Hemady (dexamethasone 20 mg) Dailymed
Generic: dexamethasone is used for the treatment of Addison Disease Asthma Brain Edema Bursitis Colitis, Ulcerative Collagen Diseases Cushing Syndrome Depressive Disorder Dermatitis Herpetiformis Hypercalcemia Iritis Keratitis Leukemia Lupus Erythematosus, Systemic Mycosis Fungoides Nasal Polyps Nausea Pemphigus Rheumatic Diseases Rhinitis, Allergic, Perennial Sarcoidosis Shock, Septic Spondylitis, Ankylosing Thyroiditis Tuberculosis, Ocular Tuberculosis, Pulmonary Vomiting Eye Infections, Fungal Eye Infections, Viral Purpura, Thrombocytopenic, Idiopathic
IMPRINT: 20
SHAPE: round
COLOR: white
All Imprints
hemady (dexamethasone) 20 mg - 20 round white
Go PRO for all pill images
1 Indications & Usage
HEMADY is indicated in combination with other anti-myeloma products for the treatment of adults with multiple myeloma (MM).
HEMADY is a corticosteroid indicated in combination with other anti-myeloma products for the treatment of adults with multiple myeloma.
2 Dosage & Administration
Recommended Dosage: 20 mg or 40 mg orally once daily, on specific days depending on the protocol regimen.
2.1 Recommended Dosage
The recommended dosage of HEMADY is 20 mg or 40 mg, orally, once daily, on specific days depending on the treatment regimen. Refer to the Prescribing Information of the other anti-myeloma products used in combination with HEMADY for specific HEMADY dosing. HEMADY can be administered with or without food.
2.2 Dose Modification for Elderly Patients
Dose-reduction for HEMADY is recommended for elderly patients, due to increased toxicity in these patients. Refer to the Prescribing Information of the other anti-myeloma products used as part of a combination regimen with HEMADY, for dosing recommendations in elderly patients.
3 Dosage Forms & Strengths
Tablets: 20 mg white, round, biconvex tablet embossed with “20” on one side.
Tablets: 20 mg
4 Contraindications
HEMADY is contraindicated in patients with:
- Hypersensitivity to dexamethasone, or any of the excipients. Rare instances of anaphylactic reactions have been reported [see Adverse Reactions (6), Description (11) ].
- Systemic fungal infections. Corticosteroids may exacerbate systemic fungal infections [see Warnings and Precautions (5.2)].
- Patients with hypersensitivity to dexamethasone (
4 )- Patients with systemic fungal infections (
4 )
5 Warnings And Precautions
- Alterations in Endocrine Function: Hypothalamic-pituitary adrenal (HPA) axis suppression, Cushing’s syndrome, and hyperglycemia can occur. Monitor patients for these conditions with chronic use. (
5.1 )- Immunosuppression and Increased Risk of Infections: Increased risk of new, exacerbation, dissemination, or reactivation of latent infections. (
5.2 )- Alteration in Cardiovascular/Renal Function: Monitor for elevated blood pressure and sodium, and for decreased potassium levels. (
5.3 )- Venous and Arterial Thromboembolism: Risk increased; consider anticoagulant prophylaxis and monitor for evidence of thromboembolism. (
5.4 )- Vaccination: Avoid the administration of live or live attenuated vaccines in patients receiving immunosuppressive doses of corticosteroids. (
5.5 )- Ophthalmic Effects: May include cataracts, infections, and glaucoma. (
5.6 )- Gastrointestinal Perforation: Avoid use in active or latent peptic ulcers, diverticulitis, fresh intestinal anastomoses, and nonspecific ulcerative colitis, since they may increase the risk of a perforation. (
5.7 )- Osteoporosis: Increased risk; monitor for changes in bone density with chronic use. (
5.8 )- Behavioral and Mood Disturbances: May include euphoria, insomnia, mood swings, personality changes, severe depression, and psychosis. Monitor for signs and symptoms and manage promptly. (
5.10 )- Kaposi’s Sarcoma: Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. (
5.11 )- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus. (
5.13 ,8.1 )5.1 Alterations in Endocrine Function
Corticosteroids , such as HEMADY, can cause serious and life-threatening alterations in endocrine function, especially with chronic use. Monitor patients receiving HEMADY for adrenal insufficiency after corticosteroid withdrawal and Cushing’s syndrome and hyperglycemia while receiving corticosteroids. In addition, patients with hypopituitarism, primary adrenal insufficiency, or congenital adrenal hyperplasia, altered thyroid function, or pheochromocytoma may be at risk for adverse endocrine events.
