VITRAKVI (larotrectinib 100 mg) Dailymed
Generic: larotrectinib is used for the treatment of Neoplasm Metastasis
Go PRO for all pill images
Recent Major Changes Section
Dosage and Administration (2.3 ,2.4 ) 11/2023
Dosage and Administration (2.6 ) 11/2022
Dosage and Administration (2.8 ) 12/2022
Warnings and Precautions (5.3 ) 11/2023
1 Indications And Usage
VITRAKVI is indicated for the treatment of adult and pediatric patients with solid tumors that:
• have a neurotrophic receptor tyrosine kinase (NTRK) gene fusion without a known acquired resistance mutation,• are metastatic or where surgical resection is likely to result in severe morbidity, and• have no satisfactory alternative treatments or that have progressed following treatment.
Select patients for therapy based on an FDA-approved test [see Dosage and Administration (2.1)].
This indication is approved under accelerated approval based on overall response rate and duration of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
VITRAKVI is a kinase inhibitor indicated for the treatment of adult and pediatric patients with solid tumors that:
• have a neurotrophic receptor tyrosine kinase (NTRK) gene fusion without a known acquired resistance mutation,• are metastatic or where surgical resection is likely to result in severe morbidity, and• have no satisfactory alternative treatments or that have progressed following treatment. Select patients for therapy based on an FDA-approved test. (1 ,2.1 )
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials (1 ,14 ).
2 Dosage And Administration
• Select patients for treatment with VITRAKVI based on the presence of a NTRK gene fusion (2.1 ,14 ).• Recommended Dosage in Adult and Pediatric Patients with Body Surface Area of 1 Meter-Squared or greater: 100 mg orally twice daily (2.2 )• Recommended Dosage in Pediatric Patients with Body Surface Area of Less Than 1 Meter-Squared: 100 mg/m2 orally twice daily (2.2 )2.1 Patient Selection
Select patients for treatment with VITRAKVI based on the presence of a NTRK gene fusion in tumor specimens [see Clinical Studies (14)]. Information on FDA-approved tests is available at http://www.fda.gov/companiondiagnostics.
2.2 Recommended Dosage
Recommended Dosage in Adult and Pediatric Patients with Body Surface Area of at Least 1 Meter-Squared
The recommended dosage of VITRAKVI is 100 mg orally twice daily, with or without food, until disease progression or until unacceptable toxicity.
Recommended Dosage in Pediatric Patients with Body Surface Area Less Than 1 Meter-Squared
The recommended dosage of VITRAKVI is 100 mg/m2 orally twice daily, with or without food, until disease progression or until unacceptable toxicity.
2.3 Dosage Modifications for Adverse Reactions
For Grade 2 and higher liver function test abnormalities, refer to Section 2.4, Table 2, Dosage Modifications for Hepatotoxicity.
For all other Grade 3 or 4 adverse reactions:
• Withhold VITRAKVI until adverse reaction resolves or improves to baseline or Grade 1. Resume at the next dosage modification if resolution occurs within 4 weeks.• Permanently discontinue VITRAKVI if an adverse reaction does not resolve within 4 weeks.
The recommended dosage reductions for VITRAKVI for adverse reactions are provided in Table 1.
Table 1 Recommended Dosage Reductions for VITRAKVI for Adverse Reactions
Dosage Reduction Adult and Pediatric Patients with Body Surface Area of 1 m2 or Greater Pediatric Patients with Body Surface Area Less Than 1 m2
First
75 mg orally twice daily
75 mg/m2 orally twice daily
Second
50 mg orally twice daily
50 mg/m2 orally twice daily
Third
100 mg orally once daily
25 mg/m2 orally twice daily a
a Pediatric patients on 25 mg/m2 orally twice daily should remain on this dosage even if body surface area becomes greater than 1 m2 during the treatment. Maximum dose should be 25 mg/m2 orally twice daily at the third dosage modification.
Permanently discontinue VITRAKVI in patients who are unable to tolerate VITRAKVI after three dose modifications.
2.4Dosage Modifications for Hepatotoxicity
The recommended dosage modifications for VITRAKVI liver function test abnormalities are provided in Table 2.
For CTCAE Grade 2 ALT and/or AST elevation, monitor liver function frequently as clinically indicated, to establish whether a dose interruption or reduction is required [see Warnings and Precautions ( 5.3)].
Table 2 Recommended Dosage Modifications for VITRAKVI for Hepatotoxicity
Severitya
Dosage Modification
AST or ALT ≥ 5 x ULN with bilirubin ≤ 2 x ULN [see Warnings and Precautions (5.3)]
• Withhold VITRAKVI until recovery to ≤ Grade 1 or return to baseline. • Resume VITRAKVI at the next lower dose level. • Permanently discontinue if a Grade 4 AST and/or ALT elevation occurs after resuming VITRAKVI.
AST or ALT > 3 × ULN with total bilirubin > 2 × ULN in the
absence of alternative causes
• Permanently discontinue VITRAKVI.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; ULN = upper limit of normal
aGrading defined by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE)
version 4.03
2.5 Dosage Modifications for Coadministration with Strong CYP3A4 Inhibitors
Avoid coadministration of strong CYP3A4 inhibitors with VITRAKVI. If coadministration of a strong CYP3A4 inhibitor cannot be avoided, reduce the VITRAKVI dose by 50%. After the inhibitor has been discontinued for 3 to 5 elimination half-lives, resume the VITRAKVI dose that was used prior to initiating the CYP3A4 inhibitor [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
2.6 Dosage Modifications for Coadministration with Strong or Moderate CYP3A4 Inducers
Avoid coadministration of strong CYP3A4 inducers with VITRAKVI. If coadministration of a strong CYP3A4 inducer cannot be avoided, double the VITRAKVI dose. Additionally, for coadministration with a moderate CYP3A4 inducer, double the VITRAKVI dose. After the inducer has been discontinued for 3 to 5 elimination half-lives, resume the VITRAKVI dose that was used prior to initiating the CYP3A4 inducer [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
2.7 Dosage Modifications for Patients with Hepatic Impairment
Reduce the starting dose of VITRAKVI by 50% in patients with moderate (Child-Pugh B) to severe (Child-Pugh C) hepatic impairment [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
2.8 Administration
VITRAKVI capsule or oral solution may be used interchangeably.
Do not make up a missed dose within 6 hours of the next scheduled dose.
If vomiting occurs after taking a dose of VITRAKVI, take the next dose at the scheduled time.
Capsules
Swallow capsules whole with water. Do not chew or crush the capsules.
Oral Solution packaged in one bottle containing 100 mL
• Store the glass bottle of VITRAKVI oral solution in the refrigerator. Discard any unused VITRAKVI oral solution remaining after 90 days of first opening the bottle.• Prior to preparing an oral dose for administration, refer to the Instructions for Use.
Oral Solution packaged in two bottles each containing 50 mL
• Store the glass bottles of VITRAKVI oral solution in the refrigerator. Discard any unused VITRAKVI oral solution remaining after 31 days of first opening the bottle. • Prior to preparing an oral dose for administration, refer to the Instructions for Use.
3 Dosage Forms And Strengths
Capsules
• 25 mg: white opaque hard gelatin capsule, size 2, with blue printing of "BAYER" cross and "25 mg" on body of capsules. 25 mg larotrectinib is equivalent to 30.7 mg larotrectinib sulfate.• 100 mg: white opaque hard gelatin capsule, size 0, with blue printing of "BAYER" cross and "100 mg" on body of capsule. 100 mg larotrectinib is equivalent to 123 mg larotrectinib sulfate.
Oral Solution packaged in one bottle containing 100 mL
• 20 mg/mL: clear yellow to orange solution. 20 mg/mL larotrectinib is equivalent to 24.6 mg/mL larotrectinib sulfate.
Oral Solution packaged in two bottles each containing 50 mL
• 20 mg/mL: colorless to yellow or orange or red or brownish solution. 20 mg/mL larotrectinib is equivalent to 24.6 mg/mL larotrectinib sulfate.
• Capsules: 25 mg, 100 mg (3 )• Oral Solution: 20 mg/mL (3 )
4 Contraindications
None.
None. (4 )
5 Warnings And Precautions
• Central Nervous System (CNS) Effects: Advise patients and caretakers of the risk of CNS adverse reactions including dizziness, cognitive impairment, mood disorders, and sleep disturbances. Advise patients not to drive or operate hazardous machinery if experiencing neurotoxicity. Withhold and modify dosage, or permanently discontinue VITRAKVI based on severity. (2.3 ,5.1 )• Skeletal Fractures: Promptly evaluate patients with signs or symptoms of fractures. (5.2 )• Hepatotoxicity: Obtain liver function tests (ALT, AST, ALP and bilirubin) before initiation of VITRAKVI and every 2 weeks during the first 2 months of treatment, then monthly thereafter or as clinically indicated. Temporarily withhold, reduce dose, or permanently discontinue VITRAKVI based on severity. (2.4 ,5.3 )• Embryo-Fetal Toxicity: Can cause fetal harm. Advise females with reproductive potential of potential risk to the fetus and to use effective contraception. (5.4 ,8.3 )5.1 Central Nervous System Effects
Central nervous system (CNS) adverse reactions occurred in patients receiving VITRAKVI, including dizziness, cognitive impairment, mood disorders, and sleep disturbances.
