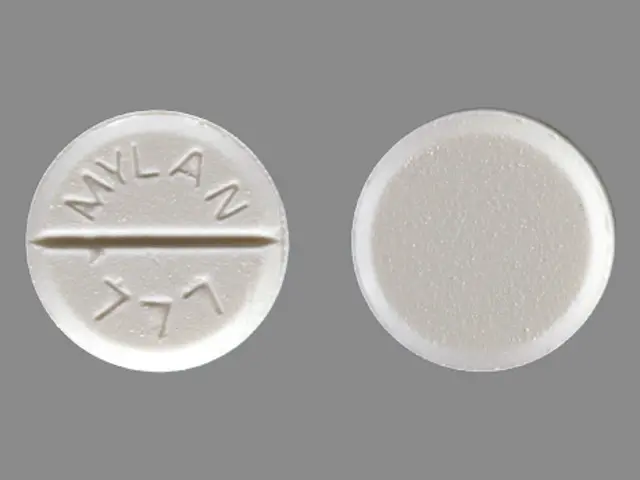
Lorazepam (lorazepam 2 mg) Dailymed
Generic: lorazepam
IMPRINT: MYLAN 777
SHAPE: round
COLOR: white SCORE: 2
All Imprints
lorazepam 0.5 mg - 321 321 m round white
lorazepam 1 mg - mylan 457 round white
lorazepam tablet - mylan 457 round white
lorazepam - lorazepam 0.5 mg oral tablet - m 321 round white
lorazepam 0.5 mg - m 321 round white
lorazepam 2 mg - mylan 777 round white
Boxed Warning
Warning: Risks From Concomitant Use With Opioids
-
• Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. -
• Limit dosages and durations to the minimum required. -
• Follow patients for signs and symptoms of respiratory depression and sedation.
Go PRO for all pill images
Warning: Risks From Concomitant Use With Opioids
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death (see WARNINGS; PRECAUTIONS: Clinically Significant Interactions).
• Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.• Limit dosages and durations to the minimum required.• Follow patients for signs and symptoms of respiratory depression and sedation.
Description
Lorazepam tablets, USP, an antianxiety agent, has the chemical formula, (±)-7-Chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-one:
![]()
C15H10Cl2N2O2 Â Â Â Â Â MW: 321.16
It is a white or almost white crystalline powder almost insoluble in water. Each lorazepam tablet, to be taken orally, contains 0.5 mg, 1 mg, or 2 mg of lorazepam, USP. The inactive ingredients present are lactose monohydrate, microcrystalline cellulose, polacrilin potassium and sodium stearyl fumarate.
Clinical Pharmacology
Studies in healthy volunteers show that in single high doses lorazepam tablets have a tranquilizing action on the central nervous system with no appreciable effect on the respiratory or cardiovascular systems.
Lorazepam tablets are readily absorbed with an absolute bioavailability of 90 percent. Peak concentrations in plasma occur approximately 2 hours following administration. The peak plasma level of lorazepam from a 2 mg dose is approximately 20 ng/mL.
The mean half-life of unconjugated lorazepam in human plasma is about 12 hours and for its major metabolite, lorazepam glucuronide, about 18 hours. At clinically relevant concentrations, lorazepam is approximately 85% bound to plasma proteins. Lorazepam tablets are rapidly conjugated at its 3-hydroxy group into lorazepam glucuronide which is then excreted in the urine. Lorazepam glucuronide has no demonstrable CNS activity in animals.
The plasma levels of lorazepam are proportional to the dose given. There is no evidence of accumulation of lorazepam on administration up to six months.
Studies comparing young and elderly subjects have shown that advancing age does not have a significant effect on the pharmacokinetics of lorazepam. However, in one study involving single intravenous doses of 1.5 mg to 3 mg of Ativan Injection, mean total body clearance of lorazepam decreased by 20% in 15 elderly subjects of 60 to 84 years of age compared to that in 15 younger subjects of 19 to 38 years of age.