Risk of Adrenal Insufficiency Following Corticosteroid Withdrawal
Corticosteroids can produce reversible hypothalamic-pituitary adrenal (HPA) axis suppression, with the potential for the development of secondary adrenal insufficiency after withdrawal of corticosteroid treatment. Acute adrenal insufficiency can occur if glucocorticoids are withdrawn abruptly and can be fatal. The degree and duration of adrenocortical insufficiently produced is variable among patients and depends on the dose, frequency, and duration of glucocorticoid therapy. The risk may be reduced by gradually tapering the corticosteroid dose when withdrawing treatment. This insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, corticosteroid therapy should be reinstituted. For patients already taking corticosteroids during times of stress, the dosage may have to be increased.
A steroid “withdrawal syndrome”, seemingly unrelated to adrenocortical insufficiency, may also occur following abrupt discontinuance of corticosteroids. This syndrome includes symptoms such as: anorexia, nausea, vomiting, lethargy, headache, fever, joint pain, desquamation, myalgia, and/or weight loss. These effects are thought to be due to the sudden change in glucocorticoid concentration rather than to low corticosteroid levels.
Cushing’s Syndrome
Cushing’s syndrome (hypercortisolism) may occur with prolonged exposure to exogenous corticosteroids, including HEMADY. Symptoms include hypertension, truncal obesity and thinning of the limbs, purple striae, facial rounding, facial plethora, muscle weakness, easy and frequent bruising with thin fragile skin, posterior neck fat deposition, osteopenia, acne, amenorrhea, hirsutism and psychiatric abnormalities. Using the lowest dose of corticosteroid for the shortest duration possible may reduce the risk.
Hyperglycemia
Corticosteroids can increase blood glucose, worsen pre-existing diabetes, and predispose those on long-term therapy to diabetes mellitus, and may reduce the effect of the antidiabetic drugs. Monitor blood glucose at regular intervals. For patients with hyperglycemia, anti-diabetic treatment should be initiated or adjusted accordingly.
Considerations for Use in Patients with Altered Thyroid Function
Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients. Changes in thyroid status of the patient may necessitate a dose adjustment of the corticosteroid. When concomitant administration of corticosteroids and levothyroxine is required, administration of corticosteroid should precede the initiation of levothyroxine therapy to avoid adrenal crisis.
Pheochromocytoma Crisis
There have been reports of pheochromocytoma crisis, which can be fatal, after administration of systemic corticosteroids. In patients with suspected or identified pheochromocytoma, consider the risk of pheochromocytoma crisis prior to administering HEMADY.
5.2 Immunosuppression and Increased Risk of Infections
Corticosteroids, including HEMADY, suppress the immune system and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic.
Corticosteroids reduce resistance to new infections, exacerbate existing infections, increase the risk of disseminated infections, increase the risk of reactivation or exacerbation of latent infections, and mask some signs of infection. These infections can be severe, and at times fatal. The degree to which the dose, route, and duration of corticosteroid administration correlates with the specific risks of infection is not well characterized; however, the rate of occurrence of infectious complications increases with increasing doses of corticosteroids.
Monitor for the development of infection and consider withdrawal of HEMADY or reduction of the dose of corticosteroids as needed.
Varicella Zoster and Measles Viral Infections
Chickenpox caused by Varicella Zoster virus and measles can have a serious or even fatal course in non-immune children or adult on corticosteroids, including HEMADY. In patients who have not had these diseases, particular care should be taken to avoid exposure. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with immune globulin (IG) may be indicated. If chickenpox develops, treatment with antiviral agents may be considered.
Hepatitis B Virus Reactivation
Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers undergoing treatment with immunosuppressive drugs including corticosteroids. Reactivation can also occur in patients who appear to have resolved hepatitis B infection.
Fungal Infections
HEMADY is contraindicated in patients with systemic fungal infections. Corticosteroids may exacerbate systemic fungal infections. For patients on chronic corticosteroids who have developed systemic fungal infections, withdrawal of corticosteroids or reduction of the dose of corticosteroids is recommended.
The following infections have been reported during the use of corticosteroids to treat other conditions that HEMADY is not indicated for:
Amebiasis
Corticosteroids may activate latent amebiasis. Rule out latent amebiasis or active amebiasis before initiating corticosteroid therapy in any patient who has spent time in the tropics or any patient with unexplained diarrhea.
Strongyloides Infestation
In patients with known or suspected Strongyloides (threadworm) infestation, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia. For patients on HEMADY who develop known or suspected Strongyloides (threadworm) infection, withdrawal of corticosteroids or reduction of the dose of corticosteroids is recommended.
Tuberculosis
The use of corticosteroids in active tuberculosis should generally be limited to cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for the management of the disease in conjunction with an appropriate anti-tuberculous regimen. If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis.
Cerebral Malaria
Corticosteroids should not be used in cerebral malaria.
5.3 Alterations in Cardiovascular/Renal Function
Corticosteroids, including HEMADY, can cause elevation of blood pressure, salt, and water retention, and increased excretion of potassium and calcium. Monitor blood pressure and assess for signs and symptoms of volume overload. Monitor serum potassium levels. Dietary salt restriction and potassium supplementation may be necessary. HEMADY should be used with caution in patients with congestive heart failure.