In patients who received VITRAKVI, all grades CNS effects including cognitive impairment, mood disorders, dizziness and sleep disorders were observed in 42% with Grades 3-4 in 3.9% of patients.
Cognitive impairment occurred in 11% of patients. The median time to onset of cognitive impairment was 5.6 months (range: 2 days to 41 months). Cognitive impairment occurring in ≥ 1% of patients included memory impairment (3.6%), confusional state (2.9%), disturbance in attention (2.9%), delirium (2.2%), cognitive disorders (1.4%), and Grade 3 cognitive adverse reactions occurred in 2.5% of patients. Among the 30 patients with cognitive impairment, 7% required a dose modification, and 20% required dose interruption.
Mood disorders occurred in 14% of patients. The median time to onset of mood disorders was 3.9 months (range: 1 day to 40.5 months). Mood disorders occurring in ≥ 1% of patients included anxiety (5%), depression (3.9%), agitation (2.9%), and irritability (2.9%). Grade 3 mood disorders occurred in 0.4% of patients. Dizziness occurred in 27% of patients, and Grade 3 dizziness occurred in 1.1% of patients. Among the 74 patients who experienced dizziness, 5% of patients required a dose modification, and 5% required dose interruption.
Sleep disturbances occurred in 10% of patients. Sleep disturbances included insomnia (7%), somnolence (2.5%), and sleep disorder (0.4%). There were no Grade 3-4 sleep disturbances. Among the 28 patients who experienced sleep disturbances, 1 patient each (3.6%) required a dose modification or dose interruption.
Advise patients and caretakers of these risks with VITRAKVI. Advise patients not to drive or operate hazardous machinery if they are experiencing neurologic adverse reactions. Withhold or permanently discontinue VITRAKVI based on the severity. If withheld, modify the VITRAKVI dosage when resumed [see Dosage and Administration (2.3)].
5.2 Skeletal Fractures
Among 187 adult patients who received VITRAKVI across clinical trials, fractures were reported in 7% and among 92 pediatric patients, fractures were reported in 9% (N=279; 8%). Median time to fracture was 11.6 months (range 0.9 to 45.8 months) in patients followed per fracture. Fractures of the femur, hip or acetabulum were reported in 4 patients (3 adult, 1 pediatric). Most fractures were associated with minimal or moderate trauma. Some fractures were associated with radiologic abnormalities suggestive of local tumor involvement. VITRAKVI treatment was interrupted due to fracture in 1.4% patients.
Promptly evaluate patients with signs or symptoms of potential fracture (e.g., pain, changes in mobility, deformity). There are no data on the effects of VITRAKVI on healing of known fractures or risk of future fractures.
5.3 Hepatotoxicity
Hepatotoxicity including drug-induced liver injury (DILI) has been reported in patients taking VITRAKVI.
In patients who received VITRAKVI, increased AST of any grade occurred in 52% of patients and increased ALT of any grade occurred in 45%. Grade 3-4 increased AST or ALT occurred in 3.1% and 2.5% of patients, respectively [see Adverse Reactions (6.1)]. The median time to onset of increased AST was 2.1 months (range: 1 day to 4.3 years). The median time to onset of increased ALT was 2.3 months (range: 1 day to 4.2 years). Increased AST and ALT leading to dose modifications occurred in 1.4% and 2.2% of patients, respectively. Increased AST or ALT led to permanent discontinuation in 3 (1.1%) of patients.
There have been reports in adult patients from clinical studies and postmarketing cases of Grade ≥ 2 increases in ALT and/or AST with increases in bilirubin ≥ 2 x ULN.
Obtain liver function tests (ALT, AST, ALP and bilirubin) before initiation of VITRAKVI and monitor every 2 weeks during the first 2 months of treatment, then monthly thereafter, or more frequently following the occurrence of Grade 2 or greater AST or ALT elevation. Temporarily withhold, reduce the dose, or permanently discontinue VITRAKVI based on severity [see Dosage and Administration ( 2.4)].
5.4 Embryo-Fetal Toxicity
Based on literature reports in human subjects with congenital mutations leading to changes in TRK signaling, findings from animal studies, and its mechanism of action, VITRAKVI can cause fetal harm when administered to a pregnant woman. Larotrectinib resulted in malformations in rats and rabbits at maternal exposures that were approximately 11- and 0.7-times, respectively, those observed at the clinical dose of 100 mg twice daily. Advise women of the potential risk to a fetus. Advise females of reproductive potential to use an effective method of contraception during treatment and for 1 week after the last dose of VITRAKVI [see Use in Specific Populations (8.1, 8.3)].
6 Adverse Reactions
The following clinically significant adverse reactions are described elsewhere in the labeling:
The most common (> 20%) adverse reactions, including laboratory abnormalities, with VITRAKVI were increased AST, increased ALT, anemia musculoskeletal pain, fatigue, hypoalbuminemia, neutropenia, increased alkaline phosphatase, cough, leukopenia, constipation, diarrhea, dizziness, hypocalcemia, nausea, vomiting, pyrexia, lymphopenia, and abdominal pain (6 ).
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Unless noted, data in WARNINGS AND PRECAUTIONS and below reflects exposure to VITRAKVI in 279 patients, including 54% patients exposed for greater than 6 months and 30% patients exposed for greater than 1 year. VITRAKVI was studied in one adult dose-finding trial [LOXO‑TRK-14001 (n = 75)], one pediatric dose-finding trial [SCOUT (n = 88)], and one single arm trial [NAVIGATE (n = 116)]. All patients had an unresectable or metastatic solid tumor and no satisfactory alternative treatment options or disease progression following treatment.
Across these 279 patients, the median age was 46 years (range: 0.1 days to 84 years); 33% were younger than 18 years; 48% were male; and 74% were White, 9% were Hispanic/Latino, 7% were Asian and, 6% were Black. The most common tumors (≥3%) in order of decreasing frequency were soft tissue sarcoma (17%), thyroid (13%), infantile fibrosarcoma (12%), primary central nervous system (CNS) (11%), salivary gland (9%), lung (9%), colon (6%), breast (4%), or melanoma (3%). NTRK gene fusions were confirmed or inferred in 75% of VITRAKVI-treated patients. Most adults (86%) received VITRAKVI 100 mg orally twice daily and 85% of pediatrics (< 18 years) received VITRAKVI 100 mg/m2 twice daily up to a maximum dose of 100 mg twice daily. The dose ranged from 50 mg daily to 200 mg twice daily in adults and 9.6 mg/m2 twice daily to 120 mg/m2 twice daily in pediatrics [see Use in Specific Populations (8.4)].
The most common adverse reactions (≥ 20%), including laboratory abnormalities, in order of decreasing frequency were increased AST, increased ALT, anemia, musculoskeletal pain, fatigue, hypoalbuminemia, neutropenia, increased alkaline phosphatase, cough, leukopenia, constipation, diarrhea, dizziness, hypocalcemia, nausea, vomiting, pyrexia, lymphopenia, and abdominal pain.
The most common serious adverse reactions (≥ 2%) were pneumonia and pyrexia. Grade 3 or 4 adverse reactions occurred in 53% of patients; adverse reactions leading to dose interruption or modification occurred in 39% and 8% of patients, respectively, and 9% permanently discontinued VITRAKVI for adverse reactions.
The most common adverse reactions (1% each) that resulted in permanent discontinuation of VITRAKVI were increased ALT, increased AST, dehydration and fatigue.
The most common adverse reactions (≥ 3%) resulting in dose interruption were increased ALT (4.7%), increased AST (4.3%), and neutrophil count decreased (4.3%). Most (70%) adverse reactions leading to dose interruption occurred during the first three months of exposure.
Adverse reactions of VITRAKVI occurring in ≥ 10% of patients and laboratory abnormalities worsening from baseline in ≥ 20% of patients are summarized in Table 3 and Table 4, respectively.
