Imbruvica (ibrutinib 280 mg) Dailymed
Generic: ibrutinib is used for the treatment of Waldenstrom Macroglobulinemia Leukemia, Lymphocytic, Chronic, B-Cell Lymphoma, B-Cell Lymphoma, Mantle-Cell
IMPRINT: IBR 140 MG
SHAPE: capsule
COLOR: white
All Imprints
ibrutinib 140 mg oral tablet [imbruvica] - ibr 140 round green
ibrutinib 420 mg oral tablet [imbruvica] - ibr 420 oval green
ibrutinib 280 mg oral tablet [imbruvica] - ibr 280 oval purple
ibrutinib 560 mg oral tablet [imbruvica] - ibr 560 oval yellow
ibrutinib 140 mg - ibr 140 mg capsule white
Go PRO for all pill images
Recent Major Changes Section
Warnings and Precautions, Hepatotoxicity,Including Drug-Induced Liver Injury ( 5.7 )     5/2024
Indications & Usage Section
IMBRUVICA is a kinase inhibitor indicated for the treatment of:
- Adult patients with chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL) (
1.1 ).- Adult patients with chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL) with 17p deletion (
1.2 ).- Adult patients with Waldenström’s macroglobulinemia (WM) (
1.3 ). - Adult and pediatric patients age 1 year and older with chronic graft versus host disease (cGVHD) after failure of one or more lines of systemic therapy (
1.4 ).
IMBRUVICA is indicated for the treatment of adult patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL).
1.
IMBRUVICA is indicated for the treatment of adult patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) with 17p deletion.
1.
IMBRUVICA is indicated for the treatment of adult patients with Waldenström’s macroglobulinemia (WM).
1.
IMBRUVICA is indicated for the treatment of adult and pediatric patients age 1 year and older with chronic graft-versus-host disease (cGVHD) after failure of one or more lines of systemic therapy.
Dosage & Administration Section
- CLL/SLL and WM: 420 mg taken orally once daily (
2.1 ).- cGVHD: ‚ó¶ Patients 12 years and older: 420 mg taken orally once daily (
2.1 ).‚ó¶ Patients 1 to less than 12 years of age: 240 mg/m2 taken orally once daily (up to a dose of 420 mg) (2.1 ).
Tablets or capsules should be taken orally with a glass of water. Do not open, break, or chew the capsules. Do not cut, crush, or chew the tablets. See full prescribing information for oral suspension administration instructions (2.1 ).
2.1
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma and Waldenström’s Macroglobulinemia
The recommended dosage of IMBRUVICA for CLL/SLL and WM is 420 mg orally once daily until disease progression or unacceptable toxicity.
For CLL/SLL, IMBRUVICA can be administered as a single agent, in combination with rituximab or obinutuzumab, or in combination with bendamustine and rituximab (BR).
For WM, IMBRUVICA can be administered as a single agent or in combination with rituximab.
When administering IMBRUVICA in combination with rituximab or obinutuzumab, consider administering IMBRUVICA prior to rituximab or obinutuzumab when given on the same day.
Chronic Graft versus Host Disease
The recommended dosage of IMBRUVICA for patients age 12 years and older with cGVHD is 420 mg orally once daily, and for patients 1 to less than 12 years of age with cGVHD is 240 mg/m2 orally once daily (up to a dose of 420 mg), until cGVHD progression, recurrence of an underlying malignancy, or unacceptable toxicity. When a patient no longer requires therapy for the treatment of cGVHD, IMBRUVICA should be discontinued considering the medical assessment of the individual patient.
Table 1: Recommended dosage based on body surface area (BSA) for patients 1 to less than 12 years of age using either IMBRUVICA capsules/tablets or oral suspension Recommended dose to achieve 240 mg/m 2 BSA* (m 2 ) Range Dose (mg) of IMBRUVICA Capsules/Tablets to Administer Volume (mL) of IMBRUVICA Oral Suspension (70 mg/mL) to Administer > 0.3 to 0.4 - 1.2 mL > 0.4 to 0.5 - 1.5 mL > 0.5 to 0.6 - 1.9 mL > 0.6 to 0.7 - 2.2 mL > 0.7 to 0.8 210 mg 2.6 mL > 0.8 to 0.9 210 mg 2.9 mL > 0.9 to 1 210 mg 3.3 mL > 1 to 1.1 280 mg 3.6 mL > 1.1 to 1.2 280 mg 4 mL > 1.2 to 1.3 280 mg 4.3 mL > 1.3 to 1.4 350 mg 4.6 mL > 1.4 to 1.5 350 mg 5 mL > 1.5 to 1.6 350 mg 5.3 mL > 1.6 420 mg 6 mL
*BSA = body surface area.
Administration
Administer IMBRUVICA at approximately the same time each day.
Swallow tablets or capsules whole with a glass of water. Do not open, break, or chew the capsules. Do not cut, crush, or chew the tablets.
Follow Instructions for Use for further administration details of IMBRUVICA oral suspension.
If a dose of IMBRUVICA is not taken at the scheduled time, it can be taken as soon as possible on the same day with a return to the normal schedule the following day. Do not take extra doses of IMBRUVICA to make up for the missed dose.
2.2
For adverse reactions uled in Table 2, interrupt IMBRUVICA therapy. Once the adverse reaction has improved to Grade 1 or baseline (recovery), follow the recommended dosage modifications (see Table 2).
Table 2: Recommended Dosage Modifications for Adverse Reactions Adverse Reaction a,b Occurrence Dose Modification for CLL/SLL, WM, and Patients 12 Years or older with cGVHD After Recovery Starting Dose = 420 mg Dose Modification for Patients 1 Year to less than 12 Years with cGVHD After Recovery Starting Dose = 240 mg/m 2 Grade 2 cardiac failure First Restart at 280 mg dailyc Restart at 160 mg/m2 dailyc Second Restart at 140 mg dailyc Restart at 80 mg/m2 dailyc Third Discontinue IMBRUVICA Discontinue IMBRUVICA Grade 3 cardiac arrhythmias First Restart at 280 mg dailyc  Restart at 160 mg/m2 dailyc Second Discontinue IMBRUVICA Discontinue IMBRUVICA Grade 3 or 4 cardiac failure Grade 4 cardiac arrhythmias First Discontinue IMBRUVICA Discontinue IMBRUVICA Other Grade 3 or 4 non-hematological toxicitiesd Grade 3 or 4 neutropeniawith infection or feverGrade 4 hematologicaltoxicities First Restart at 280 mg daily Restart at 160 mg/m2 dailyc Second Restart at 140 mg daily Restart at 80 mg/m2 dailyc Third Discontinue IMBRUVICA Discontinue IMBRUVICA
a   [see Warnings and Precautions ( 5 ) ] .  
b Grading based on National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) criteria, or International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria for hematologic toxicities in CLL/SLL.
c Evaluate the benefit-risk before resuming treatment.
d For Grade 4 non-hematologic toxicities, evaluate the benefit-risk before resuming treatment.
Table 3: Recommended dosage modifications based on BSA using either IMBRUVICA capsules/tablets or oral suspension Recommended dose to achieve 160 mg/m 2 Recommended dose to achieve 80 mg/m 2 BSA* (m 2 ) Range Dose (mg) of IMBRUVICA Capsules/Tablets to Administer Volume (mL) of IMBRUVICA Oral Suspension (70 mg/mL) to Administer Dose (mg) of IMBRUVICA Capsules/Tablets to Administer Volume (mL) of IMBRUVICA Oral Suspension (70 mg/mL) to Administer > 0.3 to 0.4 - 0.8 mL - 0.4 mL > 0.4 to 0.5 - 1 mL - 0.5 mL > 0.5 to 0.6 - 1.3 mL - 0.6 mL > 0.6 to 0.7 - 1.5 mL - 0.7 mL > 0.7 to 0.8 140 mg 1.7 mL 70 mg 0.9 mL > 0.8 to 0.9 140 mg 1.9 mL 70 mg 1 mL > 0.9 to 1 140 mg 2.2 mL 70 mg 1.1 mL > 1 to 1.1 140 mg 2.4 mL 70 mg 1.2 mL > 1.1 to 1.2 210 mg 2.6 mL - 1.3 mL > 1.2 to 1.3 210 mg 2.9 mL - 1.4 mL > 1.3 to 1.4 210 mg 3.1 mL - 1.5 mL > 1.4 to 1.5 210 mg 3.3 mL 140 mg 1.7 mL > 1.5 to 1.6 280 mg 3.5 mL 140 mg 1.8 mL > 1.6 280 mg 4 mL 140 mg 2 mL
*BSA = body surface area.
2.3
Recommended dosage modifications are described below [see Drug Interactions ( 7.1 )]:
Table 4: Recommended Dosage Modifications for Use with CYP3A Inhibitors Patient Population Coadministered Drug Recommended IMBRUVICA Dosage B-cell Malignancies
- Moderate CYP3A inhibitor
280 mg once daily Modify dose as recommended [see Dosage and Administration ( 2.2 )].
- Voriconazole 200 mg twice daily
- Posaconazole suspension 100 mg once daily, 100 mg twice daily, or 200 mg twice daily
140 mg once daily Modify dose as recommended [see Dosage and Administration ( 2.2 )].
- Posaconazole suspension 200 mg three times daily or 400 mg twice daily
- Posaconazole intravenously 300 mg once daily
- Posaconazole delayed-release tablets 300 mg once daily
70 mg once daily Interrupt dose as recommended [see Dosage and Administration ( 2.2 )].
- Other strong CYP3A inhibitors
Avoid concomitant use. If these inhibitors will be used short-term (such as anti-infectives for seven days or less), interrupt IMBRUVICA. Patients 12 years and older with cGVHD
- Moderate CYP3A inhibitor
420 mg once daily Modify dose as recommended [see Dosage and Administration ( 2.2 )].
- Voriconazole 200 mg twice daily
- Posaconazole suspension 100 mg once daily, 100 mg twice daily, or 200 mg twice daily
280 mg once daily Modify dose as recommended [see Dosage and Administration ( 2.2 )].
- Posaconazole suspension 200 mg three times daily or 400 mg twice daily
- Posaconazole intravenously 300 mg once daily
- Posaconazole delayed-release tablets 300 mg once daily
140 mg once daily Interrupt dose as recommended [see Dosage and Administration ( 2.2 )].
- Other strong CYP3A inhibitors
Avoid concomitant use. If these inhibitors will be used short-term (such as anti-infectives for seven days or less), interrupt IMBRUVICA. Patients 1 year to less than 12 years of age with cGVHD
- Moderate CYP3A inhibitors
240 mg/m2 once daily Modify dose as recommended [see Dosage and Administration ( 2.2 )].
- Voriconazole for suspension 9 mg/kg (maximum dose: 350 mg) twice daily
160 mg/m2 once daily
- Posaconazole at any dosage
80 mg/m2 once daily
- Other strong CYP3A inhibitors
Avoid concomitant use. If these inhibitors will be used short-term (such as anti-infectives for seven days or less), interrupt IMBRUVICA.
After discontinuation of a CYP3A inhibitor, resume previous dose of IMBRUVICA [see Dosage and Administration ( 2.1 ), Drug Interactions ( 7.1 )].
2.4
Adult Patients with B-cell Malignancies
The recommended dosage is 140 mg daily for patients with mild hepatic impairment (Child-Pugh class A).
The recommended dosage is 70 mg daily for patients with moderate hepatic impairment (Child-Pugh class B).
Avoid the use of IMBRUVICA in patients with severe hepatic impairment (Child-Pugh class C) [see Use in Specific Populations ( 8.6 ), Clinical Pharmacology ( 12.3 )].
Patients with cGVHD
The recommended dosage is 140 mg daily for patients 12 years of age and older with total bilirubin level >1.5 to 3 x upper limit of normal (ULN) (unless of non-hepatic origin or due to Gilbert’s syndrome).
The recommended dosage is 80 mg/m2 daily for patients 1 to less than 12 years of age with total bilirubin level >1.5 to 3 x ULN (unless of non-hepatic origin or due to Gilbert’s syndrome).
Avoid the use of IMBRUVICA in these patients with total bilirubin level > 3 x ULN (unless of non-hepatic origin or due to Gilbert’s syndrome) [see Use in Specific Populations ( 8.6 ), Clinical Pharmacology ( 12.3 )].
Dosage Forms & Strengths Section
Capsules:
Each 70 mg capsule is a yellow, opaque capsule marked with ‚Äúibr 70 mg‚ÄĚ in black ink.
Each 140 mg capsule is a white, opaque capsule marked with ‚Äúibr 140 mg‚ÄĚ in black ink.
Tablets:
Each 140 mg tablet is a yellow green to green round tablet debossed with ‚Äúibr‚ÄĚ on one side and ‚Äú140‚ÄĚ on the other side.
Each 280 mg tablet is a purple oblong tablet debossed with ‚Äúibr‚ÄĚ on one side and ‚Äú280‚ÄĚ on the other side.
Each 420 mg tablet is a yellow green to green oblong tablet debossed with ‚Äúibr‚ÄĚ on one side and ‚Äú420‚ÄĚ on the other side.
Oral Suspension:
70 mg/mL, white to off-white suspension.
Capsules: 70 mg and 140 mg (3 )
Tablets: 140 mg, 280 mg, and 420 mg (3 )
Oral suspension: 70 mg/mL (3 )
Contraindications Section
None
None (4 )
Warnings And Precautions Section
- Hemorrhage: Monitor for bleeding and manage (
5.1 ).- Infections: Monitor patients for fever and infections, evaluate promptly, and treat (
5.2 ).- Cardiac Arrhythmias , Cardiac Failure , and Sudden Death: Monitor for symptoms of arrhythmias and cardiac failure and manage (
5.3 ).- Hypertension: Monitor blood pressure and treat (
5.4 ).- Cytopenias: Check complete blood counts monthly (
5.5 ).- Second Primary Malignancies: Other malignancies have occurred in patients, including skin cancers, and other carcinomas (
5.6 ).- Hepatotoxicity, Including Drug- Induced Liver Injury: Monitor hepatic function throughout treatment (
5.7 ).- Tumor Lysis Syndrome (TLS): Assess baseline risk and take precautions. Monitor and treat for TLS (
5.8 ).- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception (
5.9 ,8.1 ,8.3 ).5.1
Fatal bleeding events have occurred in patients who received IMBRUVICA. Major hemorrhage (‚Č•¬†Grade 3, serious, or any central nervous system events; e.g., intracranial hemorrhage [including subdural hematoma], gastrointestinal bleeding, hematuria, and post procedural hemorrhage) occurred in 4.2% of patients, with fatalities occurring in 0.4% of 2,838 patients who received IMBRUVICA in 27 clinical trials. Bleeding events of any grade including bruising and petechiae occurred in 39%, and excluding bruising and petechiae occurred in 23% of patients who received IMBRUVICA, respectively¬†[see Adverse Reactions ( 6.1 )].
The mechanism for the bleeding events is not well understood.
Use of either anticoagulant or antiplatelet agents concomitantly with IMBRUVICA increases the risk of major hemorrhage. Across clinical trials, 3.1% of 2,838 patients who received IMBRUVICA without antiplatelet or anticoagulant therapy experienced major hemorrhage. The addition of antiplatelet therapy with or without anticoagulant therapy increased this percentage to 4.4%, and the addition of anticoagulant therapy with or without antiplatelet therapy increased this percentage to 6.1%. Consider the risks and benefits of anticoagulant or antiplatelet therapy when co-administered with IMBRUVICA. Monitor for signs and symptoms of bleeding.
Consider the benefit-risk of withholding IMBRUVICA for at least 3 to 7 days pre- and post-surgery depending upon the type of surgery and the risk of bleeding [see Clinical Studies ( 14 )].
5.2
Fatal and non-fatal infections (including bacterial, viral, or fungal) have occurred with IMBRUVICA therapy. Grade 3 or greater infections occurred in 21% of 1,476 patients with B-cell malignancies who received IMBRUVICA in clinical trials [see Adverse Reactions ( 6.1 , 6.2 )]. Cases of progressive multifocal leukoencephalopathy (PML) and Pneumocystis jirovecii pneumonia (PJP) have occurred in patients treated with IMBRUVICA. Consider prophylaxis according to standard of care in patients who are at increased risk for opportunistic infections. Monitor and evaluate patients for fever and infections and treat appropriately.
Fatal and serious cardiac arrhythmias and cardiac failure have occurred with IMBRUVICA. Deaths due to cardiac causes or sudden deaths occurred in 1% of 4,896 patients who received IMBRUVICA in clinical trials, including in patients who received IMBRUVICA in unapproved monotherapy or combination regimens. These adverse reactions occurred in patients with and without preexisting hypertension or cardiac comorbidities. Patients with cardiac comorbidities may be at greater risk of these events.
Grade 3 or greater ventricular tachyarrhythmias were reported in 0.2%, Grade 3 or greater atrial fibrillation and atrial flutter were reported in 3.7%, and Grade 3 or greater cardiac failure was reported in 1.3% of 4,896 patients who received IMBRUVICA in clinical trials, including in patients who received IMBRUVICA in unapproved monotherapy or combination regimens. These events have occurred particularly in patients with cardiac risk factors including hypertension and diabetes mellitus, a previous history of cardiac arrhythmias, and in patients with acute infections [see Adverse Reactions ( 6.1 )].