Indications And Usage
Lorazepam tablets are indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety or anxiety associated with depressive symptoms. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The effectiveness of lorazepam tablets in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
Contraindications
Lorazepam tablets are contraindicated in patients with
- hypersensitivity to benzodiazepines or to any components of the formulation.- acute narrow-angle glaucoma.
Warnings
Concomitant use of benzodiazepines, including lorazepam tablets, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe lorazepam tablets concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of lorazepam tablets than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking lorazepam tablets, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when lorazepam tablets are used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS: Clinically Significant Drug Interactions).
Pre-existing depression may emerge or worsen during use of benzodiazepines including lorazepam. Lorazepam tablets are not recommended for use in patients with a primary depressive disorder or psychosis.
Use of benzodiazepines, including lorazepam, both used alone and in combination with other CNS depressants, may lead to potentially fatal respiratory depression (see PRECAUTIONS: Clinically Significant Drug Interactions).
Use of benzodiazepines, including lorazepam, may lead to physical and psychological dependence.
As with all patients on CNS depressant drugs, patients receiving lorazepam should be warned not to operate dangerous machinery or motor vehicles and that their tolerance for alcohol and other CNS depressants will be diminished.
Physical and Psychological Dependence
The use of benzodiazepines, including lorazepam, may lead to physical and psychological dependence. The risk of dependence increases with higher doses and longer term use and is further increased in patients with a history of alcoholism or drug abuse or in patients with significant personality disorders. The dependence potential is reduced when lorazepam is used at the appropriate dose for short-term treatment. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving lorazepam or other psychotropic agents.
In general, benzodiazepines should be prescribed for short periods only (e.g., 2 to 4 weeks). Extension of the treatment period should not take place without reevaluation of the need for continued therapy. Continuous long-term use of product is not recommended. Withdrawal symptoms (e.g., rebound insomnia) can appear following cessation of recommended doses after as little as one week of therapy. Abrupt discontinuation of product should be avoided and a gradual dosage-tapering schedule followed after extended therapy.
Abrupt termination of treatment may be accompanied by withdrawal symptoms. Symptoms reported following discontinuation of benzodiazepines include headache, anxiety, tension, depression, insomnia, restlessness, confusion, irritability, sweating, rebound phenomena, dysphoria, dizziness, derealization, depersonalization, hyperacusis, numbness/tingling of extremities, hypersensitivity to light, noise, and physical contact/perceptual changes, involuntary movements, nausea, vomiting, diarrhea, loss of appetite, hallucinations/delirium, convulsions/seizures, tremor, abdominal cramps, myalgia, agitation, palpitations, tachycardia, panic attacks, vertigo, hyperreflexia, short-term memory loss, and hyperthermia. Convulsions/seizures may be more common in patients with pre-existing seizure disorders or who are taking other drugs that lower the convulsive threshold such as antidepressants.
There is evidence that tolerance develops to the sedative effects of benzodiazepines.
Lorazepam may have abuse potential, especially in patients with a history of drug and/or alcohol abuse.
Precautions
In patients with depression, a possibility for suicide should be borne in mind; benzodiazepines should not be used in such patients without adequate antidepressant therapy.
Lorazepam should be used with caution in patients with compromised respiratory function (e.g., COPD, sleep apnea syndrome).
Elderly or debilitated patients may be more susceptible to the sedative effects of lorazepam. Therefore, these patients should be monitored frequently and have their dosage adjusted carefully according to patient response; the initial dosage should not exceed 2 mg.
Paradoxical reactions have been occasionally reported during benzodiazepine use. Such reactions may be more likely to occur in children and the elderly. Should these occur, use of the drug should be discontinued.
The usual precautions for treating patients with impaired renal or hepatic function should be observed. As with all benzodiazepines, the use of lorazepam may worsen hepatic encephalopathy; therefore, lorazepam should be used with caution in patients with severe hepatic insufficiency and/or encephalopathy. Dosage for patients with severe hepatic insufficiency should be adjusted carefully according to patient response; lower doses may be sufficient in such patients.