Literature reports suggest an association between use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with HEMADY should be used with great caution in these patients.
5.4 Venous and Arterial Thromboembolism
Thromboembolism is a known adverse reaction of dexamethasone, including HEMADY. The risk for venous and arterial thromboembolism increases significantly when dexamethasone is administered with anti-myeloma products (e.g., thalidomide, lenalidomide, pomalidomide, and carfilzomib). Refer to the Prescribing Information of these anti-myeloma products for information about the risk of venous and arterial thromboembolism.
Consider thromboprophylaxis based on an assessment of individual patients’ underlying risk factors and the anti-myeloma drugs. Agents that also may increase the risk of thromboembolism should be used with caution in patients with multiple myeloma receiving combination regimens of HEMADY and anti-myeloma products.
5.5 Vaccination
Avoid administration of live or live attenuated vaccines in patients receiving immunosuppressive doses of corticosteroids for the treatment of multiple myeloma. Killed or inactivated vaccines may be administered. However, the response to such vaccines cannot be predicted.
5.6 Ophthalmic Effects
Use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to bacteria, fungi, or viruses. Consider referral to an ophthalmologist for patients who develop ocular symptoms or use corticosteroid-containing products for more than 6 weeks. The use of oral corticosteroids is not recommended in the treatment of optic neuritis and may lead to an increase in the risk of new episodes. Corticosteroids should not be used in patients with active ocular herpes simplex.
Intraocular pressure may become elevated in some individuals. If steroid therapy is continued for more than 6 weeks, intraocular pressure should be monitored.
5.7 Gastrointestinal Perforation
There is an increased risk of gastrointestinal perforation during corticosteroid use in patients with certain gastrointestinal disorders such as active or latent peptic ulcers, diverticulitis, fresh intestinal anastomoses, and nonspecific ulcerative colitis.
Signs of gastrointestinal perforation, such as peritoneal irritation, may be masked in patients receiving corticosteroids. Avoid corticosteroids if there is a possibility of impending perforation, abscess, or other pyrogenic infections; diverticulitis; fresh intestinal anastomoses; or active or latent peptic ulcer.
5.8 Osteoporosis
Corticosteroids decrease bone formation and increase bone resorption both through their effect on calcium regulation (i.e., decreasing absorption and increasing excretion) and inhibition of osteoblast function. This, together with a decrease in the protein matrix of the bone, secondary to an increase in protein catabolism, and reduced sex hormone production, may lead to inhibition of bone growth in pediatric patients and the development of osteoporosis at any age. Special consideration should be given to patients at increased risk of osteoporosis (e.g., postmenopausal women) before initiating HEMADY therapy.
5.9 Myopathy
An acute myopathy has been observed with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (e.g., myasthenia gravis), or in patients receiving concomitant therapy with neuromuscular blocking drugs (e.g., pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevation of creatine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.
5.10 Behavioral and Mood Disturbances
Potentially severe psychiatric adverse reactions may occur with systemic corticosteroids, including HEMADY. Symptoms typically emerge within a few days or weeks of starting treatment and may be dose-related. These reactions may improve after either dose reduction or withdrawal, although pharmacologic treatment may be necessary. Psychiatric adverse reactions usually involve hypomanic or manic symptoms (e.g., euphoria, insomnia, mood swings) during treatment and depressive episodes after discontinuation of treatment. Inform patients and caregivers of the potential for behavioral and mood changes and encourage them to seek medical attention if psychiatric symptoms develop, especially if depressed mood or suicidal ideation is suspected.
5.11 Kaposi's Sarcoma
Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy to treat other chronic conditions for which HEMADY is not indicated. Discontinuation of corticosteroids may result in clinical improvement.
5.12 HEMADY in Combination with Anti-Myeloma Products
HEMADY is administered in combination regimens with anti-myeloma products; please refer to the Prescribing Information of these products for additional information.
5.13 Embryo-Fetal Toxicity
Based on findings from clinical and animal reproduction studies, corticosteroids, including HEMADY, can cause fetal harm when administered to a pregnant woman. Dexamethasone administration to pregnant women has resulted in adverse effects on fetal growth, skeletal development/osteogenesis and low birth weight with prolonged use. Dexamethasone administration to pregnant animals during organogenesis resulted in structural abnormalities, embryo-fetal mortality, functional impairment, and alterations to growth at doses equivalent to or below the recommended doses.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with HEMADY and for at least one month after the last dose [see Use in Specific Populations (8.1, 8.3)].