Evaluate cardiac history and function at baseline, and monitor patients for cardiac arrhythmias and cardiac function. Obtain further evaluation (e.g., ECG, echocardiogram) as indicated for patients who develop symptoms of arrhythmia (e.g., palpitations, lightheadedness, syncope, chest pain), new onset dyspnea, or other cardiovascular concerns. Manage cardiac arrhythmias and cardiac failure appropriately, follow dose modification guidelines [see Dosage and Administration ( 2.2 )], and consider the risks and benefits of continued IMBRUVICA treatment.
Hypertension occurred in 19% of 1,476 patients with B-cell malignancies who received IMBRUVICA in clinical trials. Grade 3 or greater hypertension occurred in 8% of patients [see Adverse Reactions ( 6.1 )]. Based on data from a subset of these patients (N=1,124), the median time to onset was 5.9 months (range, 0 to 24 months). In a long-term safety analysis over 5 years of 1,284 patients with B-cell malignancies treated for a median of 36 months (range, 0 to 98 months), the cumulative rate of hypertension increased over time. The prevalence for Grade 3 or greater hypertension was 4% (year 0-1), 7% (year 1-2), 9% (year 2-3), 9% (year 3-4), and 9% (year 4-5); the overall incidence for the 5-year period was 11%.  
Monitor blood pressure in patients treated with IMBRUVICA, initiate or adjust anti-hypertensive medication throughout treatment with IMBRUVICA as appropriate, and follow dosage modification guidelines for Grade 3 or higher hypertension [see Dosage and Administration ( 2.2 )].
In 645 patients with B-cell malignancies who received IMBRUVICA as a single agent, grade 3 or 4 neutropenia occurred in 23% of patients, grade 3 or 4 thrombocytopenia in 8% and grade 3 or 4 anemia in 2.8%, based on laboratory measurements [see Adverse Reactions ( 6.1 )].
Monitor complete blood counts monthly.
5.6
Other malignancies (10%), including non-skin carcinomas (3.9%), occurred among the 1,476 patients with B-cell malignancies who received IMBRUVICA in clinical trials [see Adverse Reactions ( 6.1 )]. The most frequent second primary malignancy was non-melanoma skin cancer (6%).
5.7
Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of drug-induced liver injury (DILI), has occurred in patients treated with Bruton tyrosine kinase inhibitors, including IMBRUVICA.
Evaluate bilirubin and transaminases at baseline and throughout treatment with IMBRUVICA. For patients who develop abnormal liver tests after IMBRUVICA, monitor more frequently for liver test abnormalities and clinical signs and symptoms of hepatic toxicity. If DILI is suspected, withhold IMBRUVICA. Upon confirmation of DILI, discontinue IMBRUVICA.
5.
Tumor lysis syndrome has been infrequently reported with IMBRUVICA [see Adverse Reactions ( 6.2 )]. Assess the baseline risk (e.g., high tumor burden) and take appropriate precautions. Monitor patients closely and treat as appropriate.
5.
Based on findings in animals, IMBRUVICA can cause fetal harm when administered to a pregnant woman. Administration of ibrutinib to pregnant rats and rabbits during the period of organogenesis caused embryo-fetal toxicity including malformations at exposures that were 3-20 times higher than those reported in patients with hematologic malignancies. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with IMBRUVICA and for 1 month after the last dose. [see Use in Specific Populations ( 8.1 )].
Adverse Reactions Section
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hemorrhage [see Warnings and Precautions ( 5.1 )]
- Infections [see Warnings and Precautions ( 5.2 )]
- Cardiac Arrhythmias, Cardiac Failure, and Sudden Death [see Warnings and Precautions ( 5.3 )]
- Hypertension [see Warnings and Precautions ( 5.4 )]
- Cytopenias [see Warnings and Precautions ( 5.5 )]
- Second Primary Malignancies [see Warnings and Precautions ( 5.6 )]
- Hepatotoxicity, including DILI [see Warning s and Precautions ( 5.7 )]
- Tumor Lysis Syndrome [see Warnings and Precautions ( 5.8 )]
- The most common (‚Č•30%) adverse reactions in patients with B-cell malignancies¬†are thrombocytopenia, diarrhea, fatigue, musculoskeletal pain, neutropenia, rash, anemia,¬†bruising, and nausea¬†(
6 ).- The most common (‚Č•20%) adverse reactions in adult or pediatric patients with cGVHD are fatigue, anemia, bruising, diarrhea, thrombocytopenia, musculoskeletal pain, pyrexia, muscle spasms, stomatitis, hemorrhage, nausea, abdominal pain, pneumonia, and headache (
6 ).
To report SUSPECTED ADVERSE REACTIONS, contact Pharmacyclics at 1-877-877-3536 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1
Because clinical trials are conducted under widely variable conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared with rates of clinical trials of another drug and may not reflect the rates observed in practice.
Unless otherwise specified, the¬†pooled safety population described¬†in the WARNINGS AND PRECAUTIONS reflects exposure to IMBRUVICA in 6 trials. IMBRUVICA was ¬†administered as a single agent at 420 mg orally once daily (475 patients), as a single agent¬†at 560 mg orally once daily¬†[1.3 times the recommended adult dosage¬†(174 patients)], and in combination with other drugs at 420 mg orally once daily (827 patients) in patients with B-cell malignancies. In this pooled safety population of 1,476 patients, 87% were exposed for 6 months or longer and 68% were exposed for greater than one year. The most common adverse reactions (‚Č•¬†30%) were thrombocytopenia, diarrhea, fatigue, musculoskeletal pain, neutropenia, rash, anemia,¬†bruising, and nausea.
Certain subsections in the WARNINGS AND PRECAUTIONS include patients who received IMBRUVICA in unapproved monotherapy or combination regimens.
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
The data described below reflect exposure to IMBRUVICA in one single-arm, open-label clinical trial (Study¬†1102) and five randomized controlled clinical trials (RESONATE, RESONATE-2, HELIOS, iLLUMINATE, and E1912) in patients with CLL/SLL (n=2,016 total, including n=1,133¬†patients exposed to IMBRUVICA). In general, patients with creatinine clearance (CLcr) ‚ȧ 30 mL/min, AST or ALT ‚Č• 2.5 x ULN, or total bilirubin ‚Č• 1.5¬†x ULN (unless of non-hepatic origin) were excluded from these trials. In Study E1912, patients with AST or ALT > 3 x ULN or total bilirubin > 2.5 x ULN were excluded. Study 1102 included 51 patients with previously treated CLL/SLL. RESONATE included 386 randomized patients with previously treated CLL or SLL who received single agent IMBRUVICA or ofatumumab. RESONATE-2 included 267¬†randomized patients with treatment na√Įve CLL or SLL who were 65 years or older and received single agent IMBRUVICA or chlorambucil. HELIOS included 574 randomized patients with previously treated CLL or SLL who received IMBRUVICA in combination with BR or placebo in combination with BR. iLLUMINATE included 228 randomized patients with treatment na√Įve CLL/SLL who were 65 years or older or with coexisting medical conditions and received IMBRUVICA in combination with obinutuzumab or chlorambucil in combination with obinutuzumab. E1912 included 510 patients with previously untreated CLL/SLL who were 70¬†years or younger and received IMBRUVICA in combination with rituximab or received fludarabine, cyclophosphamide, and rituximab (FCR).
The most common adverse reactions in patients with CLL/SLL receiving IMBRUVICA (‚Č• 30%) were thrombocytopenia, diarrhea, fatigue, musculoskeletal pain, neutropenia, rash, anemia, bruising, and nausea.
Four to 10 percent of patients with CLL/SLL receiving IMBRUVICA discontinued treatment due to adverse reactions. These included pneumonia, hemorrhage, atrial fibrillation, neutropenia, arthralgia, rash, and thrombocytopenia. Adverse reactions leading to dose reduction occurred in approximately 9% of patients.
Study 1102
Adverse reactions and laboratory abnormalities from Study 1102 (N=51) using single agent IMBRUVICA 420 mg daily in patients with previously treated CLL/SLL occurring at a rate of ‚Č• 10% with a median duration of treatment of 15.6 months are presented in Table¬†5¬†and¬†Table¬†6.
Table 5: Non-Hematologic Adverse Reactions in ‚Č• 10% of Patients with CLL/SLL (N=51) in Study 1102 Body System Adverse Reaction All Grades (%) Grade 3 or Higher (%) Gastrointestinal disorders DiarrheaConstipationNauseaStomatitisVomitingAbdominal painDyspepsia 59222020181412 4220200 Skin and subcutaneous tissue disorders Bruising Rash Petechiae 512516 200 Infections and infestations Upper respiratory tract infectionSinusitisSkin infectionPneumoniaUrinary tract infection 4722161212 266102 General disorders and administration site conditions FatiguePyrexia Peripheral edemaAstheniaChills 3324221412 62060 Musculoskeletal and connective tissue disorders Musculoskeletal painArthralgiaMuscle spasms 252418 602 Respiratory, thoracic and mediastinal disorders CoughOropharyngeal painDyspnea 221412 000 Nervous system disorders DizzinessHeadache 2018 02 Vascular disorders Hypertension 16 8 Metabolism and nutrition disorders Decreased appetite 16 2 Neoplasms benign, malignant, unspecified Second malignancies 10 2‚Ć
†One patient death due to histiocytic sarcoma.
Table 6: Treatment-Emergent* Hematologic Laboratory Abnormalities in Patients with CLL/SLL (N=51) in Study 1102 Percent of Patients (N=51) All Grades (%) Grade 3 or 4 (%) Platelets decreased 69 12 Neutrophils decreased 53 26 Hemoglobin decreased 43 0
* Based on laboratory measurements per IWCLL criteria and adverse reactions.
Treatment-emergent Grade 4 thrombocytopenia (8%) and neutropenia (12%) occurred in patients.
RESONATE
Adverse reactions and laboratory abnormalities described below in Table 7 and Table 8 reflect exposure to IMBRUVICA with a median duration of 8.6 months and exposure to ofatumumab with a median of 5.3 months in RESONATE in patients with previously treated CLL/SLL.
Table 7: Adverse Reactions Reported in ‚Č• 10% of Patients in the IMBRUVICA Treated Arm in Patients with CLL/SLL in RESONATE Body System Adverse Reaction IMBRUVICA (N=195) Ofatumumab (N=191) All Grades (%) Grade 3 or Higher¬†(%) All Grades (%) Grade 3 or Higher¬†(%) Gastrointestinal disorders Diarrhea 48 4 18 2 Nausea 26 2 18 0 Stomatitis* 17 1 6 1 Constipation 15 0 9 0 Vomiting 14 0 6 1 Musculoskeletal and connective tissue disorders Musculoskeletal pain* 28 2 18 1 Arthralgia 17 1 7 0 Muscle spasms 13 0 8 0 Skin and subcutaneous tissue disorders Rash* 24 3 13 0 Petechiae 14 0 1 0 Bruising* 12 0 1 0 General disorders and administration site conditions Pyrexia 24 2 15 2‚Ć Respiratory, thoracic and mediastinal disorders ¬†¬†¬†Cough 19 0 23 1 ¬†¬†¬†Dyspnea 12 2 10 1 Infections and infestations Upper respiratory tract infection 16 1 11 2‚Ć Pneumonia* 15 12‚Ć 13 10‚Ć Sinusitis* 11 1 6 0 Urinary tract infection 10 4 5 1 Nervous system disorders Headache 14 1 6 0 Dizziness 11 0 5 0 Injury, poisoning and procedural complications Contusion 11 0 3 0 Eye disorders Vision blurred 10 0 3 0 The body system and individual ADR terms are sorted in descending frequency order in the IMBRUVICA arm.* Includes multiple ADR terms. ‚Ƭ†Includes 3 events of pneumonia with fatal outcome in each arm, and 1 event of pyrexia and upper respiratory tract infection with a fatal outcome in the ofatumumab arm.
Table 8: Treatment-Emergent Hematologic Laboratory Abnormalities in Patients with CLL/SLL in RESONATE          IMBRUVICA (N=195) Ofatumumab (N=191) All Grades (%) Grade 3 or 4 (%) All Grades (%) Grade 3 or 4 (%) Neutrophils decreased 51 23 57 26 Platelets decreased 52 5 45 10 Hemoglobin decreased 36 0 21 0
Treatment-emergent Grade 4 thrombocytopenia (2% in the IMBRUVICA arm vs 3% in the ofatumumab arm) and neutropenia (8% in the IMBRUVICA arm vs 8% in the ofatumumab arm) occurred in patients.
RESONATE-2
Adverse reactions and laboratory abnormalities described below in Table 9 and Table 10 reflect exposure to IMBRUVICA with a median duration of 17.4 months. The median exposure to chlorambucil was 7.1 months in RESONATE-2.
Table 9: Adverse Reactions Reported in ‚Č• 10% of Patients in the IMBRUVICA Treated Arm in Patients with CLL/SLL in RESONATE-2 Body System Adverse Reaction IMBRUVICA (N=135) Chlorambucil (N=132) All Grades (%) Grade 3 or Higher¬†(%) All Grades (%) Grade 3 or Higher¬†(%) Gastrointestinal disorders ¬†¬†¬†¬†Diarrhea 42 4 17 0 ¬†¬†¬†¬†Nausea 22 1 39 1 ¬†¬†¬†¬†Constipation 16 1 16 0 ¬†¬†¬†¬†Stomatitis* 14 1 4 1 ¬†¬†¬†¬†Vomiting 13 0 20 1 ¬†¬†¬†¬†Abdominal pain 13 3 11 1 ¬†¬†¬†¬†Dyspepsia 11 0 2 0 Musculoskeletal and connective tissue disorders ¬†¬†¬†¬†Musculoskeletal pain* 36 4 20 0 ¬†¬†¬†¬†Arthralgia 16 1 7 1 ¬†¬†¬†¬†Muscle spasms 11 0 5 0 General disorders and administration site conditions ¬†¬†¬†¬†Fatigue 30 1 38 5 ¬†¬†¬†¬†Peripheral edema 19 1 9 0 ¬†¬†¬†¬†Pyrexia 17 0 14 2 Respiratory, thoracic and mediastinal disorders ¬†¬†¬†¬†Cough 22 0 15 0 ¬†¬†¬†¬†Dyspnea 10 1 10 0 Skin and subcutaneous tissue disorders ¬†¬†¬†¬†Rash* 21 4 12 2 ¬†¬†¬†¬†Bruising* 19 0 7 0 Eye disorders ¬†¬†¬†¬†Dry eye 17 0 5 0 ¬†¬†¬†¬†Lacrimation increased 13 0 6 0 ¬†¬†¬†¬†Vision blurred 13 0 8 0 ¬†¬†¬†¬†Visual acuity reduced 11 0 2 0 Infections and infestations ¬†¬†¬†¬†Upper respiratory tract infection 17 2 17 2 ¬†¬†¬†¬†Skin infection* 15 2 3 1 ¬†¬†¬†¬†Pneumonia* 14 8 7 4 ¬†¬†¬†¬†Urinary tract infections 10 1 8 1 Vascular disorders ¬†¬†¬†¬†Hypertension* 14 4 1 0 Nervous system disorders ¬†¬†¬†¬†Headache 12 1 10 2 ¬†¬†¬†¬†Dizziness 11 0 12 1 Investigations ¬†¬†¬†¬†Weight decreased 10 0 12 0
Subjects with multiple events for a given ADR term are counted once only for each ADR term.
The body system and individual ADR terms are sorted in descending frequency order in the IMBRUVICA arm.
* Includes multiple ADR terms. 
Table 10: Treatment-Emergent Hematologic Laboratory Abnormalities in Patients with CLL/SLL in RESONATE-2 IMBRUVICA (N=135) Chlorambucil (N=132) All Grades (%) Grade 3 or 4 (%) All Grades (%) Grade 3 or 4 (%) Neutrophils Decreased 55 28 67 31 Platelets Decreased 47 7 58 14 Hemoglobin Decreased 36 0 39 2
Treatment-emergent Grade 4 thrombocytopenia (1% in the IMBRUVICA arm vs 3% in the chlorambucil arm) and neutropenia (11% in the IMBRUVICA arm vs 12% in the chlorambucil arm) occurred in patients.
HELIOS
Adverse reactions described below in Table 11 reflect exposure to IMBRUVICA + BR with a median duration of 14.7 months and exposure to placebo + BR with a median of 12.8 months in HELIOS in patients with previously treated CLL/SLL.
Table 11: Adverse Reactions Reported in ‚Č•¬†10% of Patients and ‚Č•¬†2% Greater in the IMBRUVICA Arm in Patients with CLL/SLL in HELIOS Body System Adverse Reaction IMBRUVICA + BR (N=287) Placebo + BR (N=287) All Grades (%) Grade 3 or Higher¬†(%) All Grades (%) Grade 3 or Higher¬†(%) Blood and lymphatic system disorders Neutropenia* 66 61 60 56‚Ć Thrombocytopenia* 34 16 26 16 Gastrointestinal disorders Diarrhea 36 2 23 1 Abdominal pain 12 1 8 <1 Skin and subcutaneous tissue disorders ¬† ¬†¬†¬†¬†¬†¬†Rash* 32 4 25 1 ¬†¬†¬†¬†¬†¬†Bruising¬†* 20 <1 8 <1 Musculoskeletal and connective tissue disorders ¬†¬†¬†¬†¬†¬†Musculoskeletal pain* 29 2 20 0 ¬†¬†¬†¬†¬†¬†Muscle spasms 12 <1 5 0 General disorders and administration site conditions ¬†¬†¬†¬†¬†¬†Pyrexia 25 4 22 2 Vascular disorders ¬†¬†¬†¬†¬†¬†Hemorrhage* 19 2‚Ć 9 1 ¬†¬†¬†¬†¬†¬†Hypertension* 11 5 5 2 Infections and infestations ¬†¬†¬†¬†¬†¬†Bronchitis 13 2 10 3 ¬†¬†¬†¬†¬†¬†Skin infection* 10 3 6 2 Metabolism and nutrition disorders ¬†¬†¬†¬†¬†¬†Hyperuricemia 10 2 6 0
The body system and individual ADR terms are sorted in descending frequency order in the IMBRUVICA arm.
