two hundred and forty-nine dogs ofvarious breeds, ranging in age from 11 months to20 years, and weighing 13 to 175 lbs, were randomlyadministered firocoxib or an active control drug intwo field studies. Dogs were assessed for lameness,pain on manipulation, range of motion, joint swelling,and overall improvement in a non-inferiority evalua-tionof firocoxib compared with the active control. Atthe study's end, 87% of the owners rated firoxib-treated dogs as improved. Eighty-eight percentof dogs treated with firocoxib were also judgedimproved by the veterinarians. Dogs treated withfirocoxib showed a level of improvement in veteri-narian-assessed lameness, pain on palpation, rangeof motion, and owner-assessed improvement thatwas comparable to the active control. The level ofimprovement in firocoxib-treated dogs in limb weightbearing on the force plate gait analysis assessmentwas comparable to the active control.
In a separate field study, two hundred fifty-eightclient-owned dogs of various breeds, ranging inage from 10.5 weeks to 16 years and weighing from7 to 168 lbs, were randomly administered firocoxibor a control (sham-dosed-pilled) for the control ofpostoperative pain and inflammation associated withsoft-tissue surgical procedures such as abdominalsurgery (e.g. ovariohysterectomy, abdominalcryptorchidectomy, splenectomy, cystotomy) ormajor external surgeries (e.g. mastectomy, skintumor removal ‚Č•8 cm). The study demonstratedthat firocoxib-treated dogs had significantly lowerneed for rescue medication than the control(sham-dosed-pilled) in controlling postoperativepain and inflammation associated with soft-surgery.
A multi-center field study with 226 client-owneddogs of various breeds, and ranging in age from 1to 11.9 years in the firocoxib-treated groups and0.7 to 17 years in the control group was conducted.Dogs were randomly assigned to either the firocoxibor the control (sham-dosed-pilled) group for thecontrol of postoperative pain and inflammation asso-ciated with orthopedic surgery. Surgery to repaira ruptured cruciate ligament included the followingstabilization procedures: fabellar suture and/orimbrication, fibular head transposition, tibial plateauleveling osteotomy (TPLO), and ‚Äėover the top‚Äô technique.The study (n = 220 for effectiveness) demon-strated that foirocoxib-treated dogs had signifcantlylower need for rescue medication than the control(sham-dosed-pilled) in controlling postoperativepain and inflammation associated with orthopedicsurgery.
Animal Safety:
Animal Safety: In a target animal safety study,firocoxib was administered orally to healthy adultBeagle dogs (eight dogs per group) at 5, 15, and 25mg/kg (1, 3, and 5 times the recommended total dailydose) for 180 days. At the indicated dose of 5 mg/kg, there were no treatment related adverse events.
Decreased appetite, vomiting, and diarrhea wereseen in dogs in all dose groups, including unmedicatedcontrols, although vomiting and diarrhea wereseen more often in dogs in the 5X dose group.One dog in the 3X dose group was diagnosed withjuvenile polyarteritis of unknown etiology afterexhibiting recurrent episodes of vomiting anddiarrhea, lethargy, pain, anorexia, ataxia, propriocep-tive deficits, decreased albumin lavels, decreasedand then elevated platelet counts, increased bleedingtimes, and elevated liver enzymes. On histopathologicexamination, a mild ileal ulcer was found in one5X dog. This dog also had a decreased serum albuminwhich returned to normal by study completion. Onecontrol and three 5X dogs had focal areas of inflam-mation in the pylorus or small intestine. Vacuolizationwithout inflammatory cell infiltrates was noted in thethalamic region of the brain in three control, one 3X,and three 5X dogs. Mean ALP was within the normalrange for all groups but was greater in the 3X and5X dose groups than in the control group. Transientdecreases in serum albumin were seen in multipleanimals in the 3X and 5X dose groups, and in onecontrol animal.