6 Adverse Reactions
The following clinically significant adverse reactions are described in detail in other labeling sections:
- Hypersensitivity [see Contraindications (4)]
- Alterations in Endocrine Function [see Warnings and Precautions (5.1)]
- Immunosuppression and Increased Risk of Infections [see Warnings and Precautions (5.2)]
- Alterations in Cardiovascular/Renal Function [see Warnings and Precautions (5.3)]
- Venous and Arterial Thromboembolism [see Warnings and Precautions (5.4)]
- Vaccination [see Warnings and Precautions (5.5)]
- Ophthalmic Effects [see Warnings and Precautions (5.6)]
- Gastrointestinal Perforation [see Warnings and Precautions (5.7)]
- Osteoporosis [see Warnings and Precautions (5.8)]
- Myopathy [see Warnings and Precautions (5.9)]
- Behavioral and Mood Disturbances [see Warnings and Precautions (5.10)]
- Kaposi's Sarcoma [see Warnings and Precautions (5.11)]
- HEMADY in Combination with Anti-Myeloma Products [see Warnings and Precautions (5.12)]
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.13)]
The following adverse reactions associated with the use of HEMADY or other corticosteroids were identified in clinical trials or postmarketing reports. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Allergic reactions: Allergic or hypersensitivity reaction, anaphylaxis, angioedema.
Blood and Lymphatic System Disorders: Leukocytosis.
Cardiovascular: Bradycardia, cardiac arrest, cardiac arrhythmias, cardiac enlargement, circulatory collapse, congestive heart failure, fat embolism, hypertension, hypertrophic cardiomyopathy in premature infants, myocardial rupture following recent myocardial infarction, edema, pulmonary edema, syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis.
Dermatologic: Acne, allergic dermatitis, cutaneous and subcutaneous atrophy, dry scaly skin, ecchymoses and petechiae, edema, erythema, hyperpigmentation, hypopigmentation, impaired wound healing, increased sweating, sterile abscess, rash, striae, suppressed reactions to skin tests, thin fragile skin, thinning scalp hair, urticaria.
Endocrine: Decreased carbohydrate and glucose tolerance, development of cushingoid state, hyperglycemia, glycosuria, hirsutism, hypertrichosis, increased requirements for insulin or oral hypoglycemic agents in diabetes, manifestations of latent diabetes mellitus, menstrual irregularities, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery, or illness), suppression of growth in pediatric patients.
Fluid and electrolyte disturbances: Fluid retention, hypokalemic alkalosis, potassium loss, sodium retention, increased urinary excretion of calcium, tumor lysis syndrome.
Gastrointestinal: Abdominal distention, elevation in serum liver enzyme levels (usually reversible upon discontinuation), hepatomegaly, increased appetite, nausea, pancreatitis, peptic ulcer with possible perforation and hemorrhage, perforation of the small and large intestine (particularly in patients with inflammatory bowel disease), ulcerative esophagitis.
Infection: Decreased resistance to infection, injection site infections following non-sterile administration.
Metabolic: Negative nitrogen balance due to protein catabolism.
Musculoskeletal: Osteonecrosis of femoral and humeral heads, Charcot-like arthropathy, loss of muscle mass, muscle weakness, osteoporosis, pathologic fracture of long bones, steroid myopathy, tendon rupture, vertebral compression fractures.
Neurological: Convulsions, epidural lipomatosis, headache, increased intracranial pressure with papilledema (pseudotumor cerebri) usually following discontinuation of treatment, neuritis, neuropathy, paresthesia, vertigo.
Ophthalmic: Central serous chorioretinopathy, exophthalmos, glaucoma, increased intraocular pressure, posterior subcapsular cataracts, vision blurred.
Other: Abnormal fat deposits, decreased resistance to infection, hiccups, increased or decreased motility and number of spermatozoa, malaise, moon face, weight gain.
Psychiatric: Depression, emotional instability, euphoria, insomnia, mood swings, personality changes, psychosis.
Reproductive: Alteration in motility and number of spermatozoa.
The most common adverse reactions are cardiovascular, dermatologic, endocrine, fluid and electrolyte disturbances, gastrointestinal, metabolic, musculoskeletal, neurological/psychiatric, ophthalmic, abnormal fat deposits, decreased resistance to infection, hiccups, increased or decreased motility and number of spermatozoa, malaise, moon face, and weight gain. (6 )
To report SUSPECTED ADVERSE REACTIONS, contact Acrotech Biopharma LLC at 1-888-292-9617 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch .
7 Drug Interactions
- Avoid concomitant use of strong CYP3A4 inhibitors or inducers. (
7.1 )- Concomitant therapies such as erythropoietin stimulating agents or estrogen containing therapies may have an increased risk of thromboembolism. (
7.2 )7.1 Effect of Other Drugs on HEMADY
Strong CYP3A4 inhibitors
Coadministration of strong and moderate CYP3A4 inhibitors increased dexamethasone exposure [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions [see Warnings and Precautions (5)Â and Adverse Reactions (6)]. Avoid coadministration of strong CYP3A4 inhibitors or consider alternative medication that are not strong CYP3A4 inhibitors. If concomitant use of strong CYP3A4 inhibitors cannot be avoided, closely monitor for adverse drug reactions.