* Includes multiple ADR terms.
<1 used for frequency above 0 and below 0.5%.
† Includes 2 events of hemorrhage with fatal outcome in the IMBRUVICA arm and 1 event of neutropenia with a fatal outcome in the placebo + BR arm.
Atrial fibrillation of any grade occurred in 7% of patients treated with IMBRUVICA + BR and 2% of patients treated with placebo + BR. The frequency of Grade 3 and 4 atrial fibrillation was 3% in patients treated with IMBRUVICA + BR and 1% in patients treated with placebo + BR.
iLLUMINATE
Adverse reactions described below in Table 12 reflect exposure to IMBRUVICA + obinutuzumab with a median duration of 29.3 months and exposure to chlorambucil + obinutuzumab with a median of 5.1 months in iLLUMINATE in patients with previously untreated CLL/SLL.
Table 12: Adverse Reactions Reported in ‚Č•¬†10% of Patients in the IMBRUVICA Arm in Patients with CLL/SLL in iLLUMINATE Body System Adverse Reaction IMBRUVICA + Obinutuzumab (N=113) Chlorambucil + Obinutuzumab (N=115) All Grades (%) Grade 3 or Higher¬†(%) All Grades (%) Grade 3 or Higher¬†(%) Blood and lymphatic system disorders ¬†¬†¬†¬†¬†¬†Neutropenia* 48 39 64 48 ¬†¬†¬†¬†¬†¬†Thrombocytopenia* 36 19 28 11 ¬†¬†¬†¬†¬†¬†Anemia 17 4 25 8 Skin and subcutaneous tissue disorders ¬† ¬†¬†¬†¬†¬†¬†Rash* 36 3 11 0 ¬†¬†¬†¬†¬†¬†Bruising* 32 3 3 0 Gastrointestinal disorders ¬†¬†¬†¬†¬†¬†Diarrhea 34 3 10 0 ¬†¬†¬†¬†¬†¬†Constipation 16 0 12 1 ¬†¬†¬†¬†¬†¬†Nausea 12 0 30 0 Musculoskeletal and connective tissue disorders ¬†¬†¬†¬†¬†¬†Musculoskeletal pain* 33 1 23 3 ¬†¬†¬†¬†¬†¬†Arthralgia 22 1 10 0 ¬†¬†¬†¬†¬†¬†Muscle spasms 13 0 6 0 Respiratory, thoracic and mediastinal disorders ¬†¬†¬†¬†¬†¬†Cough 27 1 12 0 Injury, poisoning and procedural complications ¬†¬†¬†¬†¬†¬†Infusion related reaction 25 2 58 8 Vascular disorders ¬†¬†¬†¬†¬†¬†Hemorrhage* 25 1 9 0 ¬†¬†¬†¬†¬†¬†Hypertension* 17 4 4 3 General disorders and administration site conditions ¬†¬†¬†¬†¬†¬†Pyrexia 19 2 26 1 ¬†¬†¬†¬†¬†¬†Fatigue 18 0 17 2 ¬†¬†¬†¬†¬†¬†Peripheral edema 12 0 7 0 Infections and infestations ¬†¬†¬†¬†¬†¬†Pneumonia* 16 9 9 4‚Ć ¬†¬†¬†¬†¬†¬†Upper respiratory tract¬†¬†¬†¬†¬†¬†¬†infection 14 1 6 0 ¬†¬†¬†¬†¬†¬†Skin infection* 13 1 3 0 ¬†¬†¬†¬†¬†¬†Urinary tract infection 12 3 7 1 ¬†¬†¬†¬†¬†¬†Nasopharyngitis 12 0 3 0 ¬†¬†¬†¬†¬†¬†Conjunctivitis 11 0 2 0 Metabolism and nutrition disorders ¬†¬†¬†¬†¬†¬†Hyperuricemia 13 1 0 0 Cardiac disorders ¬†¬†¬†¬†¬†¬†Atrial fibrillation 12 5 0 0 Psychiatric disorders ¬†¬†¬†¬†¬†¬†Insomnia 12 0 4 0
The body system and individual ADR terms are sorted in descending frequency order in the IMBRUVICA arm.
* Includes multiple ADR terms.
† Includes one event with a fatal outcome.
E1912
Adverse reactions described below in Table 13 reflect exposure to IMBRUVICA + rituximab with a median duration of 34.3 months and exposure to FCR with a median of 4.7 months in E1912 in patients with previously untreated CLL/SLL who were 70 years or younger.
Table 13: Adverse Reactions Reported in ‚Č•¬†15% of Patients in the IMBRUVICA Arm in Patients with CLL/SLL in E1912 Body System Adverse Reaction IMBRUVICA + Rituximab (N=352) Fludarabine + Cyclophosphamide + Rituximab (N=158) All Grades (%) Grade 3 or Higher¬†(%) All Grades (%) Grade 3 or Higher¬†(%) General disorders and administration site conditions ¬†¬†¬†¬†¬†¬†Fatigue 80 2 78 3 ¬†¬†¬†¬†¬†¬†Peripheral edema 28 1 17 0 ¬†¬†¬†¬†¬†¬†Pyrexia 27 1 27 1 ¬†¬†¬†¬†¬†¬†Pain 23 2 8 0 Musculoskeletal and connective tissue disorders ¬†¬†¬†¬†¬†¬†Musculoskeletal pain* 61 5 35 2 ¬†¬†¬†¬†¬†¬†Arthralgia 41 5 10 1 Gastrointestinal disorders ¬†¬†¬†¬†¬†¬†Diarrhea 53 4 27 1 ¬†¬†¬†¬†¬†¬†Nausea 40 1 64 1 ¬†¬†¬†¬†¬†¬†Stomatitis* 22 1 8 1 ¬†¬†¬†¬†¬†¬†Abdominal pain* 19 2 10 1 ¬†¬†¬†¬†¬†Vomiting 18 2 28 0 ¬†¬†¬†¬†¬†¬†Constipation 17 0 32 0 Skin and subcutaneous tissue disorders ¬†¬†¬†¬†¬†¬†Rash* 49 4 29 5 ¬†¬†¬†¬†¬†¬†Bruising* 36 1 4 1 Vascular disorders ¬†¬†¬†¬†¬†¬†Hypertension* 42 19 22 6 ¬†¬†¬†¬†¬†¬†Hemorrhage* 31 2 8 1 Nervous system disorders ¬†¬†¬†¬†¬†¬†Headache 40 1 27 1 ¬†¬†¬†¬†¬†¬†Dizziness 21 1 13 1 ¬†¬†¬†¬†¬†¬†Peripheral neuropathy* 19 1 13 1 Respiratory, thoracic and mediastinal disorders ¬†¬†¬†¬†¬†¬†Cough 32 0 25 0 ¬†¬†¬†¬†¬†¬†Dyspnea 22 2 21 1 Infections and infestations ¬†¬†¬†¬†¬†¬†Upper respiratory tract¬† 29 1 19 2 ¬†¬†¬†¬†¬†¬†infection ¬†¬†¬†¬†¬†¬†Skin infection* 16 1 3 1 Metabolism and nutrition disorders ¬†¬†¬†¬†¬†¬†Hyperuricemia 19 1 4 0 ¬†¬†¬†¬†¬†¬†Decreased appetite 15 0 20 1 Psychiatric disorders ¬†¬†¬†¬†¬†¬†Insomnia 16 1 19 1
The body system and individual ADR terms are sorted in descending frequency order in the IMBRUVICA arm.
* Includes multiple ADR terms.
Table 14: Select Laboratory Abnormalities (‚Č• 15% Any Grade), New or Worsening from Baseline in Patients Receiving IMBRUVICA (E1912) IMBRUVICA + Rituximab (N=352) Fludarabine + Cyclophosphamide + Rituximab (N=158) All Grades (%) Grade 3 or 4 (%) All Grades (%) Grade 3 or 4 (%) Hematology abnormalities ¬†¬†¬†¬†¬†Neutrophils decreased¬†¬†¬†¬†¬†Platelets decreased¬†¬†¬†¬†¬†Hemoglobin decreased 534326 3070 706951 44252 Chemistry abnormalities ¬†¬†¬†¬†¬†Creatinine increased¬†¬†¬†¬†¬†Bilirubin increased¬†¬†¬†¬†¬†AST increased 383025 123 171523 10<1
Based on laboratory measurements per IWCLL criteria.
Waldenström’s Macroglobulinemia
The data described below reflect exposure to IMBRUVICA in two¬†single-arm clinical trials (Study 1118¬†and the INNOVATE monotherapy arm) and one randomized controlled trial (INNOVATE), including a total of 169 patients with WM exposed to IMBRUVICA. Study 1118 included 63¬†patients with previously treated WM who received single agent IMBRUVICA. INNOVATE included 150 patients with treatment na√Įve or previously treated WM who received IMBRUVICA or placebo in combination with rituximab. The INNOVATE monotherapy arm included 31¬†patients with previously treated WM who received IMBRUVICA after failure of prior rituximab-containing therapy.
The most common adverse reactions in Studies 1118 and INNOVATE (‚Č•¬†20%) were neutropenia, diarrhea, bruising, thrombocytopenia, hemorrhage, musculoskeletal pain,¬†rash, and nausea.
Five percent of patients receiving IMBRUVICA across Studies 1118 and INNOVATE discontinued treatment due to adverse reactions. The most common adverse reaction leading to discontinuation was atrial fibrillation. Adverse reactions leading to dose reduction occurred in 14% of patients.
Study 1118 and INNOVATE Monotherapy Arm
Adverse reactions and laboratory abnormalities described below in Table 15 and Table 16 reflect exposure to IMBRUVICA with a median duration of 11.7 months in Study 1118 and 33 months in the INNOVATE Monotherapy Arm.
Table 15: Non-Hematologic Adverse Reactions in ‚Č• 10% in Patients with WM in Study 1118 and the INNOVATE Monotherapy Arm (N=94) Body System Adverse Reaction All Grades (%) Grade 3 or ¬† Higher¬†(%) Gastrointestinal disorders DiarrheaNauseaStomatitis*ConstipationGastroesophageal reflux disease 3821151212 20010 Skin and subcutaneous tissue disorders Bruising*Rash* 2821 11 Vascular disorders Hemorrhage*Hypertension* 2814 04 General disorders and administrative site conditions FatiguePyrexia 1812 22 Musculoskeletal and connective tissue disorders Musculoskeletal pain*Muscle spasms 2119 00 Infections and infestations Upper respiratory tract infectionSkin infection*Sinusitis*Pneumonia* 19181613 0305 Nervous system disorders HeadacheDizziness 1413 00 Respiratory, thoracic and mediastinal disorders Cough 13 0
The body system and individual ADR preferred terms are sorted in descending frequency order.
* Includes multiple ADR terms.
Table 16: Treatment-Emergent Hematologic Laboratory Abnormalities in Patients with WM in Study 1118 and the INNOVATE Monotherapy Arm (N=94) Percent of Patients (N=94) All Grades (%) Grade 3 or 4 (%) Platelets Decreased 38 11 Neutrophils Decreased 43 16 Hemoglobin Decreased 21 6
Treatment-emergent Grade 4 thrombocytopenia (4%) and neutropenia (7%) occurred in patients. 
INNOVATE
Adverse reactions described below in Table¬†17¬†reflect exposure to IMBRUVICA + R with a median duration of 25.8 months and exposure to placebo¬†+¬†R with a median duration of 15.5¬†months in patients with treatment na√Įve or previously treated WM in INNOVATE.
Table 17: Adverse Reactions Reported in ‚Č•¬†10% of Patients and ‚Č•¬†2% Greater in the IMBRUVICA Arm in Patients with WM in INNOVATE Body System Adverse Reaction IMBRUVICA + R (N=75) Placebo + R (N=75) All Grades (%) Grade 3 or Higher (%) All Grades (%) Grade 3 or Higher (%) Skin and subcutaneous tissue disorders ¬†¬†¬†¬†¬†Bruising* 37 1 5 0 ¬†¬†¬†¬†¬†Rash* 24 1 11 0 Musculoskeletal and connective tissue disorders ¬†¬†¬†¬†¬†Musculoskeletal pain* 35 4 21 3 ¬†¬†¬†¬†¬†Arthralgia 24 3 11 1 ¬†¬†¬†¬†¬†Muscle spasms 17 0 12 1 Vascular disorders ¬†¬†¬†¬†¬†Hemorrhage* 32 3 17 4‚Ć ¬†¬†¬†¬†¬†Hypertension* 20 13 5 4 Gastrointestinal disorders ¬†¬†¬†¬†¬†Diarrhea 28 0 15 1 ¬†¬†¬†¬†¬†Nausea 21 0 12 0 ¬†¬†¬†¬†¬†Dyspepsia 16 0 1 0 ¬†¬†¬†¬†¬†Constipation 13 1 11 1 Infections and infestations ¬†¬†¬†¬†¬†Pneumonia* 19 13 5 3 ¬†¬†¬†¬†¬†Skin infection* 17 3 3 0 ¬†¬†¬†¬†¬†Urinary tract infection 13 0 0 0 ¬†¬†¬†¬†¬†Bronchitis 12 3 7 0 ¬†¬†¬†¬†¬†Influenza 12 0 7 1 ¬†¬†¬†¬†¬†Viral upper respiratory tract infection 11 0 7 0 General disorders and administration site conditions ¬†¬†¬†¬†¬†Peripheral edema 17 0 12 1 Respiratory, thoracic, and mediastinal disorders ¬†¬†¬†¬†¬†Cough 17 0 11 0 Blood and lymphatic system disorders ¬†¬†¬†¬†¬†Neutropenia* 16 12 11 4 Cardiac disorders ¬†¬†¬†¬†¬†Atrial fibrillation 15 12 3 1 Nervous system disorders ¬†¬†¬†¬†¬†Dizziness 11 0 7 0 Psychiatric disorders ¬†¬†¬†¬†¬†Insomnia 11 0 4 0 Metabolism and nutrition disorders ¬†¬†¬†¬†¬†Hypokalemia 11 0 1 1
The body system and individual ADR preferred terms are sorted in descending frequency order.
* Includes multiple ADR terms.
† Includes one event with a fatal outcome.
Grade 3 or 4 infusion related reactions were observed in 1% of patients treated with IR.
Chronic Graft versus Host Disease
Study 1129
The data described below reflect exposure to IMBRUVICA in an open-label clinical trial (Study 1129) that included 42 patients with cGVHD after failure of first line corticosteroid therapy and required additional therapy [see Clinical Studies ( 14.3 )].
The most common adverse reactions in Study 1129 (‚Č• 20%) were fatigue, bruising, diarrhea, thrombocytopenia, stomatitis, muscle spasms, nausea, hemorrhage, anemia, and pneumonia. Atrial fibrillation occurred in one patient (2%) which was Grade 3.
Twenty-four percent of patients receiving IMBRUVICA in Study 1129 discontinued treatment due to adverse reactions. The most common adverse reactions leading to discontinuation were fatigue and pneumonia. Adverse reactions leading to dose reduction occurred in 26% of patients.
Adverse reactions and laboratory abnormalities described below in Table 18 and Table 19 reflect exposure to IMBRUVICA with a median duration of 4.4 months in Study 1129.
Table 18: Non-Hematologic Adverse Reactions in ‚Č• 10% of Adult Patients with cGVHD in Study 1129 (N=42) Body System Adverse Reaction All Grades (%) Grade 3 or Higher¬† (%) General disorders and administration site conditions FatiguePyrexiaEdema peripheral 571712 1250 Skin and subcutaneous tissue disorders Bruising*Rash* 4012 00 Gastrointestinal disorders DiarrheaStomatitis*NauseaConstipation 36292612 10200 Musculoskeletal and connective tissue disorders Muscle spasmsMusculoskeletal pain* 2914 25 Vascular disorders Hemorrhage* 26 0 Infections and infestations Pneumonia*Upper respiratory tract infectionSepsis* 211910 14‚Ć 010 Nervous system disorders Headache 17 5 Injury, poisoning and procedural complications Fall 17 0 Respiratory, thoracic and mediastinal disorders CoughDyspnea 1412 02 Metabolism and nutrition disorders Hypokalemia 12 7
The system organ class and individual ADR preferred terms are sorted in descending frequency order.
* Includes multiple ADR terms.
† Includes 2 events with a fatal outcome.