Table 3 Adverse Reactions Occurring in ≥ 10% of Patients Treated with VITRAKVI
Adverse Reaction* VITRAKVI NÂ =Â 279 All Grades** (%) Grade 3-4*** (%)
Musculoskeletal and Connective Tissue
 Musculoskeletal Pain1
42
3.9
 Muscular weakness
10
0.7
General
 Fatigue2
36
2.5
 Pyrexia
24
1.8
 Edema3
19
0.7
Respiratory, Thoracic and Mediastinal
 Cough4
32
0.4
 Dyspnea5
17
3
 Upper respiratory tract infection
13
0
 Nasal congestion
11
0
Nervous System
 Dizziness6
27
1.1
 Headache
15
0.4
 Cognitive Impairment7
11
2.5
Gastrointestinal
 Constipation
27
0.4
 Diarrhea
27
1.4
 Nausea
25
0.7
 Vomiting
25
0.7
 Abdominal pain8
21
2.2
Skin and Subcutaneous Tissue Disorders
 Rash9
19
0.4
Psychiatric
 Mood disorders10
14
0.4
 Sleep Disturbance11
10
0
Investigations
 Increased weight
14
3.6
Metabolism and Nutrition
 Decreased appetite
12
1.4
Infections and Infestations
 Urinary tract infection12
12
1.4
* The adverse reaction identifies a composite term:
1Includes: arthralgia, back pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal pain, musculoskeletal stiffness, myalgia, neck pain, non-cardiac chest pain, and pain in extremity
2Includes: fatigue, asthenia
3Includes: face edema, generalized edema, lip edema, localized edema, edema, edema genital, edema peripheral, periorbital edema, and swelling
4Includes: cough, productive cough, and upper-airway cough syndrome
5Includes: dyspnea, and dyspnea exertional
6Includes: dizziness, dizziness postural, and vertigo
7Includes: amnesia, aphasia, cognitive disorder, confusional state, delirium, disturbance in attention, hallucinations, memory impairment, mental impairment, mental status changes
8Includes: abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, abdominal tenderness, epigastric discomfort, and gastrointestinal pain
9Includes: dermatitis, dermatitis acneiform, dermatitis bullous, dermatitis exfoliative generalized, eczema, eczema asteatotic, palmar-plantar erythrodysaesthesia syndrome, rash, rash erythematous, rash macular, rash maculo-papular, rash papular, rash pruritic, and rash pustular
10Includes: agitation, anxiety, depression, depressed mood, euphoric mood, irritability
11Includes: insomnia, sleep disorder, somnolence
12Includes: cystitis, escherichia urinary tract infection, pyelonephritis acute, and urinary tract infection
** National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) v 4.03.
*** One Grade 4 adverse reaction of pyrexia.
Clinically relevant adverse reactions occurring in ≤ 10% of patients include fractures (8%).
Table 4 Laboratory Abnormalities Occurring in ≥ 20% Patients Treated with VITRAKVI
Laboratory Abnormality
VITRAKVI*
All Grades (%)**
Grade 3-4
(%)**
Chemistry
 Increased AST
52
3.1
 Increased ALT
45
2.5
 Hypoalbuminemia
36
1.9
 Increased alkaline phosphatase
34
1.9
 Hypocalcemia
25
2.6
Hematology
 Anemia
42
10
 Neutropenia
36
14
 Leukopenia
28
1.9
 Lymphopenia
22
6
* Based on NCI CTCAE v4.03
** Denominator for each laboratory parameter is based on the number of patients with a baseline and post-treatment laboratory value available which ranged from 272 to 277 patients.
7 Drug Interactions
• Strong CYP3A4 Inhibitors: Avoid coadministration of strong CYP3A4 inhibitors with VITRAKVI. If coadministration cannot be avoided, reduce the VITRAKVI dose. (2.5 ,7.1 )• Moderate CYP3A4 Inhibitors: Monitor for adverse reactions more frequently in patients coadministered a moderate CYP3A4 inhibitor with VITRAKVI and reduce the VITRAKVI dosage based on severity of adverse reactions. (7.1 )• Strong CYP3A4 Inducers: Avoid coadministration of strong CYP3A4 inducers with VITRAKVI. If coadministration cannot be avoided, increase the VITRAKVI dose. (2.6 ,7.1 )• Moderate CYP3A4 Inducers: Increase the VITRAKVI dose. (2.6 ,7.1 )• Sensitive CYP3A4 Substrates: Avoid coadministration of sensitive CYP3A4 substrates with VITRAKVI. (7.2 )7.1 Effects of Other Drugs on VITRAKVI
Strong and Moderate CYP3A4 Inhibitors
Coadministration of VITRAKVI with a strong or moderate CYP3A4 inhibitor may increase larotrectinib plasma concentrations, which may result in a higher incidence of adverse reactions [see Clinical Pharmacology (12.3)]. Avoid coadministration of VITRAKVI with strong CYP3A4 inhibitors, including grapefruit or grapefruit juice. If coadministration of strong CYP3A4 inhibitors cannot be avoided, modify VITRAKVI dose as recommended [see Dosage and Administration (2.5)]. In patients coadministered a moderate CYP3A4 inhibitor with VITRAKVI, monitor for adverse reactions more frequently and reduce the VITRAKVI dosage based on the severity of emergent adverse reactions [see Dosage and Administration (2.3)].
Strong and Moderate CYP3A4 Inducers
Coadministration of VITRAKVI with a strong or moderate CYP3A4 inducer may decrease larotrectinib plasma concentrations, which may decrease the efficacy of VITRAKVI [see Clinical Pharmacology (12.3)]. Avoid coadministration of VITRAKVI with strong CYP3A4 inducers, including St. John’s wort. If coadministration of strong CYP3A4 inducers cannot be avoided, modify VITRAKVI dose as recommended. For coadministration with moderate CYP3A4 inducers, modify VITRAKVI dose as recommended [see Dosage and Administration (2.6)].
7.2 Effects of VITRAKVI on Other Drugs
Sensitive CYP3A4 Substrates
Coadministration of VITRAKVI with sensitive CYP3A4 substrates may increase their plasma concentrations, which may increase the incidence or severity of adverse reactions [see Clinical Pharmacology (12.3)]. Avoid coadministration of VITRAKVI with sensitive CYP3A4 substrates. If coadministration of these sensitive CYP3A4 substrates cannot be avoided, monitor patients for increased adverse reactions of these drugs.
8 Use In Specific Populations
• Lactation: Advise not to breastfeed. (8.2 )• Hepatic Impairment: Reduce the starting dose of VITRAKVI in patients with moderate (Child-Pugh B) to severe (Child-Pugh C) hepatic impairment. (2.7 ,8.7 )8.1 Pregnancy
Risk Summary
Based on literature reports in human subjects with congenital mutations leading to changes in TRK signaling, findings from animal studies, and its mechanism of action [see Clinical Pharmacology (12.1)], VITRAKVI can cause embryo-fetal harm when administered to a pregnant woman. There are no available data on VITRAKVI use in pregnant women. Administration of larotrectinib to pregnant rats and rabbits during the period of organogenesis resulted in malformations at maternal exposures that were approximately 11- and 0.7-times, respectively, those observed at the clinical dose of 100 mg twice daily (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Published reports of individuals with congenital mutations in TRK pathway proteins suggest that decreases in TRK-mediated signaling are correlated with obesity, developmental delays, cognitive impairment, insensitivity to pain, and anhidrosis.
Animal Data
Larotrectinib crosses the placenta in animals. Larotrectinib did not result in embryolethality at maternally toxic doses [up to 40 times the human exposure based on area under the curve (AUC) at the clinical dose of 100 mg twice daily] in embryo-fetal development studies in pregnant rats dosed during the period of organogenesis; however, larotrectinib was associated with fetal anasarca in rats from dams treated at twice-daily doses of 40 mg/kg [11 times the human exposure (AUC) at the clinical dose of 100 mg twice daily]. In pregnant rabbits, larotrectinib administration was associated with omphalocele at twice-daily doses of 15 mg/kg (0.7 times the human exposure at the clinical dose of 100 mg twice daily).
8.2 Lactation
Risk Summary
There are no data on the presence of larotrectinib or its metabolites in human milk and no data on its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with larotrectinib and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating VITRAKVI [see Use in Specific Populations (8.1)].
Contraception
VITRAKVI can cause embryo-fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Females
Advise female patients of reproductive potential to use effective contraception during treatment with VITRAKVI and for 1 week after the last dose.
Males
Advise males with female partners of reproductive potential to use effective contraception during treatment with VITRAKVI and for 1 week after the last dose.
Infertility
Females
Based on histopathological findings in the reproductive tracts of female rats in a 1-month repeated-dose study, VITRAKVI may reduce fertility [See Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of VITRAKVI in pediatric patients was established based upon data from three multicenter, open-label, single-arm clinical trials in adult or pediatric patients 28Â days and older [see Adverse Reactions (6.1), Clinical Studies (14)].
The efficacy of VITRAKVI was evaluated in 12 pediatric patients and is described in the Clinical Studies section [see Clinical Studies (14)]. The safety of VITRAKVI was evaluated in 92 pediatric patients who received VITRAKVI. Of these 92 patients, 36% were <1 month to < 2 years (n = 33), 41% were 2 years to < 12 years (n = 38), and 23% were 12 years to < 18 years (n = 21); 29% had metastatic disease, 42% had locally advanced disease, and 27% had primary CNS; and 86% had received prior treatment for their cancer, including surgery, radiotherapy, or systemic therapy. The most common cancers were infantile fibrosarcoma (37%), primary CNS tumors (27%), soft tissue sarcoma (24%), and thyroid cancer (7%). The median duration of exposure was 7.4 months (range: 0.4 months to 39 months).