Strong CYP3A4 inducers
Coadministration of strong CYP3A4 inducers may decrease dexamethasone exposure [see Clinical Pharmacology (12.3)], which may result in loss of efficacy. Avoid coadministration of strong CYP3A4 inducers or consider alternative medication that are not CYP3A4 inducers. If concomitant use strong CYP3A4 inducers cannot be avoided, consider increasing the dose of HEMADY.
Cholestyramine
Cholestyramine may increase the clearance of corticosteroids and potentially decrease corticosteroid exposure. Avoid coadministration of cholestyramine and HEMADY and consider alternative agents.
Anticholinesterases
Concomitant use of anticholinesterase agents and corticosteroids may produce severe weakness in patients with myasthenia gravis. If possible, anticholinesterase agents should be withdrawn at least 24 hours before initiating corticosteroid therapy.
Ephedrine
Ephedrine may decrease dexamethasone exposure. Decreased exposure may result in loss of efficacy. Consider increasing the dose of HEMADY when used concomitantly with ephedrine.
Estrogens, Including Oral Contraceptives
Estrogens may decrease the hepatic metabolism of certain corticosteroids and increase exposures, which may increase the risk of adverse reactions [see Warnings and Precautions (5)Â and Adverse Reactions (6)].
7.2 Effect of HEMADY on Other Drugs
CYP3A4 Substrates
Coadministration of dexamethasone with drugs that are CYP3A4 substrates may decrease the concentration of these drugs. This may result in loss of efficacy of these drugs.
Oral Anticoagulants
Coadministration of anticoagulants with corticosteroids may reduce the response to anticoagulants [see Adverse Reactions (6)]. Frequently monitor coagulation indices to maintain the desired anticoagulant effect when administered with HEMADY.
Amphotericin B Injection and Potassium-Depleting Agents
Sodium retention with resultant edema and potassium loss may occur in patients receiving corticosteroids [see Warnings and Precautions (5.3), and Adverse Reactions (6)]. Closely monitor potassium levels when potassium-depleting agents are coadministered with HEMADY. In addition, there have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure.
Antidiabetics
Corticosteroids, including HEMADY, may increase blood glucose concentrations [see Warnings and Precautions (5.1)Â and Adverse Reactions (6)]. Consider adjusting the dose of antidiabetic agents, as necessary, when coadministered with HEMADY.
Isoniazid
Serum concentrations of isoniazid may be decreased with corticosteroids.
Cyclosporine
Increased activity of both cyclosporine and corticosteroids may occur when the two are used concurrently. Convulsions have been reported with this concurrent use.
Digitalis Glycosides
Patients on digitalis glycosides may be at increased risk of arrhythmias due to hypokalemia [see Warnings and Precautions (5.3)Â and Adverse Reactions (6)].
Nonsteroidal Anti-Inflammatory Agents (NSAIDS)
Concomitant use of aspirin (or other nonsteroidal anti-inflammatory agents) and corticosteroids increases the risk of gastrointestinal side effects [see Warnings and Precautions (5.7)Â and Adverse Reactions (6)]. The clearance of salicylates may be increased with concurrent use of corticosteroids. Monitor for toxicity when aspirin is used in conjunction with HEMADY in hypoprothrombinemia.
Phenytoin
In post-marketing experience, there have been reports of both increases and decreases in phenytoin levels with dexamethasone coadministration, leading to alterations in seizure control.
Vaccines
Patients on corticosteroid therapy may exhibit a diminished response to toxoids and live or inactivated vaccines due to inhibition of antibody response. Corticosteroids may also potentiate the replication of some organisms contained in live attenuated vaccines. If possible, defer routine administration of vaccines or toxoids until HEMADY therapy is discontinued [see Warnings and Precautions (5.5)].
Concomitant Therapies that May Increase the Risk of Thromboembolism
Erythropoietic agents, or other agents that may increase the risk of thromboembolism, such as estrogen containing therapies, coadministered with HEMADY may increase the risk of thromboembolism. Monitor for risk of thromboembolism in patients with MM receiving anti-myeloma products with HEMADY [see Warnings and Precautions (5.4)].
Thalidomide
Toxic epidermal necrolysis has been reported with concomitant use of thalidomide. Closely monitor for toxicity when thalidomide is coadministered with HEMADY.
7.3 Laboratory Test Interference
Skin Tests
Corticosteroids may suppress reactions to skin tests.