Table 19: Treatment-Emergent Hematologic Laboratory Abnormalities in Adult Patients with cGVHD in Study 1129 (N=42) Percent of Patients (N=42) All Grades (%) Grade 3 or 4 (%) Platelets decreased 33 0 Neutrophils decreased 10 10 Hemoglobin decreased 24 2
Treatment-emergent Grade 4 neutropenia occurred in 2% of patients.
iMAGINE
The safety of IMBRUVICA was evaluated in the iMAGINE study, which included 47 pediatric and young adult patients 1 year to less than 22 years of age with cGVHD after failure of one or more lines of systemic therapy. Patients age 12 years and older were treated with IMBRUVICA 420 mg orally once daily, and patients age 1 year to less than 12 years were treated with IMBRUVICA 240 mg/m2 orally once daily [see Clinical Studies ( 14.3 )]. The median duration of exposure to IMBRUVICA was 7.1 months (range, 0.2 to 25.9 months).
Serious adverse reactions occurred in 64% of patients who received IMBRUVICA. Serious adverse reactions in more than two patients included pneumonia, pyrexia, sepsis, and stomatitis. Fatal adverse reactions occurred in two patients who received IMBRUVICA, including sepsis and acute respiratory distress syndrome (ARDS).
Permanent discontinuation of IMBRUVICA due to an adverse reaction occurred in 23% of patients. Adverse reactions which resulted in permanent discontinuation in at least two patients included hemorrhage. Dose reductions of IMBRUVICA due to an adverse reaction occurred in 19% of patients. Adverse reactions which required dose reduction in at least two patients included stomatitis.
The most common (‚Č•¬†20%) adverse reactions, including laboratory abnormalities, were anemia, musculoskeletal pain, pyrexia, diarrhea, pneumonia, abdominal pain, stomatitis, thrombocytopenia, and headache.
Table 20 summarizes the adverse reactions in iMAGINE.
Table¬†20: Adverse Reactions (‚Č• 10%) in Patients with Previously Treated cGVHD Who Received IMBRUVICA in iMAGINE IMBRUVICA (N=47) Body System Adverse Reaction All Grades (%) Grade 3 or 4¬† (%) General disorders and administration site conditions ¬†¬†¬†Pyrexia 30 11 Musculoskeletal and connective tissue disorders ¬†¬†¬†Musculoskeletal pain* 30 2 ¬†¬†¬†Osteonecrosis 11 9 Gastrointestinal disorders ¬†¬†¬†Diarrhea 28 2 ¬†¬†¬†Abdominal pain* 23 4 ¬†¬†¬†Stomatitis* 23 9 ¬†¬†¬†Vomiting 19 2 ¬†¬†¬†Nausea 19 4 Infections and infestations ¬†¬†¬†Pneumonia* 23 13 ¬†¬†¬†Skin infection* 17 4 ¬†¬†¬†Sepsis* 11 9‚Ć Nervous system disorders ¬†¬†¬†Headache 21 2 Skin and subcutaneous tissue disorders ¬†¬†¬†Rash* 19 2 ¬†¬†¬†Pruritus 13 0 ¬†¬†¬†Petechiae 13 0 Respiratory, thoracic and mediastinal disorders ¬†¬†¬†Cough 19 2 Vascular disorders ¬†¬†¬†Hemorrhage* 17 0 ¬†¬†¬†Hypertension* 11 4 Blood and lymphatic system disorders ¬†¬†¬†Hypokalemia 15 6 ¬†¬†¬†Hypogammaglobulinemia* 11 0 Cardiac Disorders ¬†¬†¬†Sinus tachycardia 11 0 Investigations ¬†¬†¬†Alanine aminotransferase increased 11 2
The system organ class and individual ADR preferred terms are sorted in descending frequency order.
* Includes multiple ADR terms.
† Includes 1 fatal outcome.
Table 21 summarizes the laboratory abnormalities in iMAGINE.
Table 21: Select Hematologic Laboratory Abnormalities (‚Č•¬†10%) That Worsened from Baseline in Patients with Previously Treated cGVHD Who Received IMBRUVICA in iMAGINE IMBRUVICA (N=47) All Grades (%) Grade 3 or 4 (%) Hemoglobin decreased 49 13 Platelets decreased 21 4 Neutrophils decreased 13 6
Treatment-emergent Grade 4 neutropenia occurred in 3% of patients.
Additional Important Adverse Reactions
Cardiovascular Events
Data on cardiovascular events are based on randomized controlled trials with IMBRUVICA (n=2,115; median treatment duration of 19.1 months for 1,157 patients treated with IMBRUVICA and 5.3 months for 958 patients in the control arm). The incidence of ventricular tachyarrhythmias (ventricular extrasystoles, ventricular arrhythmias, ventricular fibrillation, ventricular flutter, and ventricular tachycardia) of any grade was 1.0% versus 0.4% and of Grade 3 or greater was 0.3% versus 0% in patients treated with IMBRUVICA compared to patients in the control arm. The incidence of atrial fibrillation and atrial flutter of any grade was 8.4% versus 1.6% and for Grade 3 or greater was 4.0% versus 0.5% in patients treated with IMBRUVICA compared to patients in the control arm. In addition, the incidence of cardiac failure of any grade was 1.7% versus 0.5% and for Grade 3 or greater was 1.2% versus 0.3% in patients treated with IMBRUVICA compared to patients in the control arm.
The incidence of ischemic cerebrovascular events (cerebrovascular accidents, ischemic stroke, cerebral ischemia, and transient ischemic attack) of any grade was 1% versus 0.4% and Grade 3 or greater was 0.5% versus 0.2% in patients treated with IMBRUVICA compared to patients in the control arm, respectively.
Diarrhea
In randomized controlled trials (n=2,115; median treatment duration of 19.1 months for 1,157 patients treated with IMBRUVICA and 5.3 months for 958 patients in the control arm), diarrhea of any grade occurred at a rate of 43% of patients treated with IMBRUVICA compared to 19% of patients in the control arm. Grade 3 diarrhea occurred in 3% versus 1% of IMBRUVICA-treated patients compared to the control arm, respectively. Less than 1% (0.3%) of subjects discontinued IMBRUVICA due to diarrhea compared with 0% in the control arm.
Based on data from 1,605 of these patients, the median time to first onset was 21 days (range, 0 to 708) versus 46 days (range, 0 to 492) for any grade diarrhea and 117 days (range, 3 to 414) versus 194 days (range, 11 to 325) for Grade 3 diarrhea in IMBRUVICA-treated patients compared to the control arm, respectively. Of the patients who reported diarrhea, 85% versus 89% had complete resolution, and 15% versus 11% had not reported resolution at time of analysis in IMBRUVICA-treated patients compared to the control arm, respectively. The median time from onset to resolution in IMBRUVICA-treated subjects was 7 days (range, 1 to 655) versus 4 days (range, 1 to 367) for any grade diarrhea and 7 days (range, 1 to 78) versus 19 days (range, 1 to 56) for Grade 3 diarrhea in IMBRUVICA-treated subjects compared to the control arm, respectively.
Visual Disturbance
In randomized controlled trials (n=2,115; median treatment duration of 19.1 months for 1,157 patients treated with IMBRUVICA and 5.3 months for 958 patients in the control arm), blurred vision and decreased visual acuity of any grade occurred in 11% of patients treated with IMBRUVICA (9% Grade 1, 2% Grade 2, no Grade 3 or higher) compared to 6% in the control arm (5% Grade 1 and < 1% Grade 2 and 3).
Based on data from 1,605 of these patients, the median time to first onset was 91 days (range, 0 to 617) versus 100 days (range, 2 to 477) in IMBRUVICA-treated patients compared to the control arm, respectively. Of the patients who reported visual disturbances, 60% versus 71% had complete resolution and 40% versus 29% had not reported resolution at the time of analysis in IMBRUVICA-treated patients compared to the control arm, respectively. The median time from onset to resolution was 37 days (range, 1 to 457) versus 26 days (range, 1 to 721) in IMBRUVICA-treated subjects compared to the control arm, respectively. 
6.2
The following adverse reactions have been identified during postapproval use of IMBRUVICA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hepatobiliary disorders: hepatic failure including acute and/or fatal events, hepatic cirrhosis, drug-induced liver injury
- Respiratory disorders: interstitial lung disease
- Metabolic and nutrition disorders: tumor lysis syndrome
- Immune system disorders: anaphylactic shock, angioedema, urticaria
- Skin and subcutaneous tissue disorders: Stevens-Johnson Syndrome (SJS), onychoclasis, panniculitis, neutrophilic dermatoses
- Infections: hepatitis B reactivation
- Nervous system disorders: peripheral neuropathy
Drug Interactions Section
- CYP3A Inhibitors: Modify IMBRUVICA dose as described (
2.3 ,7.1 ).- CYP3A Inducers: Avoid coadministration with strong CYP3A inducers (
7.2 ).7.1
The coadministration of IMBRUVICA with a strong or moderate CYP3A inhibitor may increase ibrutinib plasma concentrations [see Clinical Pharmacology ( 12.3 )]. Increased ibrutinib concentrations may increase the risk of drug-related toxicity.
Dose modifications of IMBRUVICA are recommended when used concomitantly with posaconazole, voriconazole and moderate CYP3A inhibitors [see Dosage and Administration ( 2.3 )]. 
Avoid concomitant use of other strong CYP3A inhibitors. Interrupt IMBRUVICA if these inhibitors will be used short-term (such as anti-infectives for seven days or less) [see Dosage and Administration ( 2.3 ) ].
Avoid grapefruit and Seville oranges during IMBRUVICA treatment, as these contain strong or moderate inhibitors of CYP3A.
7.2
The coadministration of IMBRUVICA with strong CYP3A inducers may decrease ibrutinib concentrations. Avoid coadministration with strong CYP3A inducers [see Clinical Pharmacology ( 12.3 ) ].
Use In Specific Populations Section
- Lactation: Advise not to breastfeed (
8.2 ).- Hepatic Impairment: Avoid use of IMBRUVICA in patients with severe hepatic impairment. In patients with mild or moderate impairment, reduce IMBRUVICA dose (
2.4 ,8.6 ).8.1
Risk Summary
IMBRUVICA can cause fetal harm based on findings from animal studies. There are no available data on IMBRUVICA use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. In animal reproduction studies, administration of ibrutinib to pregnant rats and rabbits during the period of organogenesis at exposures up to 3-20 times the clinical dose of 420 mg daily produced embryofetal toxicity including structural abnormalities (see Data). Advise pregnant women of the potential risk to a fetus.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Ibrutinib was administered orally to pregnant rats during the period of organogenesis at doses of 10, 40 and 80 mg/kg/day. Ibrutinib at a dose of 80 mg/kg/day was associated with visceral malformations (heart and major vessels) and increased resorptions and post-implantation loss. The dose of 80 mg/kg/day in rats is approximately 20 times the exposure in patients with CLL/SLL or WM administered a dose of 420 mg daily. Ibrutinib at doses of 40 mg/kg/day or greater was associated with decreased fetal weights. The dose of 40 mg/kg/day in rats is approximately 8 times the exposure (AUC) in patients administered a dose of 420 mg daily.
Ibrutinib was also administered orally to pregnant rabbits during the period of organogenesis at doses of 5, 15, and 45 mg/kg/day. Ibrutinib at a dose of 15 mg/kg/day or greater was associated with skeletal variations (fused sternebrae) and ibrutinib at a dose of 45 mg/kg/day was associated with increased resorptions and post-implantation loss. The dose of 15 mg/kg/day in rabbits is approximately 2.8 times the exposure in patients with CLL/SLL or WM administered a dose of  420 mg daily.
8.2
Risk Summary
There is no information regarding the presence of ibrutinib or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with IMBRUVICA and for 1 week after the last dose.
8.3
IMBRUVICA can cause fetal harm when administered to pregnant women [see Use in Specific Populations ( 8.1 )].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating IMBRUVICA.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with IMBRUVICA and for 1 month after the last dose.
Males
Advise males with female partners of reproductive potential to use effective contraception during treatment with IMBRUVICA and for 1 month following the last dose.
8.4
Chronic GVHD
The safety and effectiveness of IMBRUVICA have been established for treatment of cGVHD after failure of one or more lines of systemic therapy in pediatric patients 1 year of age and older.
Use of IMBRUVICA for this indication is supported by evidence from iMAGINE, a study which included pediatric patients age 1 year and older with previously treated cGVHD, including patients in the following age groups: one patient 1 year to less than 2 years of age, 20 patients 2 years to less than 12 years of age, and 19 patients 12 years to less than 17 years of age. Additional supportive efficacy data was provided from Study 1129 in adults [see Adverse Reactions ( 6.1 ), Clinical Pharmacology ( 12.3 ), and Clinical Studies ( 14.3 )].
The recommended dosage of IMBRUVICA in patients age 12 years and older is the same as that in adults, and the recommended dosage in patients age 1 year to less than 12 years old is based on body-surface area (BSA) [see Dosage and Administration ( 2.1 )].
The safety and effectiveness of IMBRUVICA have not been established for this indication in pediatric patients less than 1 year of age.
Mature B-cell Non-Hodgkin Lymphoma
The safety and effectiveness of IMBRUVICA in combination with chemoimmunotherapy were assessed but have not been established based on an open-label, randomized study (NCT02703272) in 35 patients, which included 26 pediatric patients age 5 to less than 17 years, with previously treated mature B-cell non-Hodgkin lymphoma. The study was stopped for futility. In the randomized population, major hemorrhage and discontinuation of chemoimmunotherapy due to adverse reactions occurred more frequently in the ibrutinib plus chemoimmunotherapy arm compared to the chemoimmunotherapy alone arm.
CLL/SLL, CLL/SLL with 17p deletion, WM
The safety and effectiveness of IMBRUVICA in pediatric patients have not been established in CLL/SLL, CLL/SLL with 17p deletion, or WM.
8.5
Of 992 patients in clinical studies of IMBRUVICA for B-cell malignancies or cGVHD, 62% were ‚Č• 65 years of age, while 22% were ‚Č•¬†75 years of age [see Clinical Studies ( 14.1 , 14.2 , 14.3 )]. No overall differences in effectiveness were observed between younger and older patients. Anemia (all grades), pneumonia (Grade 3 or higher), thrombocytopenia, hypertension, and atrial fibrillation occurred more frequently among older patients treated with IMBRUVICA [see Adverse Reactions ( 6.1 )].
8.6
Adult Patients with B-cell Malignancies
Avoid use of IMBRUVICA in patients with severe hepatic impairment (Child-Pugh class C). The safety of IMBRUVICA has not been evaluated in patients with mild to severe hepatic impairment by Child-Pugh criteria.
Reduce the recommended dose when administering IMBRUVICA to patients with mild or moderate hepatic impairment (Child-Pugh class A and B). Monitor patients more frequently for adverse reactions of IMBRUVICA [see Dosage and Administration ( 2.4 ), Clinical Pharmacology ( 12.3 )].
Patients with cGVHD
Avoid use of IMBRUVICA in patients with total bilirubin level > 3 x ULN (unless of non-hepatic origin or due to Gilbert’s syndrome). Reduce recommended dose when administering IMBRUVICA to patients with total bilirubin level > 1.5 to 3 x ULN (unless of non-hepatic origin or due to Gilbert’s syndrome) [see Dosage and Administration ( 2.4 )].
8.7
Management of hyperviscosity in WM patients may include plasmapheresis before and during treatment with IMBRUVICA. Modifications to IMBRUVICA dosing are not required.
Overdosage Section
There is no specific experience in the management of ibrutinib overdose in patients. One healthy subject experienced reversible Grade 4 hepatic enzyme increases (AST and ALT) after a dose of 1680 mg. Closely monitor patients who ingest more than the recommended dosage and provide appropriate supportive treatment.
Description Section
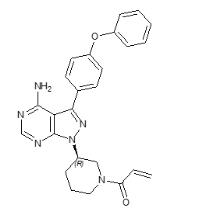
Ibrutinib is a kinase inhibitor. It is a white to off-white solid with the empirical formula C25H24N6O2 and a molecular weight 440.50. Ibrutinib is freely soluble in dimethyl sulfoxide, soluble in methanol and practically insoluble in water. The chemical name for ibrutinib is 1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidinyl]-2-propen-1-one and has the following structure:
IMBRUVICA (ibrutinib) is available as immediate-release oral capsules, immediate-release oral tablets, and immediate-release oral suspension.
IMBRUVICA (ibrutinib) capsules for oral use are available in the following dosage strengths: 70 mg and 140 mg. Each capsule contains ibrutinib (active ingredient) and the following inactive ingredients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate. The capsule shell contains gelatin, titanium dioxide, yellow iron oxide (70 mg capsule only), and black ink.
IMBRUVICA (ibrutinib) tablets for oral use are available in the following dosage strengths: 140 mg, 280 mg, and 420 mg. Each tablet contains ibrutinib (active ingredient) and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate. The film coating for each tablet contains ferrosoferric oxide (140 mg, 280 mg, and 420 mg tablets), polyvinyl alcohol, polyethylene glycol, red iron oxide (280 mg tablets), talc, titanium dioxide, and yellow iron oxide (140 mg and 420 mg tablets).
IMBRUVICA (ibrutinib) oral suspension contains 70 mg/mL ibrutinib (active ingredient) and the following inactive ingredients: benzyl alcohol, citric acid monohydrate, disodium hydrogen phosphate, hypromellose, microcrystalline cellulose and carboxymethylcellulose sodium, purified water and sucralose.
Clinical Pharmacology Section
12.1
Ibrutinib is a small-molecule inhibitor of Bruton’s tyrosine kinase (BTK). Ibrutinib forms a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. BTK is a signaling molecule of the B-cell antigen receptor (BCR) and cytokine receptor pathways. BTK’s role in signaling through the B-cell surface receptors results in activation of pathways necessary for B-cell trafficking, chemotaxis, and adhesion. Nonclinical studies show that ibrutinib inhibits malignant B-cell proliferation and survival in vivo as well as cell migration and substrate adhesion in vitro.