In a separate safety study, firocoxib was administeredorally to healthy juvenile (10-13 weeks of age)Beagle dogs at 5, 15, and 25 mg/kg (1, 3, and 5 timesthe recommended total daily dose) for 180 days. Atthe indicated (1X) dose of 5 mg/kg, on histopathologicexamination, three out of six dogs had minimalperiportal hepatic fatty change. On histopathologicexamination, one control, one 1X, and two 5X dogshad diffuse slight hepatic fatty change. These animalsshowed no clinical signs and had no liver enzymeelevations. In the 3X dose group, one dog waseuthanized because of poor clinical condition (Day63). This dog also had a mildly decreased serumalbumin. At study completion, out of five survivingand clinically normal 3X dogs, three had minimal periportalhepatic fatty change. Of twelve dogs in the 5Xdose group, one died (Day 82) and three moribunddogs were euthanized (Days 38, 78, and 79) becauseof anorexia, poor weight gain, depression, and inone dog, vomiting. One of the euthanized dogs hadingested a rope toy. Two of these 5X dogs had mildlyelevated liver enzymes. At necropsy all five of thedogs that died or were euthanized had moderateperiportal or severe panzonal hepatic fatty change;two had duodenal ulceration; and two had pancreaticedema. Of two other clinically normal 5X dogs (outof four euthanized as comparators to the clinicallyaffected dogs), one had slight and one had moderateperiportal hepatic fatty change. Drug treatment wasdiscontinued for four dogs in the 5X group. Thesedogs survived the remaining 14 weeks of the study.On average, the dogs in the 3X and 5X dose groupsdid not gain as much weight as control dogs. Rate ofweight gain was measured (instead of weight loss)because these were young growing dogs. Thalamicvacuolation was seen in three of six dogs in the3X dose group, five of twelve dogs in te 5X dosegroup, and to a lesser degree in two unmedicatedcontrols. Diarrhea was seen in all dose groups,including unmedicated controls.
In a separate dose tolerance safety study involving atotal of six dogs (two control dogs and four treateddogs), firocoxib was administered to four healthyadult Beagle dogs at 50 mg/kg (ten times the recommendeddaily dose) for twenty-two days. All dogssurvived to the end of the study. Three of the fourtreated dogs developed small intestinal erosion orulceration. Treated dogs that developed small intestinalerosion or ulceration had a higher incidence ofvomiting, diarrhea, and decreased food consumptionthan control dogs. One of these dogs had severeduodenal ulceration, with hepatic fatty change andassociated vomiting, diarrhea, anorexia, weightloss, ketonuria, and mild elevations in AST andALT. All four treated dogs exhibited progressivelydecreasing serum albumin that, with the exception ofone dog that developed hypoalbuminemia, remainedwithin normal range. Mild weight loss also occurredin the treated group. One of the two control dogs andthree of the four treated dogs exhibited transientincreases in ALP that remained within normal range.
Storage:
Store at controlled room temperaturebetween 20-25¬įC (68-77¬įF), excursions permittedbetween 15-40¬įC (59-104¬įF).
To Request a Safety Data Sheet (SDS), call 1-800-874-9764.
How Supplied:
Firocoxib Chewable Tablets forDogs is available as round, beige to tan, half-scoredtablets in two strengths, containing 57 mg or 227 mgfirocoxib. Each tablet strength is supplied in 60 countand 180 count bottles.
References Section
1 Willoughby DA, Moore AR and Colville-Nash PR.COX-1, COX-2, and COX-3 and the future treat-ment of chronic inflammatory disease. Lancet2000;355:646-648.
2 Smith, et al., Pharmacological Analysis of Cyclo-oxygenase-1 in Inflammation. Proc. Natl. Acad. Sci.USA, Pharmacology 1998;95:13313-13318.
3 Jones CJ and Budsberg SC. Physiologic characteristicsand clinical importance of the cyclooxygenaseisoforms in dogs and cats. JAVMA2000;217(5):721-729.
4 Zhang, et al., Inhibition of Cyclo-oxygenase-2Rapidly Reverses Inflammatory Hyperalgesia andProstaglandin E2 Production. JPET 1997;283:1069-1075.
5Jones and Budsberg, pp. 721-729.
6Zhang, et al., pp. 1069-1075.
7 Chandrasekharan NV, Dai H, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen andother analgesic/antipyretic drugs: Cloning, structureand expression. Proc. Natl. Acad. Sci. USA,2002;99(21):13926-13931.
8Data on file with the NADA 141-230.
Approved by FDA under ANADA # 200-751
Manufactured by:Pegasus Laboratories, Inc.Employee-OwnedPensacola, FL 32514
Se-‚ÄĘQual‚ĄĘ and PRN‚ĄĘ are trademarks ofPegasus Laboratories, Inc.
Rev. 08-2022
Sńď‚óŹQual‚ĄĘ Products
By PRN‚ĄĘ Pharmacal
Information For Dog Owners Aboutfirocoxib Chewable Tablets For Dogs
Firocoxib Chewable Tablets for Dogs
Firocoxib Chewable Tablets for Dogs are used for the control of pain and inflammation due to osteoarthritis or associated with soft- tissue and orthopedic surgery in your dog.