In patients where gastrointestinal or cardiovascular disorders coexist with anxiety, it should be noted that lorazepam has not been shown to be of significant benefit in treating the gastrointestinal or cardiovascular component.
Esophageal dilation occurred in rats treated with lorazepam for more than one year at 6 mg/kg/day. The no-effect dose was 1.25 mg/kg/day (approximately 6 times the maximum human therapeutic dose of 10 mg per day). The effect was reversible only when the treatment was withdrawn within two months of first observation of the phenomenon. The clinical significance of this is unknown. However, use of lorazepam for prolonged periods and in geriatric patients requires caution, and there should be frequent monitoring for symptoms of upper GI disease.
Safety and effectiveness of lorazepam tablets in children of less than 12 years have not been established.
Information for Patients
To assure the safe and effective use of lorazepam tablets, patients should be informed that, since benzodiazepines may produce psychological and physical dependence, it is advisable that they consult with their physician before either increasing the dose or abruptly discontinuing this drug.
Essential Laboratory Tests
Some patients on lorazepam tablets have developed leukopenia, and some have had elevations of LDH. As with other benzodiazepines, periodic blood counts and liver function tests are recommended for patients on long-term therapy.
Clinically Significant Drug Interactions
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
The benzodiazepines, including lorazepam tablets, produce increased CNS- depressant effects when administered with other CNS depressants such as alcohol, barbiturates, antipsychotics, sedative/hypnotics, anxiolytics, antidepressants, narcotic analgesics, sedative antihistamines, anticonvulsants, and anesthetics.
Concomitant use of clozapine and lorazepam may produce marked sedation, excessive salivation, hypotension, ataxia, delirium, and respiratory arrest.
Concurrent administration of lorazepam with valproate results in increased plasma concentrations and reduced clearance of lorazepam. Lorazepam dosage should be reduced to approximately 50% when coadministered with valproate.
Concurrent administration of lorazepam with probenecid may result in a more rapid onset or prolonged effect of lorazepam due to increased half-life and decreased total clearance. Lorazepam dosage needs to be reduced by approximately 50% when coadministered with probenecid.
The effects of probenecid and valproate on lorazepam may be due to inhibition of glucuronidation.
Administration of theophylline or aminophylline may reduce the sedative effects of benzodiazepines, including lorazepam.
Carcinogenesis and Mutagenesis
No evidence of carcinogenic potential emerged in rats during an 18-month study with lorazepam tablets. No studies regarding mutagenesis have been performed.
Pregnancy
Reproductive studies in animals were performed in mice, rats, and two strains of rabbits. Occasional anomalies (reduction of tarsals, tibia, metatarsals, malrotated limbs, gastroschisis, malformed skull, and microphthalmia) were seen in drug-treated rabbits without relationship to dosage. Although all of these anomalies were not present in the concurrent control group, they have been reported to occur randomly in historical controls. At doses of 40 mg/kg and higher, there was evidence of fetal resorption and increased fetal loss in rabbits which was not seen at lower doses.
The clinical significance of the above findings is not known. However, an increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam, and meprobamate) during the first trimester of pregnancy has been suggested in several studies. Because the use of these drugs is rarely a matter of urgency, the use of lorazepam during this period should be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant, they should communicate with their physician about the desirability of discontinuing the drug.
In humans, blood levels obtained from umbilical cord blood indicate placental transfer of lorazepam and lorazepam glucuronide. Infants of mothers who ingested benzodiazepines for several weeks or more preceding delivery have been reported to have withdrawal symptoms during the postnatal period.
Symptoms such as hypoactivity, hypotonia, hypothermia, respiratory depression, apnea, feeding problems, and impaired metabolic response to cold stress have been reported in neonates born of mothers who have received benzodiazepines during the late phase of pregnancy or at delivery.
Nursing Mothers
Lorazepam has been detected in human breast milk; therefore, it should not be administered to breastfeeding women, unless the expected benefit to the woman outweighs the potential risk to the infant.