Due to the small number of pediatric and adult patients, the single arm design of clinical studies of VITRAKVI, and confounding factors such as differences in susceptibility to infections between pediatric and adult patients, it is not possible to determine whether differences in the incidence of adverse reactions to VITRAKVI are related to patient age or other factors. Adverse reactions occurring more frequently (at least a 10% increase in per-patient incidence) in pediatric patients compared to adult patients were pyrexia (45% versus 13%), vomiting (42% versus 17% in adults), diarrhea (35% versus 23% in adults), rash (28% versus 15% in adults), upper respiratory tract infection (23% versus 8% in adults), nasopharyngitis (16% versus 6% in adults), and otitis media and rhinitis (each 14% versus 0.5% in adults).
Laboratory abnormalities occurring more frequently (at least a 10% increase in per-patient incidence) in pediatric patients compared to adult patients were AST increased (63% versus 49% in adults), neutrophil count decrease (60% versus 16% in adults), leukocyte count decrease (39% versus 27% in adults), hyperkalemia (36% versus 15%), and lymphocyte increase (24% versus 0.5%). Two of the 92 pediatric patients discontinued VITRAKVI due to an adverse reaction (Grade 3 increased ALT and Grade 3 decreased neutrophil count).
The pharmacokinetics of VITRAKVI in the pediatric population were similar to those seen in adults [see Clinical Pharmacology (12.3)].
Juvenile Animal Toxicity Data
Larotrectinib was administered in a juvenile toxicity study in rats at twice daily doses of 0.2, 2Â and 7.5 mg/kg from postnatal day (PND) 7 to 27 and at twice daily doses of 0.6, 6 and 22.5mg/kg between PND 28 and 70. The dosing period was equivalent to human pediatric populations from newborn to adulthood. The doses of 2/6 mg/kg twice daily [approximately 0.7Â times the human exposure (AUC) at the clinical dose of 100 mg twice daily] and 7.5/22.5 mg/kg twice daily (approximately 4 times the human exposure at the clinical dose of 100 mg twice daily) resulted in mortality between PND 9 to 99; a definitive cause of death was not identified in the majority of cases.
The main findings were transient central nervous system-related signs including head flick, tremor, and circling in both sexes. An increase in the number of errors in a maze swim test occurred in females at exposures of approximately 4 times the human exposure (AUC) at the clinical dose of 100 mg twice daily. Decreased growth and delays in sexual development occurred in the mid- and high-dose groups. Mating was normal in treated animals, but a reduction in pregnancy rate occurred at the high-dose of 7.5/22.5 mg/kg twice daily (approximately 4 times the human exposure at the clinical dose of 100 mg twice daily).
8.5 Geriatric Use
Of 279 patients in the overall safety population who received VITRAKVI, 19% of patients were ≥ 65 years of age and 5% of patients were ≥ 75 years of age. Clinical studies of VITRAKVI did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
8.6 Hepatic Impairment
No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A). Larotrectinib clearance was reduced in subjects with moderate (Child-Pugh B) to severe (Child-Pugh C) hepatic impairment [see Clinical Pharmacology (12.3)]. Reduce VITRAKVI dose as recommended [see Dosage and Administration ( 2.7 )].
8.7 Renal Impairment
No dose adjustment is recommended for patients with renal impairment of any severity [see Clinical Pharmacology (12.3)].
11 Description
Larotrectinib is a kinase inhibitor. VITRAKVI (larotrectinib) capsules and oral solution are formulated using larotrectinib sulfate. The molecular formula for larotrectinib sulfate is C21H24F2N6O6S and the molecular weight is 526.51 g/mol for the sulfate salt and 428.44 g/mol for the free base. The chemical name is (3S)-N-{5-[(2R)-2-(2,5-difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-a]pyrimidin-3-yl}-3-hydroxy-1-pyrrolidinecarboxamide sulfate. Larotrectinib sulfate has the following chemical structure:
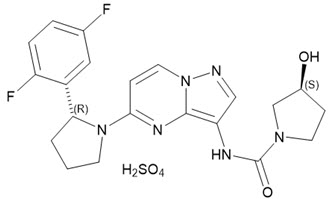
Larotrectinib sulfate is an off-white to pinkish yellow solid that is not hygroscopic. The aqueous solubility of larotrectinib at 37á´¼C is pH dependent (very soluble at pH 1.0 and freely soluble at pH 6.8, according to USP descriptive terms of solubility).
VITRAKVI (larotrectinib) capsules and oral solution are for oral use. Each capsule contains 25 mg or 100Â mg larotrectinib (30.7 mg and 123 mg larotrectinib sulfate, respectively) in a hard gelatin capsule. The capsule is composed of gelatin, titanium dioxide, and edible ink.
The oral solution packaged in one bottle containing 100 mL contains 20 mg/mL larotrectinib (24.6Â mg/mL larotrectinib sulfate) and the following inactive ingredients: purified water, hydroxypropyl betadex, sucrose, glycerin, sorbitol, citric acid, sodium phosphate, sodium citrate dihydrate, propylene glycol and flavoring. Preserved with methylparaben and potassium sorbate.
The oral solution packaged in two bottles each containing 50 mL contains 20 mg/mL larotrectinib (24.6Â mg/mL larotrectinib sulfate) and the following inactive ingredients: purified water, hydroxypropyl betadex, sucralose, sodium citrate, strawberry flavor, and citric acid. Preserved with sodium benzoate.
12 Clinical Pharmacology
12.1 Mechanism of Action
Larotrectinib is an inhibitor of the tropomyosin receptor kinases (TRK), TRKA, TRKB, and TRKC. In a broad panel of purified enzyme assays, larotrectinib inhibited TRKA, TRKB, and TRKC with IC50 values between 5-11 nM. One other kinase TNK2 was inhibited at approximately 100-fold higher concentration. TRKA, B, and C are encoded by the genes NTRK1, NTRK2, and NTRK3. Chromosomal rearrangements involving in-frame fusions of these genes with various partners can result in constitutively-activated chimeric TRK fusion proteins that can act as an oncogenic driver, promoting cell proliferation and survival in tumor cell lines.
In in vitro and in vivo tumor models, larotrectinib demonstrated anti-tumor activity in cells with constitutive activation of TRK proteins resulting from gene fusions, deletion of a protein regulatory domain, or in cells with TRK protein overexpression. Larotrectinib had minimal activity in cell lines with point mutations in the TRKA kinase domain, including the clinically identified acquired resistance mutation, G595R. Point mutations in the TRKC kinase domain with clinically identified acquired resistance to larotrectinib include G623R, G696A, and F617L.
12.2 Pharmacodynamics
Cardiac Electrophysiology
At a dose 9-fold higher than the recommended adult dose, VITRAKVI does not prolong QTc intervals to any clinically relevant extent.
12.3 Pharmacokinetics
The pharmacokinetics of larotrectinib were studied in healthy subjects and adult and pediatric patients with locally advanced or metastatic solid tumors. In healthy subjects who received a single dose of VITRAKVI capsules, systemic exposure (Cmax and AUC) of larotrectinib was dose proportional over the dose range of 100 mg to 400 mg (1 to 4 times the recommended adult dose) and slightly greater than proportional at doses of 600 mg to 900 mg (6 to 9 times the recommended adult dose). In adult patients who received VITRAKVI capsules 100 mg twice daily in Study LOXO-TRK-14001, peak plasma levels (Cmax) of larotrectinib were achieved at approximately 1 hour after dosing and steady-state was reached within 3 days. Mean steady-state larotrectinib [coefficient of variation (CV%)] for Cmax was 788 (81%) ng/mL and AUC0-24hr was 4351 (97%) ng*h/mL.
Absorption
The mean absolute bioavailability of VITRAKVI capsules was 34% (range: 32% to 37%). In healthy subjects, the AUC of VITRAKVI oral solution was similar to that of the capsules and the Cmax was 36% higher with the oral solution.
Effect of Food
The AUC of larotrectinib was similar and the Cmax was reduced by 35% after oral administration of a single 100 mg capsule of VITRAKVI to healthy subjects taken with a high-fat meal (approximately 900 calories, 58 grams carbohydrate, 56 grams fat and 43 grams protein) compared to the Cmax and AUC in the fasted state.
Distribution
The mean (CV%) volume of distribution (Vss) of larotrectinib is 48 (38%) L following intravenous administration of larotrectinib in healthy subjects.
Larotrectinib is 70% bound to human plasma proteins in vitro and binding is independent of drug concentrations. The blood-to-plasma concentration ratio is 0.9.
Elimination
The mean (CV%) clearance (CL/F) of larotrectinib is 98 (44%) L/h and the half-life is 2.9 hours following oral administration of VITRAKVI in healthy subjects.
Metabolism
Larotrectinib is metabolized predominantly by CYP3A4. Following oral administration of a single [14C] radiolabeled 100 mg dose of larotrectinib to healthy subjects, unchanged larotrectinib constituted 19% and an O-linked glucuronide constituted 26% of the major circulating radioactive drug components in plasma.
Excretion
Following oral administration of a single [14C] radiolabeled 100 mg dose of larotrectinib to healthy subjects, 58% (5% unchanged) of the administered radioactivity was recovered in feces and 39% (20% unchanged) was recovered in urine.
