Galliprant (grapiprant 60 mg) Dailymed
Generic: grapiprant
IMPRINT: G 60 MG
SHAPE: oval
COLOR: brown SCORE: 2
All Imprints
grapiprant 20 mg - g 20 mg oval brown
grapiprant 60 mg oral tablet [galliprant] - 60 mg g oval brown
grapiprant 60 mg oral tablet [galliprant] - g 60 mg oval brown
Go PRO for all pill images
(grapiprant tablets)
For oral use in dogs only
20 mg, 60 mg and 100 mg flavored tablets
A prostaglandin E2 (PGE2) EP4 receptor antagonist; a non-cyclooxygenase inhibiting, non-steroidal anti-inflammatory drug
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description:
GALLIPRANT (grapiprant tablets) is a prostaglandin E2 (PGE2) EP4 receptor antagonist; a non-cyclooxygenase (COX) inhibiting, non-steroidal anti-inflammatory drug (NSAID) in the piprant class. GALLIPRANT is a flavored, oval, biconvex, beige to brown in color tablet debossed with a âGâ that contains grapiprant and desiccated pork liver as the flavoring agent. The 20 mg and 60 mg tablets are scored. The 100 mg tablet is not scored.
The molecular weight of grapiprant is 491.61 Daltons. The empirical formula is C26H29N5O3S. Grapiprant is N-[[[2-[4-(2-Ethyl-4,6-dimethyl-1H-imidazo[4,5-c] pyridin-1-yl)phenyl]ethyl]amino]carbonyl]-4 methylbenzenesulfonamide.
The structural formula is:
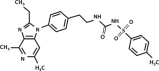
Indication:
GALLIPRANT (grapiprant tablets) is indicated for the control of pain and inflammation associated with osteoarthritis in dogs.
Dosage And Administration:
Always provide âInformation for Dog Ownersâ Sheet with prescription.
Carefully consider the potential benefits and risks of GALLIPRANT and other treatment options before deciding to use GALLIPRANT. Use the lowest effective dose for the shortest duration consistent with individual response.
The dose of GALLIPRANT (grapiprant tablets) is 0.9 mg/lb (2 mg/kg) once daily.
Only the 20 mg and 60 mg tablets of GALLIPRANT are scored.
The dosage should be calculated in half tablet increments. Dogs less than 8 lbs. (3.6 kgs) cannot be accurately dosed.
Dosing Chart
Dose
Weight in pounds
Weight in kilograms
20 mg tablet
60 mg tablet
100 mg tablet
0.9 mg/lb(2 mg/kg)once daily
8-15
3.6-6.8
0.5
15.1-30
6.9-13.6
1
30.1-45
13.7-20.4
0.5
45.1-75
20.5-34
1
75.1-150
34.1-68
1
The 100 mg tablet is not scored and should not be broken in half.
Breaking the 100 mg tablet in half will not guarantee that half of the active ingredient is contained within each half of the tablet. For dogs larger than 150 lbs (68 kgs), use a combination of tablet and half tablets to achieve the appropriate dose.
Contraindications:
GALLIPRANT should not be used in dogs that have a hypersensitivity to grapiprant.
Warnings:
Not for use in humans. Keep this and all medications out of reach of children and pets. Consult a physician in case of accidental ingestion by humans. For use in dogs only. All dogs should undergo a thorough history and physical examination before the initiation of NSAID therapy. Appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to and periodically during administration of any NSAID is recommended.
Owners should be advised to observe for signs of potential drug toxicity (see Adverse Reactions , Post-Approval Experience , and Animal Safety ) and be given an âInformation for Dog Ownersâ Sheet about GALLIPRANT.
Do not administer GALLIPRANT in conjunction with any other oral or injectable NSAID or corticosteroid.
Keep GALLIPRANT in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
Precautions:
The safe use of GALLIPRANT has not been evaluated in dogs younger than 9 months of age and less than 8 lbs (3.6 kgs), dogs used for breeding, or in pregnant or lactating dogs.
Adverse reactions in dogs receiving GALLIPRANT may include vomiting, diarrhea, decreased appetite, mucoid, watery or bloody stools, and decreases in serum albumin and total protein (see Adverse Reactions and Post-Approval Experience ).
If GALLIPRANT is used long term appropriate monitoring is recommended.
GALLIPRANT is a non-cyclooxygenase (COX) inhibiting non-steroidal anti-inflammatory drug (NSAID). As a class, NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Dogs that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction.
Anesthetic drugs may affect renal perfusion; approach concomitant use of anesthetics and NSAIDs cautiously. Appropriate monitoring procedures (including ECG, blood pressure, and temperature regulation) should be employed during all surgical procedures. The use of parenteral fluids during surgery is recommended to decrease potential renal complications when using NSAIDs perioperatively.