This summary contains important information about FirocoxibChewable Tablets for Dogs. You should read this information beforeyou start giving your dog Firocoxib Chewable Tablets for Dogsand review it each time your prescription is refilled. This sheet isprovided only as a summary and does not take the place of instructionsfrom your veterinarian. Talk to your veterinarian if you do notunderstand any of this information or you want to know more aboutFirocoxib Chewable Tablets for Dogs.
What is Firocoxib Chewable Tablets for Dogs? Firocoxib Chewable Tablets for Dogs is a veterinary prescriptionnon-steroidal anti-inflammatory drug (NSAID) used to control painand inflammation due to osteoarthritis, or associated with soft-tissueand orthopedic surgery in dogs.
Osteoarthritis is a painful condition caused by ‚Äúwear and tear‚ÄĚ ofcartilage and other parts of the joints that may result in the followingchanges or signs in your dog:‚ÄĘ Limping or lameness.‚ÄĘ Decreased activity or exercise (reluctance to stand, climb stairs,jump or run, or difficulty performing these activities).‚ÄĘ Stiffness or decreased movement of joints.
Firocoxib Chewable Tablets for Dogs is indicated for the controlof postoperative pain and inflammation following soft-tissue andorthopedic surgeries (e.g. spays, cruciate ligament repair). Yourveterinarian may administer Firocoxib Chewable Tablets for Dogsbefore the procedure and recommend that the dog be treated for afew days after going home.
What kind of results can I expect when my dog is on Firocoxib Chewable Tablets for Dogs for osteoarthritis? While Firocoxib Chewable Tablets for Dogs is not a cure forosteoarthritis, it can control the pain and inflammation and improveyour dog’s mobility.
‚ÄĘ Response varies from dog to dog, but improvement can be quitedramatic.‚ÄĘ In most dogs, improvement can be seen within days.‚ÄĘ If Firocoxib Chewable Tablets for Dogs is discontinued or notgiven as directed, your dog's pain and inflammation may return.
What kind of results can I expect when my dog is onFirocoxib Chewable Tablets for Dogs for the control of pain andinflammation following soft-tissue and orthopedic surgery?‚ÄĘ Firocoxib Chewable Tablets for Dogs allow your dog to recovermore comfortably by controlling pain and inflammation followingsoft-tissue and orthopedic surgery.‚ÄĘ Control of pain and inflammation may vary from dog to dog.‚ÄĘ If Firocoxib Chewable Tablets for Dogs are not given according toyour veterinarian‚Äôs directions, your dog‚Äôs pain may return.‚ÄĘ Consult your veterinarian if your dog appears to be uncomfortable.
Which dogs should not take Firocoxib Chewable Tablets for Dogs? Your dog should not be given Firocoxib Chewable Tablets for Dogsif he/she:‚ÄĘ Has an allergic reaction to firocoxib, the active ingredient inFirocoxib Chewable Tablets for Dogs.‚ÄĘ Has had an allergic reaction (such as hives, facial swelling, or redor itchy skin) to aspirin or other NSAIDs.‚ÄĘ Is presently taking aspirin, other NSAIDs, or corticosteroids.‚ÄĘ Is under 12.5 pounds in body weight.‚ÄĘ Has pre-existing kidney or liver disease.‚ÄĘ Has decreased appetite, vomiting or diarrhea.
Firocoxib Chewable Tablets for Dogs should only be given to dogs. People should not take Firocoxib Chewable Tablets for Dogs.Keep Firocoxib Chewable Tablets for Dogs and all medicationsout of the reach of children. Call your physician immediately if youaccidentally take Firocoxib Chewable Tablets for Dogs.
What to tell/ask your veterinarian before giving Firocoxib Chewable Tablets for Dogs. Talk to your veterinarian about:‚ÄĘ The signs of osteoarthritis you have observed in your dog, suchas limping or stiffness.‚ÄĘ The importance of weight control in the management ofosteoarthritis.‚ÄĘ What tests might be done before Firo coxib Chewable Tablets forDogs is prescribed.‚ÄĘ How often your dog may need to be examined by your veterinarian.‚ÄĘ The risks and benefits of using Firocoxib Chewable Tablets forDogs. Serious adverse reactions, including death, have beenassociated with Firocoxib Chewable Tablets for Dogs administrationat doses above the recommended dose in puppies less thanseven months of age.
Tell your veterinarian if your dog is currently experiencing or has everhad the following medical problems:‚ÄĘ Any side effects from taking Firocoxib Chewable Tablets for Dogsor other NSAIDs, such as aspirin.‚ÄĘ Any digestive upset (vomiting and/or diarrhea).‚ÄĘ Any kidney disease.‚ÄĘ Any liver disease.