12.2
In patients with recurrent B-cell lymphoma > 90% occupancy of the BTK active site in peripheral blood mononuclear cells was observed up to 24 hours after ibrutinib doses of ‚Č•¬†2.5¬†mg/kg/day (‚Č• 175 mg/day for average weight of 70 kg).
In adult patients with cGVHD, 93% occupancy of the BTK active site in peripheral blood mononuclear cells was observed at the ibrutinib recommended dose. The mean BTK occupancy in pediatric patients ranged from 95.1% to 99.6%.
In vitro Platelet Aggregation
Ibrutinib demonstrated inhibition of collagen-induced platelet aggregation, with IC50 values at 4.6 ¬ĶM (2026 ng/mL), 0.8 ¬ĶM (352 ng/mL), and 3 ¬ĶM (1321 ng/mL) in blood samples from healthy donors, donors taking warfarin, and donors with severe renal dysfunction, respectively.¬†Ibrutinib did not show meaningful inhibition of platelet aggregation for ADP, arachidonic acid, ristocetin, and TRAP-6.
Cardiac Electrophysiology
At a single dose 4 times the maximum recommended dose (1680 mg), IMBRUVICA did not prolong the QT interval to any clinically relevant extent.
12.3
Ibrutinib exposure increases with doses up to 840 mg (2 times the maximum approved recommended dosage) in patients with B-cell malignancies. The mean steady-state AUC (% coefficient of variation) observed in patients at 420 mg with CLL/SLL is 708 (71%) ng×h/mL, with WM is 707 (72%) ng×h/mL, and in adult patients with previously treated cGVHD is 1159 (50%) ng×h/mL. Steady-state concentrations of ibrutinib without CYP3A inhibitors were achieved with an accumulation ratio of 1 to 1.6 after 1 week of multiple daily doses of 420 mg.
Absorption
Absolute bioavailability of ibrutinib in fasted condition was 2.9% (90% CI: 2.1, 3.9) in healthy subjects. Ibrutinib is absorbed after oral administration with a median Tmax  of 1 hour to 2 hours.
Effect of Food
The administration of IMBRUVICA with a high-fat and high-calorie meal (800 calories to 1,000 calories with approximately 50% of total caloric content of the meal from fat) increased ibrutinib Cmax by 2- to 4-fold and AUC by approximately 2-fold, compared with administration of ibrutinib after overnight fasting.
In vitro studies suggest that ibrutinib is not a substrate of p-glycoprotein (P-gp) or breast cancer resistance protein (BCRP).
Distribution
Reversible binding of ibrutinib to human plasma protein in vitro was 97.3% with no concentration dependence in the range of 50 ng/mL to 1000 ng/mL. The volume of distribution (Vd) was 683 L, and the apparent volume of distribution at steady state (Vd,ss/F) was approximately 10,000 L.
Elimination
Intravenous clearance was 62 L/h in fasted conditions and 76 L/h in fed conditions. In line with the high first-pass effect, the apparent oral clearance is 2000 L/h in fasted conditions and 1000 L/h in fed conditions. The half-life of ibrutinib is 4 hours to 6 hours.
Metabolism
Metabolism is the main route of elimination for ibrutinib. It is metabolized to several metabolites primarily by cytochrome P450 (CYP) 3A and to a minor extent by CYP2D6. The active metabolite, PCI-45227, is a dihydrodiol metabolite with inhibitory activity towards BTK approximately 15 times lower than that of ibrutinib. The range of the mean metabolite to parent ratio for PCI-45227 at steady-state is 1 to 2.8.
Excretion
Ibrutinib, mainly in the form of metabolites, is eliminated primarily via feces. After a single oral administration of radiolabeled ibrutinib, 90% of radioactivity was excreted within 168 hours, with 80% excreted in the feces and less than 10% eliminated in urine. Unchanged ibrutinib accounted for 1% of the radiolabeled excreted dose in feces and none in urine, with the remainder of the excreted dose being metabolites.
Specific Populations
Age and Sex
Age and sex have no clinically meaningful effect on ibrutinib pharmacokinetics.
Patients with Renal Impairment
Mild and moderate renal impairment (creatinine clearance [CLcr] > 25 mL/min as estimated by Cockcroft-Gault equation) had no influence on the exposure of ibrutinib. No data is available in patients with severe renal impairment (CLcr < 25 mL/min) or in patients on dialysis.
Patients with Hepatic Impairment
The AUC of ibrutinib increased 2.7-fold in subjects with mild hepatic impairment (Child-Pugh class A), 8.2-fold in subjects with moderate hepatic impairment (Child-Pugh class B) and 9.8-fold in subjects with severe hepatic impairment (Child-Pugh class C) relative to subjects with normal liver function. The Cmax of ibrutinib increased 5.2-fold in mild hepatic impairment, 8.8-fold in moderate hepatic impairment and 7-fold in severe hepatic impairment relative to subjects with normal liver function [see Use in Specific Populations ( 8.6 )].
Pediatric Patients
In pediatric patients with cGVHD treated with ibrutinib at 240 mg/m2 once daily (patients age ‚Č•¬†1 to <¬†12 years)¬†or 420 mg once daily (patients age ‚Č•¬†12 years), the geometric mean (%CV) steady state AUC and Cmax in patients age ‚Č•¬†1 to <¬†12 years is 467 (102%) ng√óh/mL and 65.7 (96%) ng/mL, respectively, and in patients age ‚Č•¬†12 to <¬†17 years is 966 (78%) ng√óh/mL and 149 (79%) ng/mL, respectively.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Effect of CYP3A Inhibitors on Ibrutinib: The coadministration of multiple doses of ketoconazole (strong CYP3A inhibitor) increased the Cmax of ibrutinib by 29-fold and AUC by 24-fold. The coadministration of multiple doses of voriconazole (strong CYP3A inhibitor) increased steady state Cmax of ibrutinib by 6.7-fold and AUC by 5.7-fold. Simulations under fed conditions suggest that posaconazole (strong CYP3A inhibitor) may increase the AUC of ibrutinib 3-fold to 10-fold.
The coadministration of multiple doses of erythromycin (moderate CYP3A inhibitor) increased steady state Cmax of ibrutinib by 3.4-fold and AUC by 3-fold.
Effect of CYP3A Inducers on Ibrutinib: The coadministration of rifampin (strong CYP3A inducer) decreased the Cmax of ibrutinib by more than 13-fold and AUC by more than 10-fold. Simulations suggest that efavirenz (moderate CYP3A inducer) may decrease the AUC of ibrutinib by 3-fold.
In Vitro Studies
Effect of Ibrutinib on CYP Substrates: In vitro studies suggest that ibrutinib and PCI-45227 are unlikely to inhibit CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6 or 3A at clinical doses. Both ibrutinib and PCI-45227 are unlikely to induce CYP1A2, CYP2B6 or CYP3A at clinical doses.
Effect of Ibrutinib on Substrates of Transporters: In vitro studies suggest that ibrutinib may inhibit BCRP and P-gp transport at clinical doses. The coadministration of oral P-gp or BCRP substrates (e.g., digoxin, methotrexate) with IMBRUVICA may increase their concentrations.
Nonclinical Toxicology Section
13.1
Ibrutinib was not carcinogenic in a 6-month rasH2 mouse study at oral doses up to 2000 mg/kg/day resulting in exposures approximately 32 (males) to 52 (females) times higher than the exposure in humans at a dose of 420 mg daily [see Warnings and Precautions ( 5.6 )].
Ibrutinib was not mutagenic in a bacterial mutagenicity (Ames) assay, was not clastogenic in a chromosome aberration assay in mammalian (CHO) cells, nor was it clastogenic in an in vivo bone marrow micronucleus assay in mice at doses up to 2000 mg/kg.
Rats were administered oral daily doses of ibrutinib for 4 weeks prior to pairing and during pairing in males and 2 weeks prior to pairing and during pairing in females. Treatment of female rats continued following pregnancy up to gestation day (GD) 7, and treatment of male rats continued until end of study. No effects on fertility or reproductive capacities were observed in male or female rats up to the maximum dose tested, 100 mg/kg/day (Human Equivalent Dose [HED] 16 mg/kg).
Clinical Studies Section
The safety and efficacy of IMBRUVICA in patients with CLL/SLL were demonstrated in one uncontrolled trial and five randomized, controlled trials.
Study 1102
Study 1102 (NCT01105247), an open-label, multi-center trial, was conducted in 48 previously treated CLL patients. IMBRUVICA was administered orally at 420 mg once daily until disease progression or unacceptable toxicity. The ORR and DOR were assessed using a modified version of the International Workshop on CLL Criteria by an Independent Review Committee.
The median age was 67¬†years (range, 37 to 82 years), 71% were male, and 94% were White. All patients had a baseline ECOG performance status of 0 or 1. The median time since diagnosis was 80 months and the median number of prior treatments was 4 (range, 1 to 12 treatments). At baseline, 46% of subjects had at least one tumor ‚Č•¬†5 cm.
The ORR was 58.3% (95% CI: 43.2%, 72.4%), all partial responses. None of the patients achieved a complete response. The DOR ranged from 5.6 to 24.2+ months. The median DOR was not reached.
RESONATE
The RESONATE study, a randomized, multicenter, open-label, phase 3 study of IMBRUVICA versus ofatumumab (NCT01578707), was conducted in patients with previously treated CLL or SLL. Patients (n=391) were randomized 1:1 to receive either IMBRUVICA 420 mg daily until disease progression, or unacceptable toxicity or ofatumumab at an initial dose of 300 mg, followed one week later by a dose of 2000 mg weekly for 7 doses and then every 4 weeks for 4 additional doses. Fifty-seven patients randomized to ofatumumab crossed over following progression to receive IMBRUVICA.
The¬†median age was 67 years (range, 30 to 88 years), 68% were male, and 90% were White. All patients had a baseline ECOG performance status of 0 or 1. The trial enrolled 373 patients with CLL and 18 patients with SLL. The median time since diagnosis was 91 months and the median number of prior treatments was 2 (range, 1 to 13 treatments). At baseline, 58% of patients had at least one tumor ‚Č•¬†5 cm. Thirty-two percent of patients had 17p deletion.
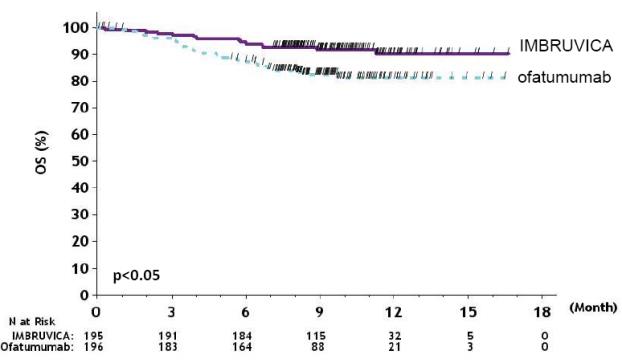
Efficacy results for RESONATE are shown in Table 22 and the Kaplan-Meier curves for PFS, assessed by an IRC according to IWCLL criteria, and OS are shown in Figure 1 and Figure 2, respectively.
Table 22: Efficacy Results in Patients with CLL/SLL in RESONATE Endpoint IMBRUVICA N=195 Ofatumumab N=196 Progression - Free Survival b Number of events (%) 35 (17.9) 111 (56.6)    Disease progression 26 93    Death events 9 18    Median (95% CI), months NE 8.1 (7.2, 8.3)    HR (95% CI) 0.22 (0.15, 0.32) Overall Survival a    Number of deaths (%) 16 (8.2) 33 (16.8)    HR (95% CI) 0.43 (0.24, 0.79) Overall Response Rate b 42.6% 4.1%
a       Median OS not evaluable for either arm.
b       IRC evaluated. All partial responses achieved; none of the patients achieved a complete response.
CI = confidence interval; HR = hazard ratio; NE = not evaluable.
Figure 1: Kaplan-Meier Curve of Progression - Free Survival (ITT Population) in Patients with CLL/SLL in RESONATE
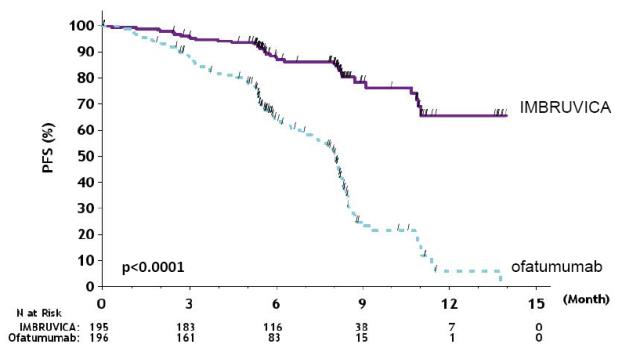
Figure 2: Kaplan-Meier Curve of Overall Survival (ITT Population) in Patients with CLL/SLL in RESONATE
63-Month Follow-Up
With an overall follow-up of 63 months, the median investigator-assessed PFS per IWCLL criteria was 44.1 months [95% CI (38.5, 56.9)] in the IMBRUVICA arm and 8.1 months [95% CI (7.8, 8.3)] in the ofatumumab arm. Overall response rate as assessed by investigators was 87.2% in the IMBRUVICA arm versus 22.4% in the ofatumumab arm.
CLL/SLL with 17p deletion (del 17p CLL/SLL) in RESONATE
RESONATE included 127 patients with del 17p CLL/SLL. The median age was 67 years (range, 30 to 84 years), 62% were male, and 88% were White. All patients had a baseline ECOG performance status of 0 or 1. PFS and ORR were assessed by an IRC. Efficacy results for del 17p CLL/SLL are shown in Table 23.
Table 23: Efficacy Results in Patients with del 17p CLL/SLL in RESONATE Endpoint IMBRUVICA N=63 Ofatumumab N=64 Progression - Free Survival a Number of events (%) 16 (25.4) 38 (59.4)    Disease progression 12 31    Death events 4 7    Median (95% CI), months NE 5.8 (5.3, 7.9)    HR (95% CI) 0.25 (0.14, 0.45) Overall Response Rate a 47.6% 4.7%
a       IRC evaluated. All partial responses achieved; none of the patients achieved a complete response.
CI = confidence interval; HR = hazard ratio; NE = not evaluable.
63-Month Follow-Up
With an overall follow-up of 63 months, the median investigator-assessed PFS in patients with del 17p per IWCLL criteria was 40.6 months [95% CI (25.4, 44.6)] in the IMBRUVICA arm and 6.2 months [95% CI (4.6, 8.1)] in the ofatumumab arm. Overall response rate as assessed by investigators in patients with del 17p was 88.9% in the IMBRUVICA arm versus 18.8% in the ofatumumab arm.
RESONATE-2
The RESONATE-2 study, a¬†randomized, multicenter, open-label, phase 3 study of IMBRUVICA versus chlorambucil (NCT01722487), was conducted in patients with treatment na√Įve CLL or SLL who were 65 years of age or older. Patients (n = 269) were randomized 1:1 to receive either IMBRUVICA 420 mg daily until disease progression or unacceptable toxicity, or chlorambucil at a starting dose of 0.5¬†mg/kg on Days 1 and 15 of each 28-day cycle for a maximum of 12 cycles, with an allowance for intrapatient dose increases up to 0.8 mg/kg based on tolerability.
The median age was 73 years (range, 65 to 90 years), 63% were male, and 91% were White. Ninety one percent of patients had a baseline ECOG performance status of 0 or 1 and 9% had an ECOG performance status of 2. The trial enrolled 249 patients with CLL and 20 patients with SLL. At baseline, 20% of patients had 11q deletion. The most common reasons for initiating CLL therapy include: progressive marrow failure demonstrated by anemia and/or thrombocytopenia (38%), progressive or symptomatic lymphadenopathy (37%), progressive or symptomatic splenomegaly (30%), fatigue (27%) and night sweats (25%).
With a median follow-up of 28.1 months, there were 32 observed death events [11 (8.1%) and 21 (15.8%) in IMBRUVICA and chlorambucil treatment arms, respectively]. With 41% of patients switching from chlorambucil to IMBRUVICA, the overall survival analysis in the ITT patient population resulted in a statistically significant HR of 0.44 [95% CI (0.21, 0.92)] and 2-year survival rate estimates of 94.7% [95% CI (89.1, 97.4)] and 84.3% [95% CI (76.7, 89.6)] in the IMBRUVICA and chlorambucil arms, respectively.
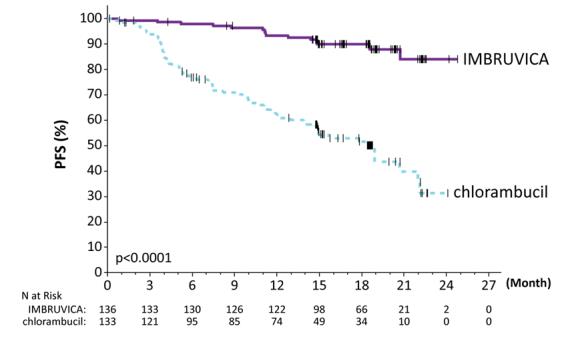
Efficacy results for RESONATE-2 are shown in Table 24 and the Kaplan-Meier curve for PFS, assessed by an IRC according to IWCLL criteria is shown in Figure 3.