8 Use In Specific Populations
Lactation: Advise not to breastfeed.(8.2 )
8.1 Pregnancy
Risk Summary
Corticosteroids, including HEMADY, readily cross the placenta. Adverse developmental outcomes including orofacial clefts (cleft lip with or without cleft palate), intrauterine growth restriction, and decreased birth weight have been reported with maternal use of corticosteroids, including HEMADY, during pregnancy. In animal developmental and reproductive toxicology studies administration of corticosteroids to pregnant animals during organogenesis resulted in structural abnormalities, embryo-fetal mortality, functional impairment, and alterations to growth at doses equivalent to or below the recommended doses (see Data).
Advise pregnant women of the potential risk to a fetus.
HEMADY is administered in combination with anti-myeloma products that can cause embryo-fetal harm and are contraindicated for use in pregnancy. Refer to the Prescribing Information of the other anti-myeloma products used in combination with HEMADY for additional information.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Human Data
HEMADY should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Multiple courses of antenatal dexamethasone had been associated with reduced birth weight, susceptibility to infections, and increase blood glucose level in the newborns. Neonatal hypoglycemia was also reported. Infants born to mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.
Animal Data
In pregnant animals administered dexamethasone during organogenesis, doses equivalent to or below the recommended human dose have caused adverse developmental outcomes including structural abnormalities (cleft palate), alterations to growth (growth restrictions including reduced bone lengths and fetal weights), functional impairment (neurodevelopmental and metabolic effects), and embryo-fetal mortality (reduced number of embryonic implantations and fewer live fetuses).
8.2 Lactation
Risk Summary
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Advise women not to breastfeed during treatment and for 2 weeks after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential prior to initiating HEMADY [see Use in Specific Populations (8.1)].
HEMADY is used in combination with other anti-myeloma products that require pregnancy testing in females of reproductive potential. Refer to the Prescribing Information for the products used in combination with HEMADY.
Contraception
Advise patients of reproductive potential to use effective contraception during treatment with HEMADY and for at least one month following the final dose of HEMADY. HEMADY is used in combination with other anti-myeloma products that require contraception in females and males of reproductive potential. Refer to the Prescribing Information for the products used in combination with HEMADY.
Infertility
Males
Steroids may increase or decrease motility and number of spermatozoa in some patients. In animals, dexamethasone affects male spermatogenesis [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
No overall differences in safety or effectiveness were observed between elderly subjects and younger subjects, and other reported clinical experience with dexamethasone has not identified differences in responses between the elderly and younger patients. However, the incidence of corticosteroid-induced adverse reactions may be increased in geriatric patients and are dose-related.
Osteoporosis is the most frequently encountered complication, which occurs at a higher incidence rate in corticosteroid-treated geriatric patients as compared to younger populations and in age-matched controls. Losses of bone mineral density appear to be greatest early on in the course of treatment and may recover over time after steroid withdrawal or use of lower doses. Higher doses increase the relative risk of both vertebral and nonvertebral fractures, even in the presence of higher bone density compared to patients with involution osteoporosis. Perform routine screening of geriatric patients, including regular assessments of bone mineral density and institution of fracture prevention strategies, along with regular review of the dose of and need for continued dexamethasone therapy [see Warnings and Precautions (5.8)].
HEMADY is used in combination with other anti-myeloma products. Refer to the Prescribing Information of the other anti-myeloma products used as part of a combination regimen with HEMADY, for information on the use of those products in elderly patients.
10 Overdosage
Treatment of overdosage is by supportive and symptomatic therapy. In the case of acute overdosage, according to the patient’s condition, supportive therapy may include gastric lavage or induced vomiting.
11 Description
HEMADY (dexamethasone, USP) is an anti-inflammatory, 9-fluoro-glucocorticoid. The chemical name is 9-fluoro-11β,17,21trihydroxy-16α-methylpregna-1,4-diene-3,20-dione. The molecular weight is 392.47 g/mol. The molecular formula is C22H29FO5. The structural formula is:Â
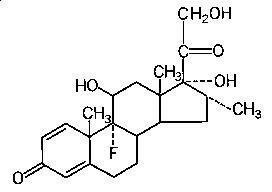
Dexamethasone is a white to practically white, odorless, crystalline powder. It is stable in air. It is practically insoluble in water. HEMADY for oral administration is available as an immediate-release tablet in a strength of 20 mg. Each tablet contains dexamethasone USP and the following inactive ingredients: corn starch NF, lactose monohydrate NF, magnesium stearate NF, povidone NF, and sodium starch glycolate NF.
12 Clinical Pharmacology
12.1 Mechanism of Action
Dexamethasone is a corticosteroid with anti-inflammatory effects and low mineralocorticoid activity. The precise mechanism of action in multiple myeloma is unknown. Dexamethasone induces apoptosis of multiple myeloma cells.