If additional pain medication is needed after a daily dose of GALLIPRANT, a non-NSAID/non-corticosteroid class of analgesic may be necessary. Concurrent administration of potentially nephrotoxic drugs should be carefully approached and monitored. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. GALLIPRANT blocks the activity of the specific prostaglandin E2 (PGE2). Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. NSAIDs possess the potential to produce gastrointestinal ulceration and/or gastrointestinal perforation. Do not use GALLIPRANT concomitantly with other anti-inflammatory drugs, such as NSAIDs or corticosteroids. Consider appropriate washout times when switching from GALLIPRANT to another NSAID (or vice versa) or when switching from corticosteroid use to GALLIPRANT use.
The concomitant use of protein-bound drugs with GALLIPRANT has not been studied in dogs. Commonly used protein-bound drugs include cardiac, anticonvulsant and behavioral medications. The influence of concomitant drugs that may inhibit metabolism of GALLIPRANT has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy.
The use of GALLIPRANT in dogs with cardiac disease has not been studied.
It is not known whether dogs with a history of hypersensitivity to sulfonamide drugs will exhibit hypersensitivity to GALLIPRANT. GALLIPRANT is a methylbenzenesulfonamide.
Adverse Reactions:
In a controlled field study, 285 dogs were evaluated for safety when given either GALLIPRANT or a vehicle control (tablet minus grapiprant) at a dose of 2 mg/kg (0.9 mg/lb) once daily for 28 days. GALLIPRANT-treated dogs ranged in age from 2 to 16.75 years. The following adverse reactions were observed:
Table 1. Adverse reactions reported in the field study. *Dogs may have experienced more than one type or occurrence during the study.
Adverse reaction*
GALLIPRANT(grapiprant tablets) N = 141
Vehicle control (tablets minus grapiprant)N = 144
Vomiting
24
9
Diarrhea, soft stool
17
13
Anorexia, inappetence
9
7
Lethargy
6
2
Buccal ulcer
1
0
Immune mediated hemolytic anemia
1
0
GALLIPRANT was used safely during the field studies with other concurrent therapies, including antibiotics, parasiticides and vaccinations.
Post-Approval Experience (2024)
The following adverse events are based on post-approval adverse drug experience reporting for GALLIPRANT. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events in dogs are categorized in order of decreasing reporting frequency by body system and in decreasing order of reporting frequency within each body system:
GI: diarrhea (with or without blood), vomiting (with or without blood), elevated pancreatic enzymes/pancreatitis, melena, bloody stool, hypoalbuminemia, abdominal pain, gastrointestinal ulcer.General: anorexia, lethargy, weight loss, panting, hyperactivity.Hepatic: elevated liver enzymes.Renal/Urinary: increased BUN or creatinine, polydipsia, urinary incontinence/inappropriate urination, polyuria, renal failure.Neurologic: ataxia, seizures.Hematologic: anemia, thrombocytopenia.Dermatologic/Immunologic: pruritus, allergic reactions (including facial edema and hives), immune mediated hemolytic anemia.
In some cases, death (including euthanasia) has been reported as an outcome of the adverse events uled above.
Contact Information:
To report suspected adverse drug experiences and/or obtain a copy of the Safety Data Sheet (SDS) or for technical assistance, contact Elanco US Inc. at 1-888-545-5973.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
Information For Dog Owners:
Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance. Adverse reactions may include diarrhea, vomiting, decreased appetite, dark or tarry stools, increased water consumption, increased urination, pale gums due to anemia, jaundice (yellowing of gums, skin or white of the eye), lethargy, incoordination, seizures, and behavioral changes such as depression or restlessness. Serious adverse reactions associated with this drug class can occur without warning and, in some cases, result in death (see Adverse Reactions and Post-Approval Experience ).
Owners should be advised to discontinue GALLIPRANT therapy and contact their veterinarian immediately if signs of intolerance are observed. The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized, the drug is withdrawn, and veterinary care, if appropriate, is initiated. Owners should be advised of the importance of periodic follow up for all dogs during administration of any NSAID.
Clinical Pharmacology:
Grapiprant is a prostaglandin E2 (PGE2) EP4 receptor antagonist; a non- cyclooxygenase inhibiting, non-steroidal, anti-inflammatory drug. Grapiprant has a canine EP4 receptor binding affinity (Ki) of 24 nM.