Tell your veterinarian about:‚ÄĘ Any other medical problems or allergies that your dog has now,or has had in the past.‚ÄĘ All medicines that you are giving or plan to give to your dog,including those you can get without a prescription and any dietarysupplements.
Tell your veterinarian if your dog:‚ÄĘ Is under 7 months of age.‚ÄĘ Is pregnant, nursing or if you plan to breed your dog.
How to give Firocoxib Chewable Tablets for Dogs to your dog. Firocoxib Chewable Tablets for Dogs should be given accordingto your veterinarian’s instructions. Do not change the way you giveFirocoxib Chewable Tablets for Dogs to your dog without firstspeaking with your veterinarian. Your veterinarian will tell you whatamount of Firocoxib Chewable Tablets for Dogs is right for your dogand for how long it should be given. Firocoxib Chewable Tablets forDogs may be offered to the dog by hand or you can place the tabletin your dog’s mouth. Firocoxib Chewable Tablets for Dogs may begiven with or without food.
What are the possible side effects that may occur in my dog during Firocoxib Chewable Tablets for Dogs therapy? Firocoxib Chewable Tablets for Dogs, like other NSAIDs, may causesome side effects. Serious side effects associated with NSAIDtherapy in dogs can occur with or without warning, and, in raresituations, result in death. The most common side effects associatedwith Firocoxib Chewable Tablets for Dogs therapy involve the digestivetract (vomiting and decreased food consumption). Liver andkidney problems have also been reported with NSAIDs. Look for thefollowing side effects that may indicate your dog is having a problemwith Firocoxib Chewable Tablets for Dogs:‚ÄĘ Decrease or increase in appetite.‚ÄĘ Vomiting.‚ÄĘ Change in bowel movements (such as diarrhea, or black, tarry orbloody stools).‚ÄĘ Change in behavior (such as decreased or increased activity level,incoordination, seizure, or aggression).‚ÄĘ Yellowing of gums, skin, or whites of the eyes (jaundice).‚ÄĘ Change in drinking habits (frequency or amount consumed).‚ÄĘ Change in urination habits (frequency, color, or smell).‚ÄĘ Change in skin (redness, scabs, or scratching).‚ÄĘ Unexpected weight loss.
It is important to stop the medication and contact yourveterinarian immediately if you think your dog has a medicalproblem or side effect while taking Firocoxib Chewable Tabletsfor Dogs. If you have additional questions about possibleside effects, talk with your veterinarian or call 1-800-874-9764.
Can Firocoxib Chewable Tablets for Dogs be given with other medications? Firocoxib Chewable Tablets for Dogs should not be given withother NSAIDs (for example, aspirin, carprofen, etodolac, deracoxib,meloxicam, or tepoxalin) or corticosteroids (for example, prednisone,cortisone, dexamethasone, or triamcinolone).
Tell your veterinarian about all medications that you have given yourdog in the past, and any medications you are planning to give withFirocoxib Chewable Tablets for Dogs. This should include othermedicines that you can get without a prescription or any dietarysupplements. Your veterinarian may want to check that all of yourdog’s medicines can be given together.
What do I do in case my dog eats more than the prescribed amount of Firocoxib Chewable Tablets for Dogs? Consult your veterinarian immediately if your dog eats more than theprescribed amount of Firocoxib Chewable Tablets for Dogs.
What else should I know about Firocoxib Chewable Tablets for Dogs? ‚ÄĘ This sheet provides a summary of information about FirocoxibChewable Tablets for Dogs. If you have any questions or concernsabout Firocoxib Chewable Tablets for Dogs, osteoarthritis pain, orpost operative pain following soft-tissue and orthopedic surgery,talk with your veterinarian.‚ÄĘ As with all prescribed medicines, Firocoxib Chewable Tabletsfor Dogs should only be given to the dog for which they wereprescribed. They should be given to your dog only for the conditionfor which they were prescribed, at the prescribed dose.‚ÄĘ It is important to periodically discuss your dog‚Äôs response toFirocoxib Chewable Tablets for Dogs tablets. Your veterinarian willdetermine if your dog is responding as expected and if your dogshould continue receiving Firocoxib Chewable Tablets for Dogs.
Contact Information: To report suspected adverse drug events, fortechnical assistance or to obtain a copy of the Safety Data Sheet(SDS), contact Pegasus Laboratories, Inc. at 1-800-874-9764 orwww.prnpharmacal.com.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
Approved by FDA under ANADA # 200-751
Rev. 08-2022
Sńď‚óŹQual‚ĄĘ Products by PRN‚ĄĘ Pharmacal
Prinicple Display Panel:
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site