12.2 Pharmacodynamics
Following oral administration of a single dose of dexamethasone tablet to healthy subjects, the decrease in mean baseline cortisol concentration was maximal by 12 hours post-dose, with mean cortisol concentrations returning to near baseline approximately 3 days after drug administration.
12.3 Pharmacokinetics
The pharmacokinetics of oral dexamethasone were dose proportional between single dose of 0.5 to 40 mg. Following a single HEMADY dose of 20 mg, the geometric mean (coefficient of variation, %CV) dexamethasone peak concentrations (Cmax) was 247 ng/mL (31%) and area under the curve over time to infinity (AUCinf) was 1271 ng.hr/mL (31%) in subjects.
Absorption
Following 20 mg dose of HEMADY, dexamethasone median time to peak concentrations (Tmax) was 1 hour (range: 0.5 to 4 hours).
Effect of Food
A high-fat, high-calorie (total 800-1000 calories: approximately 60% from fat, 25% from carbohydrate and 15% from protein) meal had no effect on AUCinf and decreased Cmax by 23% of a single 20 mg dose of HEMADY.
Distribution
Dexamethasone is about 77% bound to human plasma proteins in vitro.
Elimination
The mean terminal half-life (coefficient of variation) of dexamethasone is 4 hours (18%) and oral clearance (CL/F) was 15.7 L/hr following a single dose of HEMADY.
Metabolism Â
Dexamethasone is metabolized by CYP3A4.
Excretion Â
Renal excretion of dexamethasone is less than 10% of total body clearance. Less than 10% of dexamethasone is excreted in the urine.
Specific Populations
The effect of baseline renal and hepatic impairment on the pharmacokinetics of dexamethasone has not been studied.
Drug Interactions Studies
Effect of Strong and Moderate CYP3A4 Inhibitors
Coadministration of itraconazole (strong CYP3A4 inhibitor: 200 mg once daily x 4 days) with a single dose of oral dexamethasone (4.5 mg) increased dexamethasone AUCinf by 3.7-fold [see Drug Interactions (7.1)].
Coadministration of aprepitant (moderate CYP3A4 inhibitor: 125 mg on Day 1, and 80 mg once daily on Days 2 to 5) with oral dexamethasone (20 mg on Day 1, and 8 mg once daily on Day 2-5) increased dexamethasone AUCinf by 2.2 -fold on Day 1 and 5 [see Drug Interactions (7.1)].
Effects of Other Anti-Myeloma Products
Coadministration of thalidomide, lenalidomide, pomalidomide, ixazomib, bortezomib or carfilzomib with dexamethasone is not expected to affect the pharmacokinetics of dexamethasone.
Effect on Other Anti-Myeloma Products Â
Coadministration of dexamethasone had no effect on the mean AUCinf of lenalidomide, pomalidomide, ixazomib, and bortezomib.
Coadministration of dexamethasone with carfilzomib or thalidomide is not expected to affect the pharmacokinetics of these drugs, as these drugs are not primarily metabolized by CYP3A4 in vitro.
For additional information on the drug interaction studies with dexamethasone and other anti-myeloma products, refer to the Prescribing Information of the other anti-myeloma products.
13 Nonclinical Toxicology
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
No adequate studies have been conducted in animals to determine whether corticosteroids have a potential for carcinogenesis.
Dexamethasone was tested for in vitro and in vivo genotoxic potential and was positive in the following assays: chromosomal aberrations and sister-chromatid exchanges in human lymphocytes, and micronuclei and sister-chromatid exchanges in mouse bone marrow. The Ames/Salmonella assay, with and without S9 mix, did not show an increase in His+ revertants.
Published literature identified reduced testicular spermatozoids and reduced spermatogenesis in male mice dosed intraperitoneally for 7 days at doses equivalent to the human dose based on a mg/m2 body surface area comparison.
16 How Supplied/storage And Handling
How Supplied
20 mg tablet: white, round, biconvex tablets embossed "20" on one side.
NDC 72893-015-24: Bottle of 24,
NDC 72893-015-06: Bottle of 100
Storage
Store at 20°C to 25°C (68°F to 77°F) excursions permitted 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant, child resistant container.
17 Patient Counseling Information
Discuss the following with patients prior to treatment with HEMADY:
Administration
- HEMADY is administered as part of combination regimens with anti-myeloma products; instruct patients to take HEMADY exactly as prescribed in the Prescribing Information of the anti-myeloma products administered with HEMADY [see Dosage and Administration (2.1)Â and Warning and Precautions (5.12)].
- Inform elderly patients regarding dose-reduction, if needed [see Dosage and Administration (2.2)Â and Use in Specific Populations (8.5)].
- Warn patients to not stop taking HEMADY abruptly or without first checking with their healthcare providers as there may be a need for gradual dose reduction to decrease the risk of adrenal insufficiency [see Warnings and Precautions (5.1)].
- HEMADY may be taken with or without food.