Table 24: Efficacy Results in Patients with CLL/SLL in RESONATE-2 Endpoint IMBRUVICA N=136 Chlorambucil N=133 Progression - Free Survival a Number of events (%) 15 (11.0) 64 (48.1)    Disease progression 12 57    Death events 3 7         Median (95% CI), months NE 18.9 (14.1, 22.0)         HR b (95% CI) 0.16 (0.09, 0.28) Overall Response Rate a  (CR + PR) 82.4% 35.3%         P-value <0.0001
a       IRC evaluated; five subjects (3.7%) in the IMBRUVICA arm and two subjects (1.5%) in the Chlorambucil arm achieved complete response.
b       HR = hazard ratio; NE = not evaluable.
Figure 3: Kaplan-Meier Curve of Progression-Free Survival (ITT Population) in Patients with CLL/SLL in RESONATE-2
55-Month Follow-Up
With an overall follow-up of 55 months, the median PFS was not reached in the IMBRUVICA arm.
HELIOS
The HELIOS study, a randomized, double-blind, placebo-controlled phase 3 study of IMBRUVICA in combination with bendamustine and rituximab (BR) (NCT01611090), was conducted in patients with previously treated CLL or SLL. Patients (n = 578) were randomized 1:1 to receive either IMBRUVICA 420 mg daily or placebo in combination with BR until disease progression, or unacceptable toxicity. All patients received BR for a maximum of six 28-day cycles. Bendamustine was dosed at 70¬†mg/m2 infused IV over 30 minutes on Cycle 1, Days 2 and 3, and on Cycles 2-6, Days 1 and 2 for up to 6 cycles, and all patients had a CLcr ‚Č• 40 mL/min at baseline. Rituximab was administered at a dose of 375 mg/m2 in the first cycle, Day 1, and 500 mg/m2 Cycles 2 through 6, Day 1.
The median age was 64 years (range, 31 to 86 years), 66% were male, and 91% were White. All patients had a baseline ECOG performance status of 0 or 1. The median time since diagnosis was 5.9 years and the median number of prior treatments was 2 (range, 1 to 11 treatments). At baseline, 56% of patients had at least one tumor > 5 cm and 26% presented with del11q.
Efficacy results for HELIOS are shown in Table 25 and the Kaplan-Meier curves for PFS are shown in Figure 4.
Table 25: Efficacy Results in Patients with CLL/SLL in HELIOS Endpoint IMBRUVICA + BR N=289 Placebo + BR N=289 Progression - Free Survival a    Number of events (%) 56 (19.4) 183 (63.3)    Median (95% CI), months NE 13.3 (11.3, 13.9)    HR (95% CI) 0.20 (0.15, 0.28) Overall Response Rate a 82.7% 67.8%
a       IRC evaluated; twenty-four subjects (8.3%) in the IMBRUVICA + BR arm and six subjects (2.1%) in the placebo + BR arm achieved complete response.
BR = bendamustine and rituximab; CI = confidence interval; HR = hazard ratio; NE = not evaluable.
Figure 4: Kaplan-Meier Curve of Progression-Free Survival (ITT Population) in Patients with CLL/SLL in HELIOS
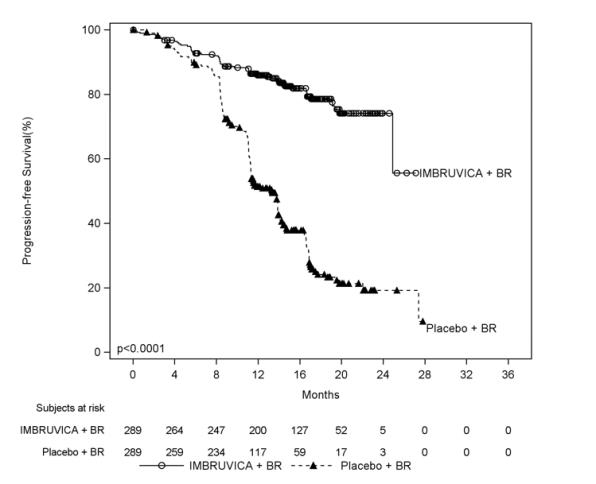
iLLUMINATE
The iLLUMINATE study, a randomized, multi-center, phase 3 study of IMBRUVICA in combination with obinutuzumab versus chlorambucil in combination with obinutuzumab (NCT02264574), was conducted in patients with treatment na√Įve CLL or SLL. Patients were 65 years of age or older or < 65 years of age with coexisting medical conditions, reduced renal function as measured by creatinine clearance < 70 mL/min, or presence of del 17p/TP53 mutation. Patients (n = 229) were randomized 1:1 to receive either IMBRUVICA 420 mg daily until disease progression or unacceptable toxicity or chlorambucil at a dose of 0.5 mg/kg on Days 1 and 15 of each 28-day cycle for 6 cycles. In both arms, patients received 1,000 mg of obinutuzumab on Days 1, 8, and 15 of the first cycle, followed by treatment on the first day of 5 subsequent cycles (total of 6¬†cycles, 28 days each). The first dose of obinutuzumab was divided between Day 1 (100 mg) and Day 2 (900 mg).
The median age was 71 years (range, 40 to 87 years), 64% were male, and 96% were White. All patients had a baseline ECOG performance status of 0 (48%) or 1-2 (52%). The trial enrolled 214 patients with CLL and 15 patients with SLL. At baseline, 65% of patients presented with CLL/SLL with high risk factors (del 17p/TP53 mutation [18%], del 11q [15%], or unmutated immunoglobulin heavy-chain variable region (unmutated IGHV) [54%]). The most common reasons for initiating CLL therapy included: lymphadenopathy (38%), night sweats (34%), progressive marrow failure (31%), fatigue (29%), splenomegaly (25%), and progressive lymphocytosis (21%).
With a median follow-up time on study of 31 months, efficacy results for iLLUMINATE assessed by an IRC according to IWCLL criteria are shown in Table 26, and the Kaplan-Meier curve for PFS is shown in Figure 5.
Table 26: Efficacy Results in Patients with CLL/SLL in iLLUMINATE Endpoint IMBRUVICA + Obinutuzumab N=113 Chlorambucil + Obinutuzumab N=116 Progression - Free Survival a   Number of events (%) 24 (21) 74 (64)     Disease progression 11 64     Death events 13 10     Median (95% CI), months NE 19.0 (15.1, 22.1)     HR (95% CI) 0.23 (0.15, 0.37)     P-valueb <0.0001 Overall Response Rate (%) a 88.5 73.3        CRc (%) 19.5 7.8        PRd (%) 69.0 65.5
a IRC-evaluated.
b P-value is from unstratified log-rank test.
c Includes 1 patient in the IMBRUVICA + obinutuzumab arm with a complete response with incomplete marrow recovery (CRi)
d PR = nPR +PR.
HR = hazard ratio; NE = not evaluable.
Figure 5: Kaplan-Meier Curve of Progression-Free Survival (ITT Population) in Patients with CLL/SLL in iLLUMINATE
 
In the high risk CLL/SLL population (del 17p/TP53 mutation, del 11q, or unmutated IGHV), the PFS HR was 0.15 [95% CI (0.09, 0.27)].
E1912
The E1912 study, a randomized, multi-center, phase 3 study of IMBRUVICA in combination with rituximab versus standard fludarabine, cyclophosphamide, and rituximab (FCR) chemoimmunotherapy (NCT02048813), was conducted in adult patients who were 70 years or younger with previously untreated CLL or SLL requiring systemic therapy. All patients had a CLcr > 40 mL/min at baseline. Patients with 17p deletion were excluded. Patients (n =529) were randomized 2:1 to receive either IMBRUVICA plus rituximab or FCR. IMBRUVICA was administered at 420 mg daily until disease progression or unacceptable toxicity. Fludarabine was administered at a dose of 25 mg/m2, and cyclophosphamide was administered at a dose of 250 mg/m2, both on Days 1, 2, and 3 of Cycles 1-6. Rituximab was initiated in Cycle 2 for the IMBRUVICA plus rituximab arm and in Cycle 1 for the FCR arm and was administered at 50 mg/m2 on Day 1 of the first cycle, 325 mg/m2 on Day 2 of the first cycle, and 500 mg/m2 on Day 1 of 5 subsequent cycles, for a total of 6 cycles. Each cycle was 28 days.
The median age was 58 years (range, 28 to 70 years), 67% were male, 90% were White and 98% had a ECOG performance status of 0-1. At baseline, 43% of patients were Rai stage 3 or 4 and 59% of patients presented with high risk factors (TP53 mutation [6%], del11q [22%], or unmutated IGHV [53%]).
With a median follow-up time on study of 37 months, efficacy results for E1912 are shown in Table 27. The Kaplan-Meier curves for PFS, assessed according to IWCLL criteria is shown in Figure 6.
Table 27: Efficacy Results in Patients with CLL/SLL in E1912 Endpoint IMBRUVICA + R  N=354 FCR N=175 Progression - Free Survival    Number of events (%) 41 (12) 44 (25)       Disease progression 39 38       Death events 2 6    Median (95% CI), months NE (49.4, NE) NE (47.1, NE)    HR (95% CI) 0.34 (0.22, 0.52)    P-valuea <0.0001
a P-value is from unstratified log-rank test.
FCR = fludarabine, cyclophosphamide, and rituximab; HR = hazard ratio; R = rituximab; NE = not evaluable.
Figure 6: Kaplan-Meier Curve of Progression-Free Survival (ITT Population) in Patients with CLL/SLL in E1912
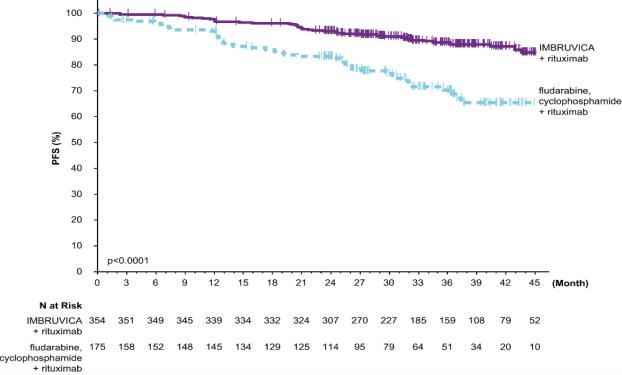
With a median follow-up time on study of 49 months, median overall survival was not reached with a total of 23 deaths: 11 (3%) in the IMBRUVICA plus rituximab and 12 (7%) in the FCR treatment arms.
Lymphocytosis
Upon initiation of single-agent IMBRUVICA, an increase in lymphocyte counts (i.e., ‚Č• 50% increase from baseline and above absolute lymphocyte count of 5,000/mcL) occurred in 66% of patients in the CLL studies. The onset of isolated lymphocytosis occurs during the first month of IMBRUVICA therapy and resolves by a median of 14 weeks (range, 0.1 to 104 weeks). When IMBRUVICA was administered in combination, lymphocytosis was 7% with IMBRUVICA + BR versus 6% with placebo + BR and 7% with IMBRUVICA¬†+¬†obinutuzumab versus 1% with chlorambucil + obinutuzumab.
14.
The safety and efficacy of IMBRUVICA in patients with WM were demonstrated in two single-arm trials and one randomized, controlled trial.
Study 1118 and INNOVATE Monotherapy Arm
Study 1118 (NCT01614821), an open-label, multi-center, single-arm trial was conducted in 63 previously treated patients with WM. IMBRUVICA was administered orally at 420 mg once daily until disease progression or unacceptable toxicity. The responses were assessed by investigators and an IRC using criteria adopted from the International Workshop of Waldenström’s Macroglobulinemia.  
The median age was 63 years (range, 44 to 86 years), 76% were male, and 95% were White. All patients had a baseline ECOG performance status of 0 or 1. The median time since diagnosis was 74 months, and the median number of prior treatments was 2 (range, 1 to 11 treatments). At baseline, the median serum IgM value was 3.5 g/dL (range, 0.7 to 8.4 g/dL).
Responses, defined as partial response or better, per IRC are shown in Table 28.
Table 28: Response Rate and Duration of Response (DOR) Based on IRC Assessment in Patients with WM in Study 1118 Total (N=63) Response rate (CR+VGPR+PR), (%) 61.9     95% CI (%) (48.8, 73.9)     Complete Response (CR) 0     Very Good Partial Response (VGPR), (%) 11.1     Partial Response (PR), (%) 50.8 Median duration of response, months (range) NE (2.8+, 18.8+) CI = confidence interval; NE = not evaluable.
The median time to response was 1.2 months (range, 0.7-13.4 months).
The INNOVATE monotherapy arm included 31 patients with previously treated WM who failed prior rituximab-containing therapy and received single-agent IMBRUVICA. The median age was 67 years (range, 47 to 90 years). Eighty-one percent of patients had a baseline ECOG performance status of 0 or 1, and 19% had a baseline ECOG performance status of 2. The median number of prior treatments was 4 (range, 1 to 7 treatments). With an overall follow-up of 61 months, the response rate observed in the INNOVATE monotherapy arm per IRC assessment was 77% (0% CR, 29% VGPR, 48% PR). The median duration of response was 33 months (range, 2.4 to 60.2+ months). 
INNOVATE
The INNOVATE study, a¬†randomized, double-blind, placebo-controlled, phase 3 study of IMBRUVICA or placebo in combination with rituximab (NCT02165397), was conducted in treatment na√Įve or previously treated patients with WM. Patients (n = 150) were randomized 1:1 to receive either IMBRUVICA 420¬†mg daily or placebo in combination with rituximab until disease progression or unacceptable toxicity. Rituximab was administered weekly at a dose of 375 mg/m2 for 4¬†consecutive weeks (weeks 1-4) followed by a second course of weekly rituximab for 4 consecutive weeks (weeks¬†17-20). The major efficacy outcome measure is progression-free survival (PFS) assessed by an IRC with additional efficacy measure of response rate.
The median age was 69 years (range, 36 to 89 years), 66% were male, and 79% were White. Ninety-three¬†percent of patients had a baseline ECOG performance status of 0 or 1, and 7% of patients had a baseline ECOG performance status of 2. Forty-five percent of patients were treatment na√Įve, and 55% of patients were previously treated. Among previously treated patients, the median number of prior treatments was 2 (range, 1 to 6 treatments). At¬†baseline, the median serum IgM value was 3.2 g/dL (range, 0.6 to 8.3¬†g/dL), and MYD88 L265P mutations were present in 77% of patients, absent in 13% of patients, and 9% of patients were not evaluable for mutation status.
An exploratory analysis demonstrated a sustained hemoglobin improvement (defined as increase of ‚Č• 2 g/dL over baseline for at least 8 weeks without blood transfusions or growth factor support) in 65% of patients in the IMBRUVICA + R group and 39% of patients in the placebo¬†+¬†R group.
With an overall follow-up of 63 months, efficacy results as assessed by an IRC at the time of the final analysis for INNOVATE are shown in Table 29, and the Kaplan-Meier curves for PFS are shown in Figure 7.
Table 29: Efficacy Results in Patients with WM by IRC in INNOVATE (Final Analysis) Endpoint IMBRUVICA + R N=75 Placebo + R N=75 Progression - Free Survival    Number of events (%) 22 (29) 50 (67)    Median (95% CI), months NE (57.7, NE) 20.3 (13.0, 27.6)    HR (95% CI) 0.25 (0.15, 0.42)    P-valuea <0.0001 Response Rate (CR+VGPR+PR) b 76% 31%    95% CI (%) (65, 85) (21, 42)    Complete Response (CR) 1% 1%    Very Good Partial Response (VGPR) 29% 4%    Partial Response (PR) 45% 25% Median duration of response, months (range) NE (1.9+, 58.9+) NE (4.6+, 49.7+)
CI = confidence interval; HR = hazard ratio; NE = not evaluable; R = rituximab.
a P-value is from the stratified log-rank test.
b P-value associated with response rate was <0.0001.
Figure 7: Kaplan-Meier Curve of Progression-Free Survival (ITT Population) in Patients with WM in INNOVATE
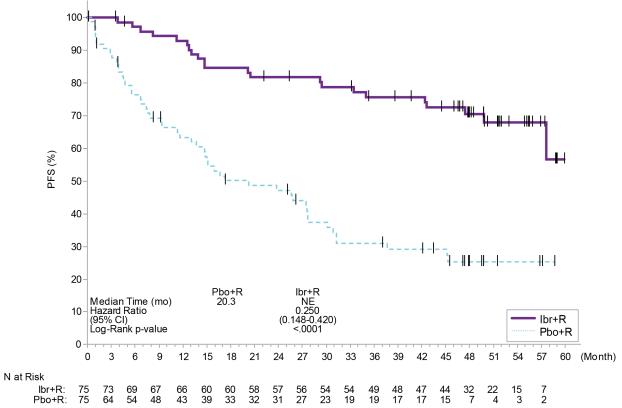
Median overall survival was not reached for either treatment arm. With an overall follow-up of 63 months, 9 (12%) patients on IMBRUVICA + R and 10 (13.3%) patients on placebo + R had died. Forty-seven percent of patients randomized to the placebo + R arm crossed over to receive IMBRUVICA.