Specific Populations
Age (range: 28 days to 82 years), sex, and body weight (range: 3.8 kg to 179 kg) had no clinically meaningful effect on the pharmacokinetics of larotrectinib.
Pediatric Patients
In pediatric patients, the larotrectinib geometric mean (%CV) AUC0-24hr by age subgroup was: 3348 (66%) ng*h/mL in patients 1 month to < 2 years (n = 9), 4135 (36%) ng*h/mL in patients 2 to < 12 years (n = 15), and 3108 (69%) ng*h/mL and in patients 12 to < 18 years (n = 9).
Patients with Renal Impairment
Following oral administration of a single 100 mg dose of VITRAKVI capsules in subjects with end-stage renal disease (e.g., subjects who required dialysis), the AUC0-INF of larotrectinib increased 1.5-fold and Cmax increased 1.3-fold as compared to that in subjects with normal renal function (creatinine clearance ≥ 90 mL/min as estimated by Cockcroft-Gault). The pharmacokinetics of VITRAKVI in patients with moderate to severe renal impairment (creatinine clearance ≤ 60 mL/min) have not been studied.
Patients with Hepatic Impairment
Following oral administration of a single 100 mg dose of VITRAKVI capsules, the AUC0-INF of larotrectinib increased 1.3-fold in subjects with mild hepatic impairment (Child-Pugh A), 2-fold in subjects with moderate hepatic impairment (Child-Pugh B) and 3.2-fold in subjects with severe hepatic impairment (Child-Pugh C) as compared to that in subjects with normal hepatic function. The Cmax was similar in subjects with mild and moderate hepatic impairment and the Cmax of larotrectinib increased 1.5-fold in subjects with severe hepatic impairment as compared to that in subjects with normal hepatic function [see Dosage and Administration ( 2.7 ), Use in Specific Populations (8.6)].
Drug Interaction Studies
Clinical Studies
Effect of CYP3A Inhibitors: Coadministration of a single 100 mg dose of VITRAKVI capsules with itraconazole (strong CYP3A inhibitor) increased the AUC0-INF of larotrectinib by 4.3-fold and the Cmax by 2.8-fold as compared to VITRAKVI administered alone [see Dosage and Administration (2.5), Drug Interactions (7.1)].
Coadministration of VITRAKVI with fluconazole (moderate CYP3A4 inhibitor) is predicted to increase VITRAKVI steady state AUC by 2.7-fold and Cmax by 1.9-fold.
Effect of CYP3A Inducers: Coadministration of a single 100 mg dose of VITRAKVI capsules with rifampin (strong CYP3A inducer) decreased the AUC0-INF of larotrectinib by 81% and the Cmax by 71% as compared to VITRAKVI administered alone [see Dosage and Administration (2.6), Drug Interactions (7.1)].
Coadministration of VITRAKVI with efavirenz (moderate CYP3A4 inducer) is predicted to decrease steady state AUC of VITRAKVI by approximately 72% and Cmax by 60% compared to VITRAKVI administered alone [see Dosage and Administration (2.6), Drug Interactions (7.1)].
Effect of Strong P-glycoprotein (P-gp) Inhibitors: Coadministration of a single 100 mg dose of VITRAKVI capsules with a P-gp inhibitor (rifampin) increased the AUC0-INF of larotrectinib by 1.7-fold and the Cmax by 1.8-fold as compared to VITRAKVI administered alone.
Effect of Larotrectinib on CYP3A4 Substrates: Coadministration of VITRAKVI capsules 100 mg twice daily with a sensitive CYP3A4 substrate (midazolam) increased both the AUC0-INF and Cmax of midazolam by 1.7-fold as compared to midazolam administered alone. The AUC0-INF and Cmax of 1-hydroxymidazolam, the main metabolite of midazolam, were both increased 1.4-fold as compared to when midazolam was administered alone [see Drug Interactions (7.2)].
In Vitro Studies
Effect of Transporter on Larotrectinib: Larotrectinib is a substrate for P-gp and BCRP. Larotrectinib is not a substrate of OAT1, OAT3, OCT1, OCT2, OATP1B1, or OATP1B3.
Effect of Larotrectinib on Transporters: Larotrectinib is not an inhibitor of BCRP, P-gp, OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, BSEP, MATE1 and MATE2-K at clinically relevant concentrations.
Effect of Larotrectinib on CYP Substrates: Larotrectinib is not an inhibitor or inducer of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2D6 at clinically relevant concentrations.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with larotrectinib. Larotrectinib was not mutagenic in the in vitro bacterial reverse mutation (Ames) assays, with or without metabolic activation, or in the in vitro mammalian mutagenesis assays, with or without metabolic activation. In vivo, larotrectinib was negative in the mouse micronucleus test.
Fertility studies with larotrectinib have not been conducted. In a 3-month repeat-dose toxicity study in the rat, larotrectinib had no effects on spermatogenesis at 75 mg/kg/day (approximately 7 times the human exposure at the 100 mg twice daily dose). Additionally, larotrectinib had no histological effects on the male reproductive tract in rats or monkeys at doses resulting in exposures up to 10 times the human exposure (AUC0-24hr) at the 100 mg twice daily clinical dose.
In a 1-month repeat-dose study in the rat, decreased uterine weight and uterine atrophy were seen at 200 mg/kg/day [approximately 45 times the human exposure (AUC) at the 100 mg twice daily dose]. Fewer corpora lutea and increased incidence of anestrus were also noted at doses ≥ 60 mg/kg/day (approximately 10 times the human exposure at the 100 mg twice daily dose). Decreased fertility occurred in a juvenile animal study [see Use in Specific Populations (8.4)]. There were no findings in female reproductive organs in repeat-dose studies in monkeys at exposures up to 22 times the human exposure at the 100 mg twice daily dose.
13.2 Animal Toxicology and/or Pharmacology
In general toxicology studies conducted in rats and monkeys and in reproductive toxicology studies conducted in rats and rabbits, administration of larotrectinib led to increased food consumption and increased body weight at doses resulting in exposures 0.6 times the human exposure at the 100 mg twice daily clinical dose. Obesity has also been one phenotypic outcome of some human syndromes resulting from congenital mutations in NTRK2 resulting in altered TRK signaling.
14 Clinical Studies
The efficacy of VITRAKVI was evaluated in pediatric and adult patients with unresectable or metastatic solid tumors with a NTRK gene fusion enrolled in one of three multicenter, open-label, single-arm clinical trials: Study LOXO-TRK-14001 (NCT02122913), SCOUT (NCT02637687), and NAVIGATE (NCT02576431). All patients were required to have progressed following systemic therapy for their disease, if available, or would have required surgery with significant morbidity for locally advanced disease.
Adult patients received VITRAKVI 100 mg orally twice daily and pediatric patients (18 years or younger) received VITRAKVI 100 mg/m2 up to a maximum dose of 100 mg orally twice daily until unacceptable toxicity or disease progression. Identification of positive NTRK gene fusion status was prospectively determined in local laboratories using next generation sequencing (NGS) or fluorescence in situ hybridization (FISH). NTRK gene fusions were inferred in three patients with infantile fibrosarcoma who had a documented ETV6 translocation identified by FISH. The major efficacy outcome measures were overall response rate (ORR) and duration of response (DOR), as determined by a blinded independent review committee (BIRC) according to RECIST v1.1.
The assessment of efficacy was based on the first 55 patients with solid tumors with an NTRK gene fusion enrolled across the three clinical trials. Baseline characteristics were: median age 45 years (range 4 months to 76 years); 22% <18 years of age, and 78% ≥18 years of age; 53% male; 67% White; 7% Hispanic/Latino, 4% Asian, 4% Black; and ECOG performance status (PS) 0-1 (93%) or 2 (7%). Eighty-two percent of patients had metastatic disease, including patients with brain metastases, and 18% had locally advanced, unresectable disease. Ninety-eight percent of patients had received prior treatment for their cancer, including surgery, radiotherapy, or systemic therapy. Of these, 82% (n = 45) received prior systemic therapy with a median of two prior systemic regimens and 35% (n = 19) received three or more prior systemic regimens. The most common cancers were salivary gland tumors (22%), soft tissue sarcoma (20%), infantile fibrosarcoma (13%), and thyroid cancer (9%). A total of 50 patients had NTRK gene fusions detected by NGS and 5 patients had NTRK gene fusions detected by FISH.
Efficacy results are summarized in Tables 5, 6, and 7.
Table 5 Efficacy Results for Patients with Solid Tumors Harboring NTRK Gene Fusions
Efficacy Parameter
VITRAKVI
NÂ =Â 55
Overall response rate (95% CI)
75% (61%, 85%)
 Complete response rate
25%*
 Partial response rate
49%
Duration of response (DOR)
N = 41
 Median DOR
32.9** (14.8, NE***)
 Range (months)
1.6+, 50.6+
 % with Observed DOR > 12 months
63%
 % with Observed DOR > 24 months
49%
+ Denotes ongoing response.