 Alterations in Endocrine Function Advise patients to inform any medical attendants that they are taking corticosteroids, as prolonged use may cause adrenal insufficiency, Cushing's syndrome and make patients dependent on corticosteroids. Instruct patients to notify their healthcare provider if they have diabetes, or thyroid gland problems as the dose of medications used to control these other conditions may need to be adjusted while they are taking HEMADY [see Warnings and Precautions (5.1)].
Advise the patient that, following prolonged therapy, withdrawal of corticosteroids may result in symptoms of the corticosteroid withdrawal syndrome including myalgia, arthralgia, and malaise. Advise patients to not discontinue use of HEMADY abruptly or without medical supervision [see Warnings and Precautions (5.1)].
Immunosuppression and Increased Risk of Infections Advise patients that they are at increased risk of infection. Tell patients to inform their healthcare provider if they have had recent or ongoing infections or if they have recently received a vaccine. Medical advice should be sought immediately if the patient develops fever or other signs of infection. Patients should be made aware that some infections can potentially be severe and fatal [see Warnings and Precautions (5.2)].
Warn patients who are on corticosteroids to avoid exposure to chickenpox or measles and to alert their healthcare provider immediately if they are exposed [see Warnings and Precautions (5.2)].
Alterations in Cardiovascular/Renal Function Inform patients that HEMADY can cause an increase in blood pressure and water retention. If this occurs, dietary salt restriction and potassium supplementation may be needed [see Warnings and Precautions (5.3)].
Venous and Arterial Thromboembolism Inform patients of the potential risk of developing venous and arterial thromboembolism and discuss the need for appropriate prophylactic treatment [see Warnings and Precautions (5.4)].
Vaccination Inform patients that they may receive concurrent vaccinations with use of HEMADY, except for live-attenuated or live vaccines [see Warnings and Precautions (5.5)].
Ophthalmic Effects Inform patients that HEMADY may cause cataracts or glaucoma and advise monitoring if corticosteroid therapy is continued for more than 6 weeks [see Warnings and Precautions (5.6)].
Gastrointestinal Perforation HEMADY may increase the risk of developing gastrointestinal perforation. Advise patients to promptly seek medical attention if they develop unusually severe, persistent or worsening abdominal pain. Warn patients to avoid corticosteroids if there is a possibility of gastrointestinal perforation [see Warnings and Precautions (5.7)].
Osteoporosis Advise patients about the risk of osteoporosis with prolonged use of HEMADY, which can predispose the patient to vertebral and long bone fractures [see Warnings and Precautions (5.8)].
Myopathy Advise patients to contact their healthcare provider if they experience new or worsening symptoms of myopathy such as unexplained muscle pain, tenderness or weakness [see Warnings and Precautions (5.9)].
Behavioral and Mood Disturbances Advise patients about the potential for severe behavioral and mood changes with HEMADY and encourage them to seek medical attention if psychiatric symptoms develop [see Warnings and Precautions (5.10)].
Kaposi’s Sarcoma Advise patients about the risk of Kaposi’s sarcoma in patients receiving corticosteroid therapy. Advise patients to discontinue HEMADY in case Kaposi’s sarcoma is diagnosed [see Warnings and Precautions (5.11)].
HEMADY in Combination with Anti-Myeloma Products Advise patients about the risk of adverse reactions which may occur when HEMADY is taken in combination with anti-myeloma products. Inform patients of the possible adverse reactions that could occur with the prescribed combination regimen, as detailed in the Prescribing Information of these products [see Warnings and Precautions (5.12)].
Embryo-Fetal Toxicity Advise females of reproductive potential of the potential risk to a fetus. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, during treatment with HEMADY [see Warnings and Precautions (5.13)Â and Use in Specific Populations (8.1)].
Drug Interactions Certain medications can cause an interaction with HEMADY. Advise patients to inform their healthcare provider of all the medicines the patient is taking, including over-the-counter medicines, dietary supplements, and herbal products. Inform patients that alternate therapy, dosage adjustment, and/or special test(s) may be needed during the treatment [see Drug Interactions (7.1, 7.2)].
Females and Males of Reproductive Potential Advise patients of reproductive potential to use effective contraception during treatment with HEMADY and for at least one month after the last dose [see Use in Specific Populations (8.3)].
Lactation Advise women not to breastfeed during treatment with HEMADY and for 2 weeks after the last dose [see Use in Specific Populations (8.2)].
Manufactured for: Dexcel Pharma Technologies Ltd Nahum Haftzadi 21, Givat Shaul Jerusalem, Israel 9548402
Distributed by: Acrotech Biopharma Inc East Windsor, NJ 08520
Package Label.principal Display Panel
Carton-24 Tablets-20 mg
Carton-100 Tablets-20 mg
 Label-24 Tablets-20 mg

Label-100 Tablets-20 mg

Â
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