Study 1129
The safety and efficacy of IMBRUVICA in cGVHD were evaluated in Study 1129 (NCT02195869), an open-label, multi-center, single-arm trial of 42 patients with cGVHD after failure of first line corticosteroid therapy and requiring additional therapy. IMBRUVICA was administered orally at 420 mg once daily. The responses were assessed by investigators using the 2005 National Institute of Health (NIH) Consensus Panel Response Criteria with two modifications to align with the updated 2014 NIH Consensus Panel Response Criteria.
The median age was 56 years (range, 19 to 74¬†years), 52% were male, and 93% were White. The most common underlying malignancies leading to transplantation were acute lymphocytic leukemia, acute myeloid leukemia, and CLL. The median time since cGVHD diagnosis was 14¬†months, the median number of prior cGVHD treatments was 2 (range, 1 to 3¬†treatments), and 60% of patients had a Karnofsky performance score of ‚ȧ 80. The majority of patients (88 %) had at least 2 organs involved at baseline, with the most commonly involved organs being mouth (86%), skin (81%), and gastrointestinal tract (33%). The median daily corticosteroid dose (prednisone or prednisone equivalent) at baseline was 0.3¬†mg/kg/day, and 52% of patients were receiving ongoing immunosuppressants in addition to systemic corticosteroids at baseline. Prophylaxis for infections were managed per institutional guidelines with 79% of patients receiving combinations of sulfonamides and trimethoprim and 64% receiving triazole derivatives.
Efficacy results are shown in Table 30.
Table 30: Best Overall Response Rate (ORR) and Sustained Response Rate¬†Based on Investigator Assessmenta in Patients with cGVHD in Study 1129 Total (N=42) ORR 28 (67%) ¬†¬†¬†¬†95% CI (51%, 80%) ¬†¬†¬†¬†Complete Response (CR) 9 (21%) ¬†¬†¬†¬†Partial Response (PR) 19 (45%) Sustained response rate b ¬† 20 (48%) CI = confidence interval. a Investigator assessment based on the 2005 NIH Response Criteria with two modifications (added ‚Äúnot evaluable‚ÄĚ for organs with non-cGVHD abnormalities, and organ score change from 0 to 1 was not considered disease progression.) b Sustained response rate is defined as the proportion of patients who achieved a CR or PR that was sustained for at least 20 weeks.
The median time to response coinciding with the first scheduled response assessment was 12.3 weeks (range, 4.1 to 42.1 weeks). Responses were seen across all organs involved for cGVHD (skin, mouth, gastrointestinal tract, and liver).
ORR results were supported by exploratory analyses of patient-reported symptom bother which showed at least a 7-point decrease in Lee Symptom Scale overall summary score in 24% (10/42) of patients on at least 2 consecutive visits.
iMAGINE
The safety and efficacy of IMBRUVICA were evaluated in iMAGINE (NCT03790332), an open-label, multi-center, single-arm trial of IMBRUVICA for the treatment of pediatric and young adult patients age 1 year to less than 22 years with moderate or severe cGVHD as defined by NIH Consensus Criteria. The study included 47 patients who required additional therapy after failure of one or more prior lines of systemic therapy. All patients had platelets ‚Č• 30 x 109/L; absolute neutrophil count ‚Č• 1.0 x 109/L; AST or ALT ‚ȧ 3 x ULN; total bilirubin of ‚ȧ 1.5 x ULN; and estimated creatinine clearance ‚Č• 30 mL/min. Patients were excluded if single organ genitourinary involvement was the only manifestation of cGVHD.
Patients age 12 years and older were treated with IMBRUVICA 420 mg orally once daily, and patients age 1 year to less than 12 years were treated with IMBRUVICA 240 mg/m2 orally once daily. Concomitant treatment with supportive care therapies for cGVHD was permitted. Initiation of new systemic cGVHD therapy while on study was not permitted.
The median age was 13 years (range, 1 to 19 years). Of the 47 patients, 70% of patients were male, and 36% were White, 9% were Black or African American, 55% were other or unreported. The median time since cGVHD diagnosis was 16.1 months, the median number of prior cGVHD treatments was 2 (range, 1 to 12). The majority of patients (87%) had at least 2 organs involved at baseline, with lung involvement at baseline in 49% of patients; 26% of patients had a Karnofsky/Lansky performance score of <80. The median daily corticosteroid dose (prednisone or prednisone equivalent) at baseline was 0.47 mg/kg/day, and 61% (19 of 31) patients were receiving ongoing immunosuppressants in addition to systemic corticosteroids at baseline. Prophylaxis for infections was managed per institutional guidelines, with 72% of patients receiving combinations of sulfonamides and trimethoprim and 70% receiving systemic antifungal agents.
The efficacy of IMBRUVICA was established based on overall response rate (ORR) through Week 25, where overall response included complete response or partial response according to the 2014 National Institutes of Health (NIH) Consensus Development Project Response Criteria. The efficacy results are shown in Table 31. 
Table 31: Efficacy Results in Patients with Previously Treated cGVHDa in iMAGINE Total (N=47) ORR by Week 25 28 (60%)    95% CI (%) (44, 74)    Complete Response (CR) 2 (4%)    Partial Response (PR) 26 (55%) Median d uration of r esponse, months (95% CI) b 5.3 (2.8, 8.8)
CI = confidence interval; ORR = overall response rate.
a Assessment based on 2014 NIH Consensus Development Project Response Criteria.
b Based on all responders in the study, calculated from first response to progression, death, or new systemic therapies for cGVHD. 
The median time to first response was 0.9 month (range, 0.9 to 6.1 months). The median time from first response to death or new systemic therapies for cGVHD was 14.8 months (95% CI: 4.6, not evaluable).
ORR results were supported by exploratory analyses of patient-reported symptom bother which showed at least a 7-point decrease in Lee Symptom Scale overall summary score through Week 25 in 50% (13/26) of patients age 12 years and older.
How Supplied Section
Capsules
The 70 mg capsules are supplied as yellow opaque capsules, marked with ‚Äúibr 70 mg‚ÄĚ in black ink, in white HDPE bottles with a child-resistant closure:
- 28 capsules per bottle: NDC 57962-070-28
The 140 mg capsules are supplied as white opaque capsules, marked with ‚Äúibr 140 mg‚ÄĚ in black ink, in white HDPE bottles with a child-resistant closure:
- 90 capsules per bottle: NDC 57962-140-09
- 120 capsules per bottle: NDC 57962-140-12
Store bottles at room temperature 20¬įC to 25¬įC (68¬įF to 77¬įF). Brief exposure to 15¬įC to 30¬įC (59¬įF to 86¬įF) permitted (see USP Controlled Room Temperature). Retain in original package until dispensing.
Tablets
The IMBRUVICA (ibrutinib) tablets are supplied in 3 strengths in the following packaging configurations:
- 140 mg tablets: Yellow green to green round tablets debossed with ‚Äúibr‚ÄĚ on one side and ‚Äú140‚ÄĚ on the other side. Carton of one folded buler card containing two 14-count buler strips for a total of 28 tablets: NDC 57962-014-28
- 280 mg tablets: Purple oblong tablets debossed with ‚Äúibr‚ÄĚ on one side and ‚Äú280‚ÄĚ on the other side. Carton of one folded buler card containing two 14-count buler strips for a total of 28¬†tablets: NDC 57962-280-28
- 420 mg tablets: Yellow green to green oblong tablets debossed with ‚Äúibr‚ÄĚ on one side and ‚Äú420‚ÄĚ on the other side. Carton of one folded buler card containing two 14-count buler strips for a total of 28 tablets: NDC 57962-420-28
Store tablets in original packaging at room temperature 20¬įC to 25¬įC (68¬įF to 77¬įF). Brief exposure to 15¬įC to 30¬įC (59¬įF to 86¬įF)¬†permitted (see USP Controlled Room Temperature).
Oral Suspension
The IMBRUVICA (ibrutinib) oral suspension is a white to off-white suspension supplied as 108 mL in a 150 mL amber glass bottle with a pre-inserted bottle adapter and a child-resistant closure. Each mL contains 70 mg of ibrutinib. The oral suspension bottle is provided in a carton with two 3 mL reusable oral dosing syringes: NDC 57962-007-12.
Store the oral suspension bottle at 2¬įC to 25¬įC (36¬įF to 77¬įF). Do not freeze. Dispense in original sealed container. Do not use if the carton seal is broken or missing.
Discard any unused IMBRUVICA oral suspension remaining 60 days after first opening the bottle.
Information For Patients Section
Advise the patients and caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hemorrhage: Inform patients of the possibility of bleeding, and to report any signs or symptoms (severe headache, blood in stools or urine, prolonged or uncontrolled bleeding). Inform the patient that IMBRUVICA may need to be interrupted for medical or dental procedures [see Warnings and Precautions ( 5.1 )].
Infections: Inform patients of the possibility of serious infection, and to report any signs or symptoms (fever, chills, weakness, confusion) suggestive of infection [see Warnings and Precautions ( 5.2 )].
Cardiac arrhythmias , cardiac failure , and sudden death :
Inform patients of the possibility of irregular heart rhythm, heart failure and sudden death.Counsel patients to report any signs of palpitations, lightheadedness, dizziness, fainting, shortness of breath, chest discomfort, or edema [see Warning s and Precautions ( 5.3 )] .
Hypertension : Inform patients that high blood pressure has occurred in patients taking IMBRUVICA, which may require treatment with anti-hypertensive therapy [see Warnings and Precautions ( 5.4 )].
Second primary malignancies: Inform patients that other malignancies have occurred in patients who have been treated with IMBRUVICA, including skin cancers and other carcinomas [see Warnings and Precautions ( 5.6 )].
Hepatotoxicity, including drug-induced liver injury:
Inform patients that liver problems, including drug-induced liver injury and abnormalities in liver tests, may develop during IMBRUVICA treatment. Advise patients to contact their healthcare provider immediately if they experience abdominal discomfort, dark urine, or jaundice [see Warnings and Precautions ( 5.7 )].
Tumor lysis syndrome: Inform patients of the potential risk of tumor lysis syndrome and to report any signs and symptoms associated with this event to their healthcare provider for evaluation [see Warnings and Precautions ( 5.8 )].
Embryo-fetal toxicity: Advise women of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions ( 5.9 ), Use in Specific Populations ( 8.1 )].Advise females of reproductive potential to use effective contraception during treatment with IMBRUVICA and for 1 month after the last dose [see Use in Specific Populations ( 8.3 ) ].Advise males with female partners of reproductive potential to use effective contraception during treatment with IMBRUVICA and for 1 month after the last dose [see Use in Specific Populations ( 8.3 ), Nonclinical Toxicology ( 13.1 )].
Lactation: Advise women not to breastfeed during treatment with IMBRUVICA and for 1 week after the last dose [see Use in Specific Populations ( 8.2 )].
Other Important Information:
Inform patients to take IMBRUVICA orally once daily according to their physician’s instructions and that the oral dosage (capsules or tablets) should be swallowed whole with a glass of water without opening, breaking or chewing the capsules or cutting, crushing or chewing the tablets approximately the same time each day [see Dosage and Administration ( 2.1 )].
Advise patients that in the event of a missed daily dose of IMBRUVICA, it should be taken as soon as possible on the same day with a return to the normal schedule the following day. Patients should not take extra doses to make up the missed dose [see Dosage and Administration ( 2.1 )].
For IMBRUVICA oral suspension, instruct patients or caregivers to read and follow the Instructions for Use for proper preparation, administration, storage and disposal [see Dosage and Administration ( 2.1 )].
Advise patients of the common side effects associated with IMBRUVICA [see Adverse Reactions ( 6 )]. Direct the patient to a complete ul of adverse drug reactions in PATIENT INFORMATION.
Advise patients to inform their health care providers of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions ( 7 )].
Advise patients that they may experience loose stools or diarrhea and should contact their doctor if their diarrhea persists. Advise patients to maintain adequate hydration [see Adverse Reactions ( 6.1 )].
Distributed and Marketed by:Pharmacyclics LLCSouth San Francisco, CA 94080 USAandMarketed by:Janssen Biotech, Inc.Horsham, PA 19044 USA
Patent http://www.imbruvica.com IMBRUVICA¬ģ is a registered trademark owned by Pharmacyclics LLC
© Pharmacyclics LLC 2024© Janssen Biotech, Inc. 2024
20085244
Spl Patient Package Insert Section
PATIENT INFORMATION IMBRUVICA (im-BRU-vih-kuh) (ibrutinib)capsules IMBRUVICA (im-BRU-vih-kuh) (ibrutinib)tablets IMBRUVICA (im-BRU-vih-kuh) (ibrutinib)oral suspension What is IMBRUVICA? IMBRUVICA is a prescription medicine used to treat: It is not known if IMBRUVICA is safe and effective in children under 1 year of age.
- Adults with chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL).
- Adults with chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL) with 17p deletion.
- Adults with Waldenström’s macroglobulinemia (WM).
- Adults and children 1 year of age and older with chronic graft versus host disease (cGVHD) after failure of 1 or more lines of systemic therapy.
Before taking IMBRUVICA, tell your healthcare provider about all of your medical conditions, including if you: Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking IMBRUVICA with certain other medicines may affect how IMBRUVICA works and can cause side effects.
- have had recent surgery or plan to have surgery. Your healthcare provider may stop IMBRUVICA for any planned medical, surgical, or dental procedure.
- have bleeding problems or are taking a blood thinner medicine.
- have an infection.
- have or had heart rhythm problems, smoke, or have a medical condition that increases your risk of heart disease, such as high blood pressure, high cholesterol, or diabetes.
- have liver problems.
- are pregnant or plan to become pregnant. IMBRUVICA can harm your unborn baby. If you are able to become pregnant, your healthcare provider will do a pregnancy test before starting treatment with IMBRUVICA. Tell your healthcare provider if you are pregnant or think you may be pregnant during treatment with IMBRUVICA. ‚óč Females who are able to become pregnant should use effective birth control (contraception) during treatment with IMBRUVICA and for 1 month after the last dose. ‚óč Males with female partners who are able to become pregnant¬†should use effective birth control, such as condoms, during treatment with IMBRUVICA and for 1 month after the last dose.
- are breastfeeding or plan to breastfeed. Do not breastfeed during treatment with IMBRUVICA and for 1 week after the last dose.
How should I take or give IMBRUVICA? IMBRUVICA comes as capsules, tablets, and oral suspension.
- Take or give IMBRUVICA exactly as your healthcare provider tells you to take or give it.
- Take or give IMBRUVICA 1 time a day at about the same time each day.
- If your healthcare provider prescribes IMBRUVICA capsules or tablets: ‚ó¶ Swallow IMBRUVICA capsules or tablets whole with a glass of water. ‚ó¶ Do not open, break, or chew IMBRUVICA capsules. ‚ó¶ Do not cut, crush, or chew IMBRUVICA tablets.
- If your healthcare provider prescribes IMBRUVICA oral suspension: ‚ó¶ See the detailed Instructions for Use that comes with IMBRUVICA oral suspension for information about the correct way to take or give a dose. If you have questions about how to take or give IMBRUVICA oral suspension, talk to your healthcare provider.‚ó¶ Do not use if the carton seal is broken or missing.
- If you miss a dose of IMBRUVICA, take or give it as soon as you remember on the same day. Take or give the next dose of IMBRUVICA at the regular time on the next day. Do not take or give extra doses of IMBRUVICA to make up for a missed dose.
- If you take too much IMBRUVICA, call your healthcare provider, or go to the nearest hospital emergency room right away.
What should I avoid while taking IMBRUVICA? You should not drink grapefruit juice, eat grapefruit, or eat Seville oranges (often used in marmalades) during treatment with IMBRUVICA. These products may increase the amount of IMBRUVICA in your blood. What are the possible side effects of IMBRUVICA? IMBRUVICA may cause serious side effects, including:
- Bleeding problems (hemorrhage) are common during treatment with IMBRUVICA and can also be serious and may lead to death. Your risk of bleeding may increase if you are also taking a blood thinner medicine. Tell your healthcare provider if you have any signs of bleeding, including:
- blood in your stools or black stools (looks like tar)
- pink or brown urine
- unexpected bleeding, or bleeding that is severe or that you cannot control
- vomit blood or vomit looks like coffee grounds
- cough up blood or blood clots
- increased bruising, or small red or purple spots on the skin
- dizziness
- weakness
- confusion
- change in your speech
- headache that lasts a long time or severe headache
- Infections can happen during treatment with IMBRUVICA. These infections can be serious and may lead to death. Tell your healthcare provider right away if you have fever, chills, weakness, confusion, or other signs or symptoms of an infection during treatment with IMBRUVICA.
- Heart problems. Serious heart rhythm problems (ventricular arrhythmias, atrial fibrillation, and atrial flutter), heart failure and death have happened in people treated with IMBRUVICA, especially in people who have an infection, an increased risk for heart disease, or have had heart rhythm problems in the past. Your heart function will be checked before and during treatment with IMBRUVICA. Tell your healthcare provider if you get any symptoms of heart problems, such as:
- feeling as if your heart is beating fast and irregular
- lightheadedness
- dizziness
- shortness of breath
- swelling of the feet, ankles, or legs
- chest discomfort
- feeling faint
If you develop any of these symptoms, your healthcare provider may do tests to check your heart and may change your IMBRUVICA dose. The most common side effects of IMBRUVICA in adults with B-cell malignancies (CLL/SLL and WM) include:
- High blood pressure (hypertension). New or worsening high blood pressure has happened in people treated with IMBRUVICA. Your healthcare provider may start you on blood pressure medicine or change current medicines to treat your blood pressure.