* 5% were pathological complete response. Patients undergoing a surgical resection whose post-operative pathologic assessment showed no viable tumor cells and negative margins were pathological complete responders provided that no other sites of disease were present. **Kaplan-Meier estimates
***NE: Not evaluable
Table 6 Efficacy Results by Tumor Type
Tumor Type
Patients (N=55)
ORR
DOR
%
95% CI
Range (months)
Salivary gland
12
83%
(52%, 98%)
7.7, 44.7+
Soft tissue sarcoma
11
91%
(59%, 100%)
3.6+, 50.6+
Infantile fibrosarcoma
7
100%
(59%, 100%)
1.6+, 28.6+
Thyroid
5
100%
(48%, 100%)
3.7, 32.9
Lung
4
75%
(19%, 99%)
8.2, 36.8+
Melanoma
4
50%
NA
1.9+, 23.2+*
Colon
4
25%
NA
5.6*
Gastrointestinal stromal tumor
3
100%
(29%, 100%)
9.5, 31.1+
Cholangiocarcinoma
2
SD, NE
NA
NA
Appendix
1
SD
NA
NA
Breast
1
PD
NA
NA
Pancreas
1
SD
NA
NA
NA = not applicable due to small numbers or lack of response; CR = complete response; PR = partial response; NEÂ =Â not evaluable; SD = stable disease; PD = progressive disease; NR = not reached.+ Denotes ongoing response.
* Observed values at data cutoff, not a range.
Table 7 Efficacy Results by NTRK Fusion Partner
NTRK Partner*
Â
Patients(N=55)
ORR
DOR
%
95% CI
Range
(months)
ETV6-NTRK3
25
84%
(64%, 96%)
3.7, 44.7+
TPM3-NTRK1
9
56%
(21%, 86%)
3.7, 27.5+
LMNA-NTRK1
5
40%
(5%, 85%)
5.6, 50.6+
Inferred ETV6-NTRK3
3
100%
(29%, 100%)
1.6+, 28.6+**
IRF2BP2-NTRK1
2
CR, PR
NA
3.7, 36.8+
SQSTM1-NTRK1
2
pCR, PR
NA
9.9, 12.9+
PDE4DIP-NTRK1
1
PR
NA
3.6+ ***
PPL-NTRK1
1
CR
NA
28.2+ ***
STRN-NTRK2
1
PR
NA
5.6 ***
TPM4-NTRK3
1
pCR
NA
25.6+ ***
TPR-NTRK1
1
PR
NA
8.2 ***
TRIM63-NTRK1
1
PR
NA
1.9+ ***
CTRC-NTRK1
1
SD
NA
NA
GON4L-NTRK1
1
NE
NA
NA
PLEKHA6-NTRK1
1
SD
NA
NA
CR = complete response; PR = partial response; pCR = pathological complete response; NE = not evaluable; SD = stable disease; NA = not applicable; NR = Not reached.
+ Denotes ongoing response.
* Fusion partners identified in the primary analysis set (N=55) may not represent all potential fusion partners.
 ** Duration of response censored at the time of surgery for one pediatric patient with unresectable infantile fibrosarcoma who underwent resection following partial response and who remained disease-free at data cutoff.
***Observed values at data cutoff, not a range.
16 How Supplied/storage And Handling
Capsules
25 mg: Hard gelatin opaque white capsule size #2 with blue printing of "BAYER" cross and "25 mg" on the body of the capsule.
• 60 count bottle       NDC# 50419-390-01
100 mg: Hard gelatin opaque white capsule size #0 with blue printing of "BAYER" cross and "100 mg" on the body of the capsule.
• 60 count bottle       NDC# 50419-391-01
Store capsules at room temperature 20°C to 25°C (68°F to 77°F); temperature excursions between 15°C and 30°C (59°F to 86°F) are permitted [see USP Controlled Room Temperature].Â
Oral Solution Packaged in One Bottle Containing 100 mL
20 mg/mL: Clear yellow to orange solution.
• One bottle containing 100 mL       NDC# 50419-392-01
Refrigerate oral solution at 2°C to 8°C (36°F to 46°F). Do not freeze.
Oral Solution Packaged in Two Bottles Each Containing 50 mL
20 mg/mL: Colorless to yellow or orange or red or brownish solution.
• Two bottles each containing 50 mL NDC# 50419-393-03
Refrigerate oral solution at 2°C to 8°C (36°F to 46°F). Do not freeze.
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Central Nervous System Effects
Advise patients to notify their healthcare provider if they experience new or worsening neurotoxicity. Advise patients not to drive or operate hazardous machinery if they are experiencing neurologic adverse reactions [see Warnings and Precautions (5.1 )].
Skeletal Fractures
Inform patients that bone fractures have been reported in patients taking VITRAKVI. Advise patients to report symptoms such as pain, changes in mobility, or deformity to their healthcare provider [see Warnings and Precautions (5.2)].
Hepatotoxicity
Advise patients that they will need to undergo laboratory tests to monitor liver function [see Warnings and Precautions (5.3)].
Embryo-Fetal Toxicity
Advise males and females of reproductive potential of the potential risk to a fetus [see Warnings and Precautions (5.4), Use in Specific Populations (8.1)].
Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy and to use effective contraception during the treatment with VITRAKVI and for 1 week after the last dose [see Use in Specific Populations (8.3 )].
Advise males with female partners of reproductive potential to use effective contraception during treatment with VITRAKVI and for 1 week after the last dose [see Use in Specific Populations (8.3 )].
Lactation
Advise women not to breastfeed during treatment with VITRAKVI and for 1 week following the last dose [see Use in Specific Populations (8.2 )].
Infertility
Advise females of reproductive potential that VITRAKVI may impair fertility [See Nonclinical Toxicology (13.1)].
Drug Interactions
Advise patients and caregivers to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products. Inform patients to avoid St. John’s wort, grapefruit or grapefruit juice while taking VITRAKVI [see Drug Interactions (7.1, 7.2)].
Manufactured for Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981
Spl Patient Package Insert Section
PATIENT INFORMATION
VITRAKVI (vi trak vee)
(larotrectinib)
capsules
VITRAKVI (vi trak vee)
(larotrectinib)
oral solution
What is VITRAKVI? VITRAKVI is a prescription medicine that is used to treat adults and children with solid tumors (cancer) that:
• are caused by certain abnormal NTRK genes and• have spread or if surgery to remove their cancer is likely to cause severe complications, and• there is no acceptable treatment option or the cancer grew or spread on other treatment.
Your healthcare provider will perform a test to make sure that VITRAKVI is right for you.It is not known if VITRAKVI is safe and effective in children younger than 28 days of age.
Before taking VITRAKVI, tell your healthcare provider about all of your medical conditions, including if you:
• have liver problems• have nervous system (neurological) problems• are pregnant or plan to become pregnant. VITRAKVI can harm your unborn baby. Tell your healthcare provider right away if you become pregnant during treatment with VITRAKVI or think you may be pregnant.
• If you are able to become pregnant, your healthcare provider may do a pregnancy test before you start treatment with VITRAKVI.• Females who are able to become pregnant should use effective birth control (contraception) during treatment and for 1 week after the last dose of VITRAKVI. Talk to your healthcare provider about birth control methods that may be right for you.• Males with female partners who are able to become pregnant should use effective birth control during treatment with VITRAKVI and for 1 week after the last dose of VITRAKVI.• are breastfeeding or plan to breastfeed. It is not known if VITRAKVI passes into your breast milk. Do not breastfeed during treatment and for 1 week after the last dose of VITRAKVI.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain other medicines may affect how VITRAKVI works and VITRAKVI may affect how other medicines work. Know the medicines you take. Keep a ul of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take VITRAKVI?
• Take VITRAKVI exactly as your healthcare provider tells you.• Do not change your dose or stop taking VITRAKVI unless your healthcare provider tells you.• VITRAKVI comes in capsules and as an oral solution.• If your healthcare provider prescribes VITRAKVI oral solution:
• Your healthcare provider will provide you with the VITRAKVI oral solution, oral syringes and bottle adaptors or send you to a pharmacy that can provide you with VITRAKVI oral solution, oral syringes and bottle adaptors.• Your healthcare provider should show you how to correctly measure and give a dose of VITRAKVI oral solution.• See the detailed Instructions for Use that comes with VITRAKVI oral solution for information about the correct way to measure and give a dose of VITRAKVI oral solution. If you have any questions, talk to your healthcare provider or pharmacist.• VITRAKVI is usually taken by mouth 2 times a day.• Swallow VITRAKVI capsules whole with water. Do not chew or crush the capsules.• Take VITRAKVI with or without food.• If you vomit after taking a dose of VITRAKVI, wait and take the next dose at your scheduled time.• If you miss a dose of VITRAKVI, take it as soon as you remember. If your next scheduled dose is due within 6 hours, skip the missed dose and take your next dose at your regular time.• If you take too much VITRAKVI, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking VITRAKVI?