Prostaglandins have a wide variety of physiologic effects. Prostaglandin E2 (PGE2) is a prostanoid that exerts its effects via four receptors, EP1, EP2, EP3, and EP4. PGE2 is involved in mediating inflammatory pain, vasodilation, increasing vascular permeability; as well as gastrointestinal homeostasis, renal function and reproductive functions. The EP4 receptor is important in mediating pain and inflammation as it is the primary mediator of the PGE2-elicited sensitization of sensory neurons1 and PGE2-elicited inflammation.2 Grapiprant blocks PGE2-elicited pain and inflammation by antagonizing the EP4 receptor.
The EP4 receptor, along with the EP1, EP2 and EP3 receptors, is involved in PGE2 mediated effects on gastrointestinal homeostasis and renal function. PGE2 effects mediated solely by the EP4 receptor are stimulation of mucus secretion in the stomach and large intestine, stimulation of acid secretion in the stomach, inhibition of small intestine motility and inhibition of cytokine expression in the large intestine.3 While PGE2 gastroprotective action is mediated by EP1, the healing- promoting action of PGE2 in the stomach is mediated by the EP4 receptor.4 In the kidney, the PGE2 antinatiuretic effect is mediated by the EP4 receptor.5
EP4 receptors are abundantly expressed in the heart of dogs,6 the clinical relevance of which is unknown. The EP4 receptor is not involved in generation of pyrexia.
Grapiprant is not a potential inhibitor of CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 mediated metabolism pathways. Grapiprant is a substrate of P-glycoprotein transport. In vitro metabolism with dog liver microsomes identified two oxidative metabolites, M3 (hydroxyl) and M5 (N-dealkylation).
The pharmacokinetic characterization of grapiprant following oral administration of GALLIPRANT tablets to healthy Beagles is provided in the table below.
Table 2. Mean (±SD) Plasma Pharmacokinetic Parameters for Grapiprant in Beagles after single oral dose of GALLIPRANT tablet formulation 1Study 1 was a food effect determination study. 2Study 2 was a PK bridging study conducted using 60 mg GALLIPRANT tablets at 6 mg/kg dose and 5 X 100 mg GALLIPRANT tablets at 50 mg/kg dose. 3Median (Range)
Study
Study 11
Study 11
Study 22
Study 22
PK Parameter
2 mg/kg(n = 10)(Fasted)
2 mg/kg(n = 10)(Fed)
6 mg/kg(n = 8)(Fasted)
50 mg/kg(n = 8)(Fasted)
Tmax3 (hr)
1.0(0.5 â 1.03)
1.0(0.5 â 8.07)
1.0(1.0 â 2.0)
2.0(1.0 â 4.0)
Cmax (ng/mL)
1210(341)
278(179)
5720(3220)
98500(13100)
AUC(0-inf) (ng*hr/mL)
2790(982)
1200(523)
17800(5520)
414000(73700)
T1/2(hr)
4.60(4.19)
5.67(3.27)
5.01(1.95)
5.21(1.66)
Fed/Fasted RelativeBioavailability GeometricMean Ratio of AUC(90% Confidence Limits)
0.37 (0.28 â 0.46)
NA
Grapiprant is absorbed rapidly following an oral dose of the GALLIPRANT; with Cmax values achieved within approximately 2 hr post-dose (Tmax). Intake of the tablet with food significantly reduces the oral bioavailability, with mean Cmax and AUC grapiprant values reduced 4-fold and 2-fold, respectively. The systemic grapiprant exposure increases in a greater than dose proportional manner.
The mean terminal elimination half-life (T1/2) ranges between 4.60 to 5.67 hr. Following once daily dosing, negligible drug accumulation in the blood is anticipated. Following an oral dose of radiolabeled grapiprant to dogs, the majority of the dose was excreted within the first 72 hr (84%) and approximately 88.7% of the dose was excreted in 192 hr. In a bile duct cannulated dog study, approximately 55.6%, 15.1% and 19.1% of the dose was excreted in bile, urine and feces, respectively, suggesting the high oral bioavailability of grapiprant in dogs (> 70%). Four metabolites were identified; two hydroxylated metabolites, one N-deamination metabolite (major metabolite urine (3.4%) and feces (7.2%)) and one N-oxidation metabolite. Metabolite activity is not known. Plasma protein binding of grapiprant was ~95%.
Effectiveness:
Two hundred and eighty five (285) client-owned dogs were enrolled in the study and evaluated for field safety. GALLIPRANT-treated dogs ranging in age from 2 to 16.75 years and weighing between 4.1 and 59.6 kgs (9 â 131 lbs) with radiographic and clinical signs of osteoarthritis were enrolled in a placebo-controlled, masked field study. Dogs had a 7-day washout from NSAID or other current OA therapy. Two hundred and sixty two (262) of the 285 dogs were included in the effectiveness evaluation. Dogs were assessed for improvements in pain and function by the owners using the Canine Brief Pain Inventory (CBPI) scoring system.7 A statistically significant difference in the proportion of treatment successes in the GALLIPRANT group (63/131 or 48.1%) was observed compared to the vehicle control group (41/131 or 31.3%). GALLIPRANT demonstrated statistically significant differences in owner assessed pain and function. The results of the field study demonstrate that GALLIPRANT, administered at 2 mg/kg (0.9 mg/pound) once daily for 28 days, was effective for the control of pain and inflammation associated with osteoarthritis.