- Decrease in blood cell counts. Decreased blood counts (white blood cells, platelets, and red blood cells) are common with IMBRUVICA, but can also be severe.  Your healthcare provider should do monthly blood tests to check your blood counts.
- Second primary cancers. New cancers have happened during treatment with IMBRUVICA, including cancers of the skin or other organs.
- Liver problems. Liver problems, which may be severe or life-threatening, or lead to death, can happen in people treated with IMBRUVICA. Your healthcare provider will do blood tests to check your liver before and during treatment with IMBRUVICA. Tell your healthcare provider or get medical help right away if you have any signs of liver problems, including stomach pain or discomfort, dark-colored urine, or yellow skin and eyes.
- Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure, and sometimes death. Your healthcare provider may do blood tests to check you for TLS.
- low platelet count
- muscle, bone, and joint pain
- low red blood cell count (anemia)
- diarrhea
- low white blood cell count
- bruising
- tiredness
- rash
- nausea
The most common side effects of IMBRUVICA in adults or children 1 year of age and older with cGVHD include:
- tiredness
- muscle, bone, and joint pain
- nausea
- low red blood cell count (anemia)
- fever
- stomach pain
- bruising
- muscle spasms
- pneumonia
- diarrhea
- mouth sores (stomatitis)
- headache
- low platelet count
- bleeding
Diarrhea is a common side effect in people who take IMBRUVICA. Drink plenty of fluids during treatment with IMBRUVICA to help reduce your risk of losing too much fluid (dehydration) due to diarrhea. Tell your healthcare provider if you have diarrhea that does not go away. These are not all the possible side effects of IMBRUVICA.Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store IMBRUVICA? Keep IMBRUVICA and all medicines out of the reach of children.
- Store IMBRUVICA capsules and tablets at room temperature between 68¬įF and 77¬įF (20¬įC and 25¬įC).
- Keep IMBRUVICA capsules in the original container with the lid tightly closed.
- Keep IMBRUVICA tablets in the original carton.
- Store IMBRUVICA oral suspension bottle between 36¬įF and 77¬įF (2¬įC and 25¬įC). Do not freeze.
- Use IMBRUVICA oral suspension within 60 days after first opening the bottle. Throw away (dispose of) any unused portion 60 days after opening.
- IMBRUVICA capsules and oral suspension come in a bottle with a child-resistant cap.
General information about the safe and effective use of IMBRUVICA. Medicines are sometimes prescribed for purposes other than those uled in a Patient Information leaflet. Do not use IMBRUVICA for a condition for which it was not prescribed. Do not give IMBRUVICA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about IMBRUVICA that is written for health professionals. What are the ingredients in IMBRUVICA? Active ingredient: ibrutinib Inactive ingredients: IMBRUVICA capsules: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The 70 mg capsule shell contains gelatin, titanium dioxide, yellow iron oxide, and black ink. The 140 mg capsule shell contains gelatin, titanium dioxide, and black ink. IMBRUVICA tablets: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate. The film coating for each tablet contains ferrosoferric oxide (140 mg, 280 mg, and 420 mg tablets), polyvinyl alcohol, polyethylene glycol, red iron oxide (280 mg tablets), talc, titanium dioxide, and yellow iron oxide (140 mg and 420 mg tablets). IMBRUVICA oral suspension: benzyl alcohol, citric acid monohydrate, disodium hydrogen phosphate, hypromellose, microcrystalline cellulose and carboxymethylcellulose sodium, purified water, and sucralose. Distributed and Marketed by: Pharmacyclics LLC South San Francisco, CA 94080 USAMarketed by: Janssen Biotech, Inc. Horsham, PA 19044 USA© Pharmacyclics LLC 2024© Janssen Biotech, Inc. 202420085244For more information, go to www.imbruvica.com or call 1-877-877-3536.
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 5/2024
Instructions For Use Section
INSTRUCTIONS FOR USE
IMBRUVICA (im-BRU-vih-kuh)
  (ibrutinib)
oral suspension
This Instructions for Use contains information about how to prepare and take or give a dose of IMBRUVICA oral suspension .
Read this Instructions for Use before you take or give IMBRUVICA and each time you get a refill. There may be new information.
This Instructions for Use does not take the place of talking to your healthcare provider about your or your child’s medical condition or treatment.
Call your healthcare provider or 1-877-877-3536 if you need help or have any questions about how to take or give IMBRUVICA the right way.
Important information you need to know before taking or giving IMBRUVICA.
- IMBRUVICA is for oral use only.
- Take or give IMBRUVICA exactly as your healthcare provider tells you to.
- If you miss a dose of IMBRUVICA, it can be taken or given as soon as possible on the same day. Do not take or give more than the prescribed dose in 1 day. 

If you or your child take too much IMBRUVICA, call your healthcare provider for help.
- Keep these instructions for future use.
Each IMBRUVICA carton contains (see Figure A):
- 1 bottle of IMBRUVICA (called ‚Äėbottle‚Äô in this¬†Instructions for Use) with pre-inserted bottle adapter (called ‚Äėadapter‚Äô in this¬†Instructions for Use).¬†Do not remove the bottle adapt e r.
- 2¬†reusable¬†3 mL oral dosing syringes (called ‚Äėsyringe‚Äô in this Instructions for Use) measuring in 0.1 mL increments.

Only use the syringes that come with IMBRUVICA. Do not use the syringes for other patients or with other medicines.  
If you cannot read the markings on the syringes, throw them away and call 1-877-877-3536 to get new ones.
P reparing and taking or giving a dose of   IMBRUVICA
Step 1: Gather and check supplies .
- Check the prescribed dose in milliliters (mLs). Find this mL marking on the syringe.
- If the dose is more than the marking on the syringe, split the dose between syringes as prescribed.
- Gather bottle and syringe(s) (see Figure A ).
- Check the bottle and make sure that the bottle has IMBRUVICA Oral Suspension printed on it and the expiration date (‚ÄúEXP‚ÄĚ) has not passed.
Figure A

Do not use IMBRUVICA after the ‚ÄúEXP‚ÄĚ date printed on the carton and on the bottle. 
Do not use if the IMBRUVICA carton seal appears to be tampered with. Step 2: Record or check the discard date .
- When opening the bottle for the first time, record the date that is 60 days from the day the bottle is opened underneath the words ‚ÄúDiscard Date‚ÄĚ (see Figure B).
- Use IMBRUVICA within 60 days after first opening the bottle.
Figure B

Do not use IMBRUVICA past the discard date recorded on the bottle. Step 3: Shake the bottle .  
- Shake the bottle well before each use (see Figure C ).
Figure C
Step 4: Remove the cap from the bottle . Do not remove the bottle adapter.
- Press down and twist the cap counterclockwise to remove it from the bottle (see Figure D).
- If there is fluid on top of the adapter, you may wipe it with a clean disposable tissue.
Figure D
Step 5: Attach the syringe   to the bottle .
- Make sure the syringe is clean and dry before use.
- Push the plunger down all the way.
- Gently insert tip of the syringe into the adapter.
- Turn the assembled bottle and syringe upside down (see Figure E).
Figure E
Step 6: Fill the syringe .
- Slowly pull the syringe plunger down, past the number of mLs for your prescribed dose (see Figure F).
- Check for air bubbles and proceed to Step 7 for instructions on how to remove air bubbles. 
Figure F
Step 7: Remove air bubbles and adjust to the prescribed dose (mL) .
- Hold the syringe and tap the sides to send bubbles to the tip.
- With the syringe attached to the bottle, push the plunger up to remove the air bubbles from the top (see  Figure G).
- After the bubbles are removed, push the plunger up until the top of the colored plunger is even with the markings on the syringe for the prescribed dose. 
Figure G

Air bubbles must be removed to ensure the correct dose. Note: Repeat steps 6 and 7 if any air bubbles remain.   Step 8: Remove the syringe   from the bottle .
- Turn the assembled bottle upright.
- Hold the middle of the syringe and carefully remove it from the bottle (see Figure H).
- Place the bottle aside.
Figure H

Do not touch the plunger of the syringe to avoid accidentally spilling the medicine before you are ready to take or give the dose. Note: If more than 1 syringe is needed to take or give the full dose, repeat steps 5 to 8 with the second syringe to complete the prescribed dose. Step 9: Take or give IMBRUVICA . Note: IMBRUVICA must be taken or given as soon as possible after being drawn from the bottle.  Note: After swallowing the dose of medicine make sure to drink water.
- Place the tip of the syringe along the inside of the cheek.
- Slowly push the plunger all the way in to take or give the entire dose (see Figure I).
- Repeat with second syringe if needed to complete the prescribed dose.
Figure I
Step 10: Recap the bottle .
- Place the cap back on the IMBRUVICA bottle (see Figure J ).  
- Make sure the bottle is tightly closed between each use.
Figure J
Step 11: Rinse the   syringe .
- Remove the plunger from the syringe.
- Rinse the plunger and the syringe only with water and air dry (see Figure K).
- Store the syringe in a clean, dry place.
Figure K

Do not clean the syringe with soap or in the dishwasher.
Turn over for more   information
How to s tore IMBRUVICA   Oral Suspension
- Store the bottle between 36¬įF and 77¬įF (2¬įC¬†and 25¬įC).

Do not freeze.
- IMBRUVICA oral suspension comes in a bottle with a child-resistant cap.
- Store IMBRUVICA and all medications out of   the reach of children.
How to d ispose of IMBRUVICA  

Throw away (dispose of) any unused medicine within 60 days after first opening of the bottle. At the same time, throw away any used or unused syringes.
- Ask your pharmacist how to properly dispose of the medicine.
- For syringe disposal, rinse and place in household trash.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed and Marketed by:Pharmacyclics LLCSouth San Francisco, CA 94080 USAandMarketed by:Janssen Biotech, Inc.Horsham, PA 19044 USA
Patent http://www.imbruvica.comIMBRUVICA¬ģ is a registered trademark owned by Pharmacyclics LLC¬†
© 2024 Pharmacyclics LLC © 2024 Janssen Biotech, Inc. 20080493Revised: 2/2024
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL   - 28 Capsule Bottle Carton
NDC 57962-070-28
Imbruvica ¬ģ (ibrutinib) capsules
70 mg
Each capsule contains: ibrutinib 70 mgSwallow capsules whole with water
28 Capsules
Rx Only
p harmacyclics ¬ģ An AbbVie Company
j anssen
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 90 Capsule Bottle Carton
NDC 57962-140-09
Imbruvica ¬ģ (ibrutinib) capsules
140 mg
Each capsule contains: ibrutinib 140 mg Swallow capsules whole with water
90 Capsules
Rx Only
p harmacyclics ¬ģ An AbbVie Company
j anssen

Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 120 Capsule Bottle Carton
NDC 57962-140-12
Imbruvica ¬ģ (ibrutinib) capsules
140 mg
Each capsule contains: ibrutinib 140 mgSwallow capsules whole with water
120 Capsules
Rx Only
p harmacyclics ¬ģ An AbbVie Company
j anssen
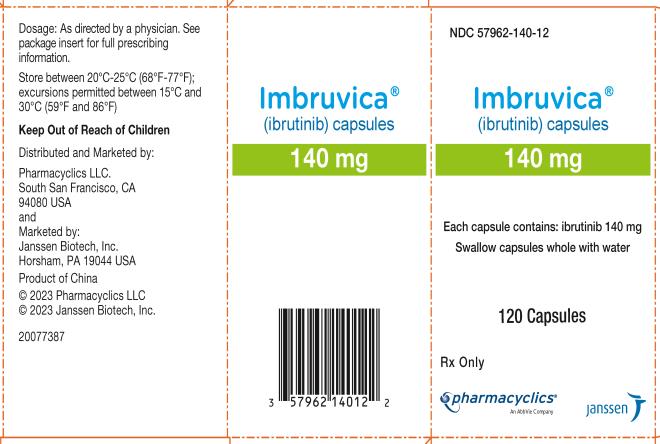
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 28 Tablet Buler Carton
NDC 57962-014-28
Imbruvica ¬ģ (ibrutinib) tablets
140 mg  per tablet
Each tablet contains ibrutinib 140 mg Wallet card contains 28 tablets
Rx Only
p harmacyclics ¬ģ An AbbVie Company
j anssen
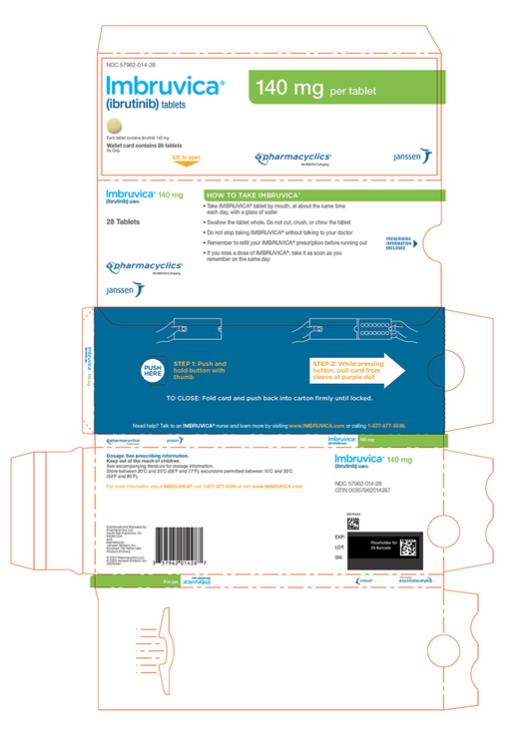
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 28 Tablet Buler Carton
NDC 57962-280-28
Imbruvica ¬ģ (ibrutinib) tablets
280 mg  per tablet
Each tablet contains ibrutinib 280 mg Wallet card contains 28 tablets
Rx Only
p harmacyclics ¬ģ
An AbbVie Company
j anssen
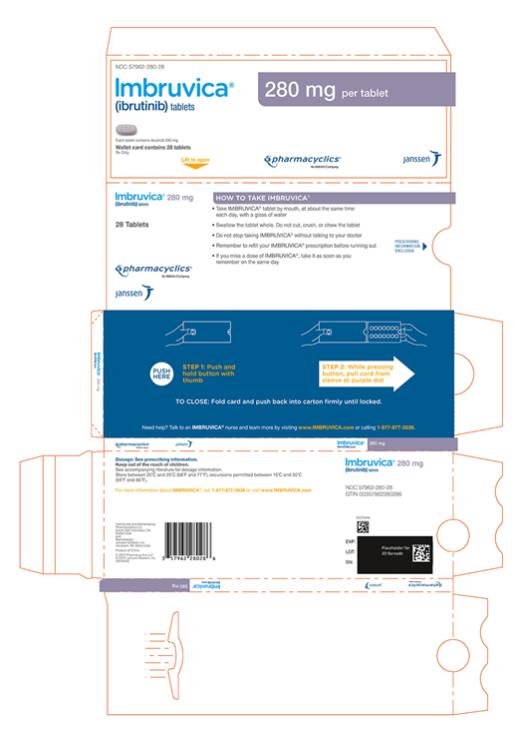
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 28 Tablet Buler Carton
NDC 57962-420-28
Imbruvica ¬ģ (ibrutinib) tablets
420 mg  per tablet
Each tablet contains ibrutinib 420 mg Wallet card contains 28 tablets
Rx Only
p harmacyclics ¬ģ An AbbVie Company
j anssen
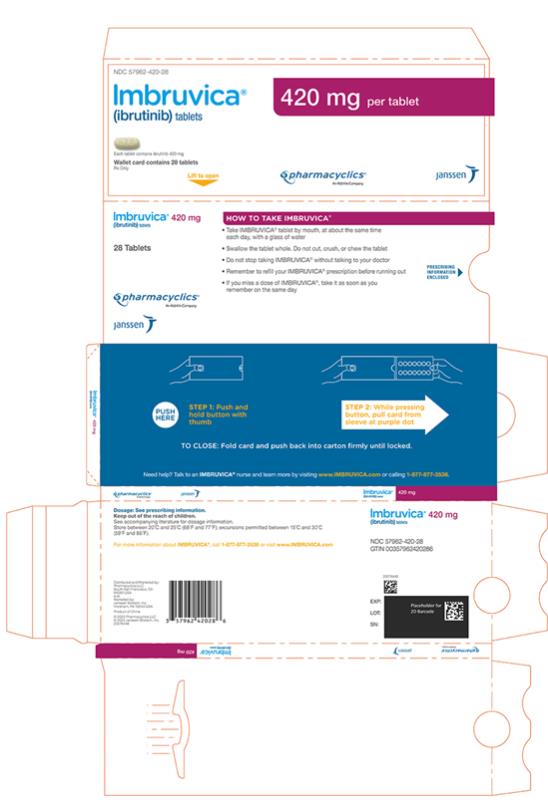
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 28 Tablet Buler Carton
NDC 57962-560-28
Imbruvica ¬ģ (ibrutinib) tablets
560 mg  per tablet
Each tablet contains ibrutinib 560 mg Wallet card contains 28 tablets
Rx Only
p harmacyclics ¬ģ An AbbVie Company
j anssen

Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL
NDC 57962-007-12
Imbruvica ¬ģ (ibrutinib) Oral Suspension
70 mg/mL
For Oral Use Only Shake well before each use Discard after 60 Days
Rx Only
108mL per Bottle
pharmacyclics ¬ģ An AbbVie Company
j anssen

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