Sedation and inability to suckle have occurred in neonates of lactating mothers taking benzodiazepines. Infants of lactating mothers should be observed for pharmacological effects (including sedation and irritability).
Geriatric Use
Clinical studies of lorazepam tablets generally were not adequate to determine whether subjects aged 65 and over respond differently than younger subjects; however, the incidence of sedation and unsteadiness was observed to increase with age (see ADVERSE REACTIONS).
Age does not appear to have a significant effect on lorazepam kinetics (see CLINICAL PHARMACOLOGY).
Clinical circumstances, some of which may be more common in the elderly, such as hepatic or renal impairment, should be considered. Greater sensitivity (e.g., sedation) of some older individuals cannot be ruled out. In general, dose selection for an elderly patient should be cautious, and lower doses may be sufficient in these patients (see DOSAGE AND ADMINISTRATION).
Adverse Reactions
Most adverse reactions to benzodiazepines, including CNS effects and respiratory depression, are dose dependent, with more severe effects occurring with high doses.
In a sample of about 3500 patients treated for anxiety, the most frequent adverse reaction to lorazepam tablets was sedation (15.9%), followed by dizziness (6.9%), weakness (4.2%), and unsteadiness (3.4%). The incidence of sedation and unsteadiness increased with age.
Other adverse reactions to benzodiazepines, including lorazepam are fatigue, drowsiness, amnesia, memory impairment, confusion, disorientation, depression, unmasking of depression, disinhibition, euphoria, suicidal ideation/attempt, ataxia, asthenia, extrapyramidal symptoms, convulsions/seizures, tremor, vertigo, eye function/visual disturbance (including diplopia and blurred vision), dysarthria/slurred speech, change in libido, impotence, decreased orgasm; headache, coma; respiratory depression, apnea, worsening of sleep apnea, worsening of obstructive pulmonary disease; gastrointestinal symptoms including nausea, change in appetite, constipation, jaundice, increase in bilirubin, increase in liver transaminases, increase in alkaline phosphatase; hypersensitivity reactions, anaphylactoid reactions; dermatological symptoms, allergic skin reactions, alopecia; SIADH, hyponatremia; thrombocytopenia, agranulocytosis, pancytopenia; hypothermia; and autonomic manifestations.
Paradoxical reactions, including anxiety, excitation, agitation, hostility, aggression, rage, sleep disturbances/insomnia, sexual arousal, and hallucinations may occur. Small decreases in blood pressure and hypotension may occur but are usually not clinically significant, probably being related to the relief of anxiety produced by lorazepam tablets.
To report SUSPECTED ADVERSE REACTIONS, contact Mylan Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Overdosage
In postmarketing experience, overdose with lorazepam has occurred predominantly in combination with alcohol and/or other drugs. Therefore, in the management of overdosage, it should be borne in mind that multiple agents may have been taken.
Symptoms
Overdosage of benzodiazepines is usually manifested by varying degrees of central nervous system depression ranging from drowsiness to coma. In mild cases, symptoms include drowsiness, mental confusion, paradoxical reactions, dysarthria and lethargy. In more serious cases, and especially when other drugs or alcohol were ingested, symptoms may include ataxia, hypotonia, hypotension, cardiovascular depression, respiratory depression, hypnotic state, coma, and death.
Management
General supportive and symptomatic measures are recommended; vital signs must be monitored and the patient closely observed. When there is a risk of aspiration, induction of emesis is not recommended. Gastric lavage may be indicated if performed soon after ingestion or in symptomatic patients. Administration of activated charcoal may also limit drug absorption. Hypotension, though unlikely, usually may be controlled with norepinephrine bitartrate injection. Lorazepam is poorly dialyzable. Lorazepam glucuronide, the inactive metabolite, may be highly dialyzable.
The benzodiazepine antagonist flumazenil may be used in hospitalized patients as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use.
Dosage And Administration
Lorazepam tablets are administered orally. For optimal results, dose, frequency of administration, and duration of therapy should be individualized according to patient response. To facilitate this, 0.5 mg, 1 mg, and 2 mg tablets are available.