Animal Safety:
In a 9-month toxicity study, grapiprant in a methylcellulose suspension was administered by oral gavage once daily to healthy Beagles at doses of 1, 6, and 50 mg/kg/day. Based on a relative bioavailability study comparing grapiprant in methylcellulose suspension to GALLIPRANT tablets, the corresponding equivalent doses were 0.75 mg/kg (0.12X â 0.25X), 4.44 mg/kg (0.72X â 1.48X) and 30.47 mg/kg (4.88X â 10.16X) of the GALLIPRANT tablets. Four animals/sex were used in each dose group and 2 additional animals/sex were used in the 50 mg/kg dose group to evaluate recovery after drug cessation. Vomiting and soft-formed or mucus stool were observed in all groups, including controls, with higher incidence in grapiprant-treated dogs. Decreases in serum albumin and total protein were seen with increasing doses of grapiprant. Hypoalbuminemia and hypoproteinemia were reversible when treatment was discontinued. Three treated dogs and one control dog had elevated alkaline phosphatase values. One animal in the 50 mg/kg group (equivalent to 30.47 mg/kg of tablet formulation) had mild regeneration of the mucosal epithelium of the ileum.
In a field study conducted in 366 client-owned dogs to evaluate GALLIPRANT at doses of 2 mg/kg once daily, 5 mg/kg once daily, 4 mg/kg twice daily, or placebo twice daily, the most common adverse reactions related to treatment were diarrhea, vomiting and inappetence. Changes in clinical pathology included concurrent elevations of alkaline phosphatase and alanine aminotransferase values on Day 28, and dose-dependent decreases in total protein values. There was no clinical impact related to these clinical pathology changes.
Storage Conditions:
Store at or below 86° F (30° C)
How Supplied:
20 mg, 60 mg and 100 mg flavored tablets in 7, 30 and 90 count bottles
Approved by FDA under NADA # 141-455
Manufactured for: Elanco US Inc. Greenfield, IN 46140
References:
1. Nakao, K., Murase, A., et al. CJ-023,423, a novel, potent and selective prostaglandin EP4 receptor antagonist with antihyperalgesic properties. The Journal of Pharmacology and Experimental Therapeutics. 2007; 322(2), 686-694.2. Murase, A., Okumura, T., et al. Effect of prostanoid EP4 receptor antagonist, CJ-042,794, in rat models of pain and inflammation. European Journal of Pharmacology. 2008; 580(1-2), 116-121.3. Takeuchi, K., S. Kato, et al. Prostaglandin EP receptors involved in modulating gastrointestinal mucosal integrity. Journal of Pharmacological Sciences. 2010; 114(3): 248-261.4. Hatazawa R, Tanaka A, Tanigami M, et al. Cyclooxygenase-2/prostaglandin E2 accelerates the healing of gastric ulcers via EP4 receptors. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2007; 293: G788-G797.5. Nasrallah R, Hassouneh R, and Hebert R. Chronic kidney disease: targeting prostaglandin E2 receptors. American Journal of Physiology Renal Physiology. 2014; 307: F242-250.6. Castleberry TA, Lu B, et al. Molecular cloning and functional characterization of the canine prostaglandin E2 receptor EP4 subtype. Prostaglandins and Other Lipid Mediators. 2001; 65: 167-187.7. http://www.vet.upenn.edu/docs/default-source/ VCIC/canine-bpi_userguide.pdf?sfvrsn=0
Galliprant, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates. © 2024 Elanco or its affiliates
October 2024
PA104149X
Elanco TM
Information For Owners/caregivers Section
Galliprant TM (grapiprant tablets)
For oral use in dogs onlyInformation for Dog Owners
GALLIPRANT is indicated for the control of pain and inflammation due to osteoarthritis.
This sheet contains important information about GALLIPRANT. You should read this information before starting your dog on GALLIPRANT and review it each time the prescription is refilled. This information provides a summary and does not take the place of the instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or you want more information about GALLIPRANT.
What is GALLIPRANT?
GALLIPRANT is an EP4 prostaglandin receptor antagonist non-steroidal, anti-inflammatory drug (NSAID). As an anti-inflammatory, GALLIPRANT is indicated for the control of pain and inflammation associated with osteoarthritis in dogs.
Control of pain and inflammation may vary from dog to dog. Consult your veterinarian if your dog appears to be uncomfortable. GALLIPRANT may need to be given for an extended period of time. Use the lowest dose to provide adequate pain control. Always consult with your veterinarian before altering the dose.
It is important to periodically discuss your dogâs response to GALLIPRANT with your veterinarian. Your veterinarian will discuss appropriate monitoring while your dog is on GALLIPRANT.
Your dog should not take GALLIPRANT if:
⢠Your dog is presently taking aspirin, other NSAIDs (non-steroidal anti-inflammatory drugs such as carprofen or meloxicam) or corticosteroids (such as prednisone) unless directed by your veterinarian.⢠Your dog has had an allergic reaction (such as hives, facial swelling, or itchy skin) to GALLIPRANT.⢠Your dog has bloody stool or vomit.⢠Your dog has a pre-existing kidney or liver condition.⢠Your dog has any condition predisposing to dehydration.⢠Your dog is anorexic (loss of appetite).⢠Your dog is under 8 pounds in body weight.
GALLIPRANT should only be given to dogs.Do not use in cats.
People should not take GALLIPRANT. Keep GALLIPRANT and all medications out of reach of children. Consult a physician if you accidentally take GALLIPRANT.
Keep GALLIPRANT in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
Tell your veterinarian about:
⢠Any side effects your dog has experienced from GALLIPRANT or other NSAIDs. Dogs that have experienced side effects from one NSAID may experience adverse reactions from another NSAID.⢠Any digestive issues (bloody or mucoid stool, vomiting, diarrhea, or decreased appetite) your dog has had.⢠If your dog has no appetite or a decreased appetite.⢠Any heart, kidney, or liver disease your dog has had.⢠Any other medical problems or allergies that your dog has now or has had in the past.⢠All medications that you are giving your dog or plan to give your dog, including those you can get without a prescription and any dietary supplements.⢠If you plan to breed your dog, or if your dog is pregnant or nursing.
Talk to your veterinarian about:
⢠The signs of osteoarthritis you have observed (for example, limping or stiffness).⢠What tests should be done prior to administering GALLIPRANT.⢠How often your dog may need to be examined by your veterinarian.⢠The risks and benefits of using GALLIPRANT.
What are the possible side effects that may occur in my dog during therapy with GALLIPRANT?
GALLIPRANT may cause some side effects in individual dogs. Serious side effects associated with this drug can occur with or without warning and, in some cases, result in death. The most common side effects associated with GALLIPRANT therapy involve the digestive tract (diarrhea, soft, mucoid stools, vomiting, and decreased appetite). Liver and kidney problems have also been reported.
Look for the following side effects that may indicate that your dog is having a problem with GALLIPRANT or may have another medical problem:
⢠Change in bowel movements such as diarrhea, soft mucoid stool, or change in stool color (black, tarry, or bloody stool).⢠Vomiting.⢠Decrease in appetite.⢠Change in behavior, such as depression or restlessness.⢠Incoordination or seizures.⢠Change in drinking or urination.⢠Yellowing of gums, skin, or whites of the eyes (jaundice).
It is important to stop the medication and contact your veterinarian immediately if you think your dog may have a medical problem or side effect while on GALLIPRANT.
If you have additional questions about possible side effects, talk with your veterinarian or call Elanco US Inc. at 1-888-545-5973. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
Can GALLIPRANT be given with other medications?
GALLIPRANT should not be given with aspirin, other non-steroidal anti-inflammatory drugs (such as carprofen or meloxicam) or corticosteroids (such as prednisone).
Tell your veterinarian about all medications that you have given your dog in the past and any medications that you are planning to give with GALLIPRANT. This should include any medications that you can get without a prescription and any dietary supplements. Your veterinarian may want to evaluate the potential for any drug interactions and to assure drug compatibility.
What can I do in case my dog eats more than the prescribed amount of GALLIPRANT?
Contact your veterinarian immediately if your dog eats more than the prescribed amount of GALLIPRANT.
What else should I know about GALLIPRANT?
Keep GALLIPRANT in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
If you have any questions or concerns about GALLIPRANT or osteoarthritis, talk to your veterinarian. GALLIPRANT should only be given to the dog for which it was prescribed. It should be given to your dog only for the condition for which it was prescribed, at the prescribed dose, as directed by your veterinarian. Your veterinarian will determine if your dog is responding as expected and if your dog should continue receiving GALLIPRANT.
Galliprant, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
October 2024
PA104149X
Elanco TM
Principal Display Panel - Galliprant 20 Mg 7 Tablets Carton Label
GalliprantTM (grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restrictsthis drug to use by or on theorder of a licensed veterinarian.
7 FLAVOREDTABLETS
Elanco