• VITRAKVI can make you feel dizzy. Do not drive or operate machinery until you know how VITRAKVI affects you.• Avoid taking St. John’s wort, eating grapefruit, or drinking grapefruit juice during treatment with VITRAKVI.
What are the possible side effects of VITRAKVI? VITRAKVI may cause serious side effects, including:
 •Central nervous system (CNS) problems. VITRAKVI may cause dizziness, confusion, problems with concentration, attention, and memory, changes in your mood, and sleep problems. Tell your healthcare provider if you develop any of these symptoms or they get worse. • Bone fractures . Bone fractures can happen with VITRAKVI. Tell your healthcare provider if you develop pain, changes in your ability to move around, or bone abnormalities. •Liver problems . Abnormal liver blood tests may occur with VITRAKVI and can sometimes become serious. Your healthcare provider will do blood tests to check your liver function before starting and during treatment with VITRAKVI as needed. Tell your healthcare provider right away if you develop new or worsening symptoms of liver problems including:• yellowing of your skin or the white part of your eyes (jaundice)• dark or brown urine• pain in the upper right side of your stomach area (abdomen)• bruising or bleeding more easily than normal• tiredness• nausea or vomiting• loss of appetite
Your healthcare provider may decrease your dose, temporarily stop or permanently stop your treatment with VITRAKVI if you develop serious side effects.
The most common side effects of VITRAKVI include:
• low red blood cell and white blood cell counts• muscle and bone pain• tiredness• low levels of protein called albumin in the blood• increased levels of enzyme called alkaline phosphatase in the blood (test for liver or bone problems)• increase in certain liver blood tests• cough• constipation• diarrhea• dizziness• low levels of calcium in the blood• nausea• vomiting• fever• stomach (abdomen) pain
VITRAKVI may affect fertility in females and may affect your ability to become pregnant. Talk to your healthcare provider if this is a concern for you.These are not all the possible side effects with VITRAKVI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store VITRAKVI?
• Store VITRAKVI capsules at room temperature between 68°F to 77°F (20°C to 25°C).• Store VITRAKVI oral solution in the refrigerator between 36° F to 46° F (2° C to 8° C). Do not freeze.• Throw away (dispose of) any unused VITRAKVI oral solution:
â–ª Bottle of 100 mL: remaining 90 days after first opening the bottleâ–ª Bottle of 50 mL: remaining 31 days after first opening the bottle
Keep VITRAKVI and all medicines out of the reach of children.
General information about the safe and effective use of VITRAKVI. Medicines are sometimes prescribed for purposes other than those uled in a Patient Information leaflet. Do not use VITRAKVI for a condition for which it was not prescribed. Do not give VITRAKVI to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about VITRAKVI that is written for health professionals.
What are the ingredients in VITRAKVI? Active ingredient: larotrectinib Inactive ingredients: Capsule: gelatin, titanium dioxide and edible ink Oral Solution Packaged in One Bottle Containing 100 mL: purified water, hydroxypropyl betadex, sucrose, glycerin, sorbitol, citric acid, sodium phosphate, sodium citrate dihydrate, propylene glycol and flavoring. Preserved with methylparaben and potassium sorbate.
Oral Solution Packaged in Two Bottles Each Containing 50 mL: purified water, hydroxypropyl betadex, sucralose, sodium citrate, strawberry flavor, and citric acid. Preserved with sodium benzoate.
Â
Manufactured for Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981
For more information, go to www.VITRAKVI.com or call 1-888-842-2937
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 11/2023
Instructions For Use Section
INSTRUCTIONS FOR USE VITRAKVI (vi trak vee) (larotrectinib) oral solution
Read this Instructions for Use before you take or give a dose of VITRAKVI oral solution for the first time and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.Your healthcare provider should show you how to correctly measure the prescribed dose of VITRAKVI oral solution before you take or give a dose for the first time. Important information about measuring VITRAKVI oral solution:
• Always use the oral syringes provided with VITRAKVI to make sure that you correctly measure the prescribed dose.• When you receive VITRAKVI oral solution from your healthcare provider or pharmacist, you will get a box that contains VITRAKVI oral solution. You may receive more than 1 box of VITRAKVI oral solution.• Your healthcare provider or pharmacist will also provide you bottle adaptors and syringes. You will receive one bottle adaptor per bottle of VITRAKVI oral solution. After inserting the bottle adaptor into the bottle, do not remove the bottle adaptor.• You will receive five 1 mL or 5 mL oral syringes that are marked to help you correctly measure the prescribed dose of VITRAKVI oral solution. Each oral syringe may be used over a 7-day period. Do not use a household teaspoon to measure the dose.
Supplies needed to take or give a dose of VITRAKVI oral solution
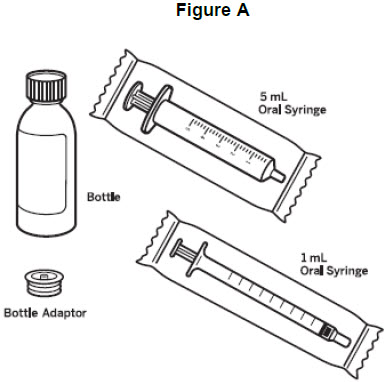
How to prepare a dose of VITRAKVI oral solution:
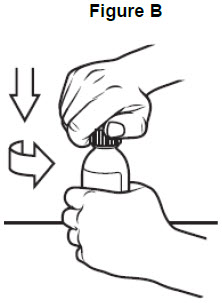
Step 1: Remove the VITRAKVI oral solution bottle from the box. Place the bottle on a flat work surface. Open the bottle by pushing down firmly on the child-resistant cap and turning it in the direction of the arrow (counter-clockwise) See Figure B. Do not throw away the child-resistant cap.
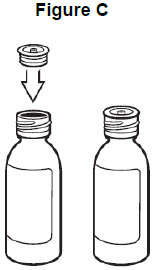
Step 2: Insert the bottle adaptor by pressing it into the bottle neck and make sure it is secure. See Figure C. Do not remove the bottle adaptor. If the bottle adaptor is missing, talk to your healthcare provider.
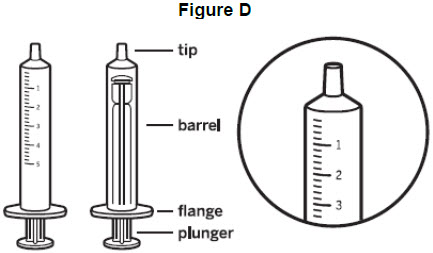
Step 3: Remove the oral syringe from the wrapper. Throw the wrapper away in your household trash. Look at the markings on the barrel of the oral syringe and find the marking that matches the VITRAKVI oral solution dose in mL prescribed by your healthcare provider. See Figure D.
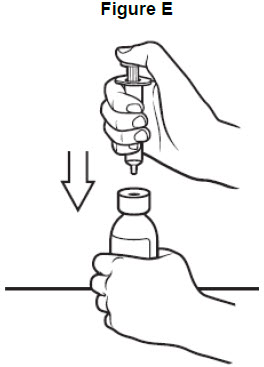
Step 4: With the bottle on your flat work surface, use 1 hand to hold the bottle upright. Using your other hand, push the air out of the oral syringe by pushing the plunger down. Then, insert the tip of the oral syringe into the bottle adaptor at the top of the bottle. See Figure E. The tip of the oral syringe should fit snugly into the hole of the bottle adaptor.
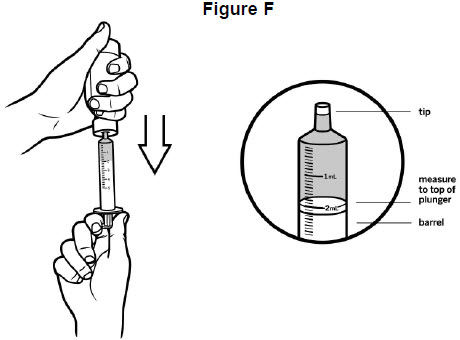
Step 5: Use 1 hand to hold the oral syringe in place. With the other hand, turn the bottle upside down. Pull back on the plunger until the top of the plunger lines up with the marking on the barrel of the oral syringe that matches the dose of VITRAKVI oral solution prescribed by your healthcare provider. See Figure F. Your dose may be different than the dose shown in Figure F.
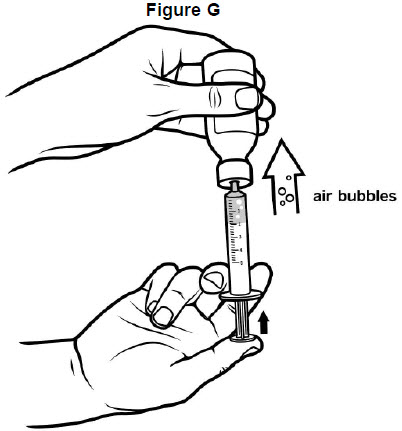
Step 6: Check for air bubbles in the oral syringe. If you see air bubbles, push up gently on the plunger to push any large air bubbles back into the bottle. Then, pull back on the plunger to the prescribed dose. See Figure G.