The usual range is 2 mg/day to 6 mg/day given in divided doses, the largest dose being taken before bedtime, but the daily dosage may vary from 1 mg/day to 10 mg/day.
For anxiety, most patients require an initial dose of 2 mg/day to 3 mg/day given two times a day or three times a day.
For insomnia due to anxiety or transient situational stress, a single daily dose of 2 mg to 4 mg may be given, usually at bedtime.
For elderly or debilitated patients, an initial dosage of 1 mg/day to 2 mg/day in divided doses is recommended, to be adjusted as needed and tolerated.
The dosage of lorazepam tablets should be increased gradually when needed to help avoid adverse effects. When higher dosage is indicated, the evening dose should be increased before the daytime doses.
How Supplied
Lorazepam Tablets, USP are available containing 0.5 mg, 1 mg or 2 mg of lorazepam, USP.
The 0.5 mg tablets are white to off-white, round, unscored tablets debossed with M on one side of the tablet and 321 on the other side. They are available as follows:
NDC 0378-2321-01bottles of 100 tablets
NDC 0378-2321-05bottles of 500 tablets
The 1 mg tablets are white to off-white, round, scored tablets debossed with MYLAN above the score and 457 below the score on one side of the tablet and blank on the other side. They are available as follows:
NDC 0378-2457-01bottles of 100 tablets
NDC 0378-2457-10bottle of 1000 tablets
The 2 mg tablets are white to off-white, round, scored tablets debossed with MYLAN above the score and 777 below the score on one side of the tablet and blank on the other side. They are available as follows:
NDC 0378-2777-01bottles of 100 tablets
NDC 0378-2777-05bottles of 500 tablets
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
PHARMACIST:Â Dispense a Medication Guide with each prescription.
Medication Guide
Lorazepam Tablets, USPÂ Â Â Â Â CIV(lor az' e pam)
What is the most important information I should know about lorazepam tablets?
• Lorazepam tablets are a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death.• Lorazepam tablets can make you sleepy or dizzy, and can slow your thinking and motor skills.
o Do not drive, operate heavy machinery, or do other dangerous activities until you know how lorazepam tablets affect you.o Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking lorazepam tablets without first talking to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, lorazepam tablets may make your sleepiness or dizziness much worse.• Do not take more lorazepam tablets than prescribed.
What are lorazepam tablets?
• Lorazepam tablets are a prescription medicine used:
o to treat anxiety disorderso for the short-term relief of the symptoms of anxiety or anxiety that can happen with symptoms of depression• Lorazepam tablets are a federal controlled substance (C-IV) because it can be abused or lead to dependence. Keep lorazepam tablets in a safe place to prevent misuse or abuse. Selling or giving away lorazepam tablets may harm others, and is against the law. Tell your healthcare provider if you have abused or been dependent on alcohol, prescription medicines or street drugs.• It is not known if lorazepam tablets are safe and effective in children less than 12 years of age.• It is not known if lorazepam tablets are safe and effective for use for longer than 4 months.
Do not take lorazepam tablets if you:
• are allergic to lorazepam, other benzodiazepines, or any of the ingredients in lorazepam tablets. See the end of this Medication Guide for a complete ul of ingredients in lorazepam tablets.
Before you take lorazepam tablets, tell your healthcare provider about all of your medical conditions, including if you:
• have or have had depression, mood problems, or suicidal thoughts or behavior• have a history of drug or alcohol abuse or addiction• have lung disease or breathing problems (such as COPD, sleep apnea syndrome)• have liver or kidney problems• have or have had seizures• are pregnant or plan to become pregnant. Lorazepam tablets may harm your unborn baby. You and your healthcare provider should decide if you should take lorazepam tablets while you are pregnant.• are breastfeeding or plan to breastfeed. Lorazepam passes into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take lorazepam tablets. You should not breastfeed while taking lorazepam tablets.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking lorazepam tablets with certain other medicines can cause side effects or affect how well lorazepam tablets or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
How should I take lorazepam tablets?