Principal Display Panel - Galliprant 20 Mg 7 Tablets Bottle Label
GalliprantTM (grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
7 FLAVOREDTABLETS

Principal Display Panel - Galliprant 20 Mg 30 Tablets Carton Label
GalliprantTM (grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
30 FLAVOREDTABLETS
Elanco

Principal Display Panel - Galliprant 20 Mg 30 Tablets Bottle Label
GalliprantTM (grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
30 FLAVOREDTABLETS

Principal Display Panel - Galliprant 20 Mg 90 Tablets Carton Label
GalliprantTM (grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
90 FLAVOREDTABLETS
Elanco

Principal Display Panel - Galliprant 20 Mg 90 Tablets Bottle Label
GalliprantTM (grapiprant tablets)
20 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
90 FLAVOREDTABLETS

Principal Display Panel - Galliprant 60 Mg 7 Tablets Carton Label
GalliprantTM (grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
7 FLAVOREDTABLETS
Elanco

Principal Display Panel - Galliprant 60 Mg 7 Tablets Bottle Label
GalliprantTM (grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
7 FLAVOREDTABLETS

Principal Display Panel - Galliprant 60 Mg 30 Tablets Carton Label
GalliprantTM (grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
30 FLAVOREDTABLETS
Elanco

Principal Display Panel - Galliprant 60 Mg 30 Tablets Bottle Label
GalliprantTM (grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
30 FLAVOREDTABLETS

Principal Display Panel - Galliprant 60 Mg 90 Tablets Carton Label
GalliprantTM (grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
90 FLAVOREDTABLETS
Elanco

Principal Display Panel - Galliprant 60 Mg 90 Tablets Bottle Label
GalliprantTM (grapiprant tablets)
60 mg
For oral use in dogs only
EP4 Prostaglandin ReceptorAntagonist NSAID
Caution: Federal law restricts this drug to use by or on the orderof a licensed veterinarian.
90 FLAVOREDTABLETS
Principal Display Panel - Galliprant 100 Mg 7 Tablets Carton Label
GalliprantTM (grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
7 FLAVOREDTABLETS
Elanco

Principal Display Panel - Galliprant 100 Mg 7 Tablets Bottle Label
GalliprantTM (grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
7 FLAVOREDTABLETS

Principal Display Panel - Galliprant 100 Mg 30 Tablets Carton Label
GalliprantTM (grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
30 FLAVOREDTABLETS
Elanco

Principal Display Panel - Galliprant 100 Mg 30 Tablets Bottle Label
GalliprantTM (grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin ReceptorAntagonist NSAID
Caution: Federal law restricts this drug to use by or on the orderof a licensed veterinarian.
30 FLAVOREDTABLETS

Principal Display Panel - Galliprant 100 Mg 90 Tablets Carton Label
GalliprantTM (grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin Receptor Antagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
90 FLAVOREDTABLETS
Elanco

Principal Display Panel - Galliprant 100 Mg 90 Tablets Bottle Label
GalliprantTM (grapiprant tablets)
100 mg
For oral use in dogs only
EP4 Prostaglandin ReceptorAntagonist NSAID
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
90 FLAVOREDTABLETS

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