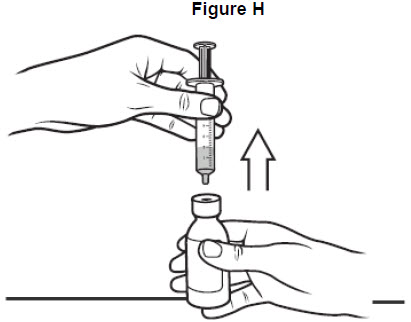
Step 7: Turn the bottle upright again and place it on your work surface. Remove the oral syringe from the bottle adaptor by gently pulling up on the syringe barrel. See Figure H. Do not push on the plunger during this step. The bottle adaptor should stay attached to the bottle.
Giving a dose of VITRAKVI oral solution by mouth:
Step 8: Place the tip of the oral syringe into the child’s mouth against the inside of the cheek. Slowly squirt VITRAKVI oral solution into the mouth by pressing down on the plunger and allow the child to swallow. See Figure I.
• The child should be kept in an upright position for a few minutes right after giving a dose of VITRAKVI.• If the child spits up a dose or you are not sure the entire dose was given, do not give another dose. Wait until the next scheduled dose.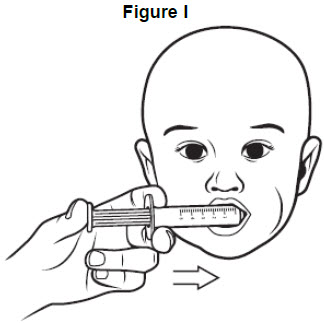
Step 9: Replace the child-resistant cap on the bottle of VITRAKVI oral solution. Do not remove the bottle adaptor. Close the bottle by turning the bottle cap in the direction of the arrow (clockwise). See Figure J.

Cleaning instructions for oral syringes
Follow the instructions below for cleaning the oral syringe (Step 10 through Step 16). After 7 days of use, throw away the oral syringe in your household trash. Use a new oral syringe for the next 7 days.
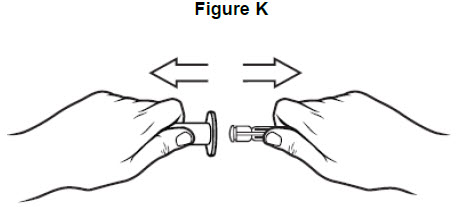
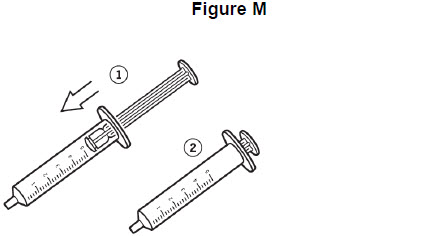
Step 10: Remove plunger from the barrel of the oral syringe. See Figure K.
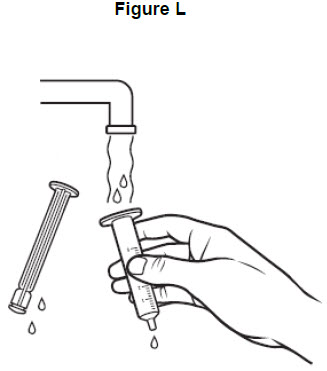
Step 11: Rinse the barrel and plunger in warm running water to help make sure that all of the medicine has been removed from the oral syringe. See Figure L. Do not boil the oral syringe.
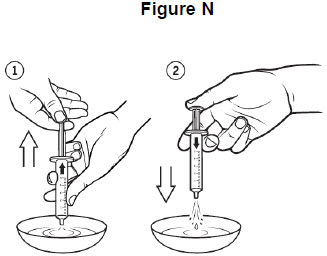
Step 12: Re-insert the plunger into the barrel of the oral syringe. See Figure M.
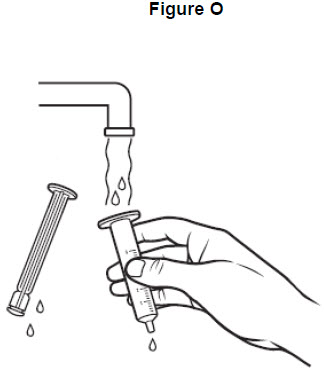
Step 13: Draw warm water several times into the oral syringe and squirt out again until all of the medicine has been removed from the oral syringe. See Figure N.
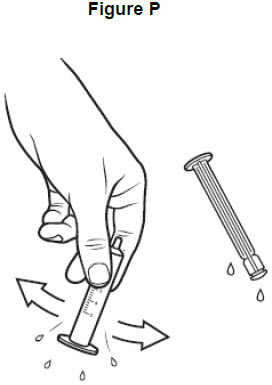
Step 14: Repeat steps 10 and 11 to rinse the barrel and plunger again with warm water. See Figure O.
Step 15: Shake off excess water or wipe off the outside. See Figure P. Place the barrel and plunger on a clean, dry paper towel to dry.
Step 16: Repeat step 12 to assemble the oral syringe and store in a clean place until the next use.Replace the oral syringe after 7 days of use, or if:
• there is any damage to the barrel, plunger, or tip• the dosage marking is no longer clearly recognizable or• it becomes difficult to move the plunger
How should I store VITRAKVI oral solution?
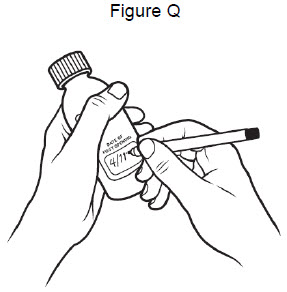
• Store VITRAKVI oral solution in a refrigerator between 36° F to 46° F (2° C to 8° C). Do not freeze.• Write the date that you opened the bottle of VITRAKVI oral solution on the bottle. See Figure Q.• Throw away (dispose of) any unused VITRAKVI oral solution:• Bottle of 100 mL: remaining 90 days after first opening the bottle• Bottle of 50 mL: remaining 31 days after first opening the bottle
Keep VITRAKVI oral solution and all medicines out of the reach of children.
Talk to your healthcare provider if you have questions about how to use VITRAKVI oral solution.
For more information, go to www.VITRAKVI.com or call 1-888-842-2937.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981
Revised: 12/2022
Package Label.principal Display Panel
Package Label - 25 mg - 60 Capsules
PRINCIPAL DISPLAY PANEL
NDC 71777-390-01
VITRAKVI®
(larotrectinib) capsules
25 mg
Usual Dosage: See prescribing information. Rx only. 60 capsules. Keep out of reach of children. Store at 20°C to 25°C (68°F to 77°F).Excursions permitted from 15°C to 30°C (59° to 86°F).
Manufactured for Loxo Omcology, Inc. Stamford, CT 06901Â Â Â Â Â Â 86579626
(01)10371777390012
Bayer
LOXO
Each capsule contains25 mg larotrectinib(equivalent to 30.7 mglarotrectinib sulfate).

Package Label.principal Display Panel
Package Label - 100 mg - 60 Capsules
PRINCIPAL DISPLAY PANEL
NDC 71777-391-01
VITRAKVI®
(larotrectinib) capsules
100 mg
Usual Dosage: See prescribing information.
Rx only. 60 capsules. Keep out of reach of children. Store at 20°C to 25°C (68°F to 77°F).Excursions permitted from 15°C to 30°C (59° to 86°F).
Manufactured for Loxo Omcology, Inc. Stamford, CT 06901Â Â Â Â Â Â 86579634
(01)10371777391019
Bayer
LOXO
Each capsulecontains 100 mglarotrectinib(equivalent to123 mglarotrectinibsulfate).

Package Label.principal Display Panel
Package Label - 20 mg/mL - 100 mL Oral Solution
PRINCIPAL DISPLAY PANEL
NDC 71777-392-01
VITRAKVI®
(larotrectinib) oral solution
20 mg/mL
Usual Dosage: See prescribing information. Keep refrigerated. Store at 2°C to 8°C (36°F to 46°F). Rx only. Date of first opening ____/____/____. Discard unused portion 90 days after first opening. 100 mL oral solution. Keep out of reach of children.
Manufactured for Loxo Omcology, Inc. Stamford, CT 06901Â Â Â Â Â Â 86659069
(01)10371777392016
Bayer
LOXO
Each bottle contains100 ml of 20 mg/mLlarotrectinib (equivalentto 24.6 mg/mLlarotrectinib sulfate).

Package/label Display Panel
Package Label - 20 mg/mL - 50 mL Oral Solution
NDC 50419-393-02
VITRAKVI®
(larotrectinib) oral solution
20 mg/mL
RECOMMENDED DOSAGE: See prescribing information. Keep refrigerated. Store at 2°C to 8°C (36°F to 46°F). Rx only. Date of first opening ____/____/____. Discard unused portion 31 days after first opening. 50 mL oral solution. For Oral Administration Only. Keep out of reach of children.
Manufactured for Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981 88735951
(01)10350419393024
Bayer
Each bottle contains50 mL of 20 mg/mLlarotrectinib (equivalentto 24.6 mg/mLlarotrectinib sulfate).

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