• Take lorazepam tablets exactly as your healthcare provider tells you to take it. Your healthcare provider will tell you how many lorazepam tablets to take and when to take it.• If you take too many lorazepam tablets, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking lorazepam tablets?
• Lorazepam tablets can cause you to be drowsy. Do not drive a car or operate heavy machinery until you know how lorazepam tablets affect you.• You should not drink alcohol while taking lorazepam tablets. Drinking alcohol can increase your chances of having serious side effects.
What are the possible side effects of lorazepam tablets?
Lorazepam tablets may cause serious side effects, including:
• See “What is the most important information I should know about lorazepam tablets?”• Depression. Pre-existing depression may emerge or worsen during use of benzodiazepines including lorazepam tablets.• Abuse and Dependence. Taking lorazepam tablets can cause physical and psychological dependence. Physical and psychological dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical and psychological dependence and drug addiction.• Withdrawal Symptoms. You may have withdrawal symptoms if you stop taking lorazepam tablets suddenly. Withdrawal symptoms can be serious and include seizures. Mild withdrawal symptoms include a depressed mood and trouble sleeping. Talk to your healthcare provider about slowly stopping lorazepam tablets to avoid withdrawal symptoms.
The most common side effects of lorazepam tablets include:
• sedation
• dizziness
• weakness
• unsteadiness
These are not all the possible side effects of lorazepam tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store lorazepam tablets?
• Store lorazepam tablets in a tightly closed container at 20° to 25°C (68° to 77°F). Protect from light.• Keep lorazepam tablets and all medicines out of the reach of children.
General information about the safe and effective use of lorazepam tablets.
Medicines are sometimes prescribed for purposes other than those uled in a Medication Guide. Do not use lorazepam tablets for a condition for which it was not prescribed. Do not give lorazepam tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about lorazepam tablets that is written for health professionals.
What are the ingredients in lorazepam tablets?
Active ingredient: lorazepam
Inactive ingredients: lactose monohydrate, microcrystalline cellulose, polacrilin potassium and sodium stearyl fumarate.
Manufactured by: Mylan Pharmaceuticals Inc., Morgantown, WV 26505 U.S.A.
For more information, call Mylan Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
This Medication Guide has been approved by the U.S. Food and Drug Administration
Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
Revised: 4/2017LORZ:R4mmh
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 0.5 mg
NDC 0378-2321-01
Lorazepam Tablets, USP CIV 0.5 mg
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only      100 Tablets
Each tablet contains:Lorazepam, USPÂ Â Â Â Â Â Â Â Â Â 0.5 mg
Dispense in a tight, light-resistantcontainer as defined in the USPusing a child-resistant closure.
Keep container tightly closed.
Keep this and all medication out of the reach of children.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Usual Dosage: One to six mgper day, given in divided doses.
See accompanying prescribinginformation.
Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
Mylan.com
RM2321A6
![]()
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 1 mg
NDC 0378-2457-01
Lorazepam Tablets, USP CIV 1 mg
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only      100 Tablets
Each tablet contains:Lorazepam, USP Â Â Â Â Â Â Â Â Â Â 1 mg
Dispense in a tight, light-resistantcontainer as defined in the USPusing a child-resistant closure.
Keep container tightly closed.
Keep this and all medication out of the reach of children.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Usual Dosage: One to six mgper day, given in divided doses.
See accompanying prescribinginformation.
Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
Mylan.com
RM2457A6
![]()
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 2 mg
NDC 0378-2777-01
Lorazepam Tablets, USP CIV 2 mg
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only     100 Tablets
Each tablet contains:Lorazepam, USP Â Â Â Â Â Â Â Â Â 2 mg
Dispense in a tight, light-resistantcontainer as defined in the USPusing a child-resistant closure.
Keep container tightly closed.
Keep this and all medication out of the reach of children.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Usual Dosage: One to six mgper day, given in divided doses.
See accompanying prescribinginformation.
Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
Mylan.com
RM2777A6
![]()
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site