Marplan (isocarboxazid 10 mg) Dailymed
Generic: isocarboxazid is used for the treatment of Cerebrovascular Disorders Depressive Disorder Hypertension Kidney Diseases Liver Diseases Pheochromocytoma
Boxed Warning
Boxed Warning Section
Go PRO for all pill images
brand of isocarboxazid t ablets
Boxed Warning Section
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies with Major Depressive Disorder (MDD) and other psychiatric disorders.   Anyone considering the use of Marplan or any other antidepressant in a child, adolescent or young adult must balance this risk with the clinical need.   Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older.    Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide.   Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior.   Families and caregivers should be advised of the need for close observation and communication with the prescriber.   Marplan is not approved for use in pediatric patients( see Warnings: Clinical Worsening and Suicide Risk , Precautions: Information for Patients , and Precautions: Pediatric Use ) .
Pooled analyses of short-term (4 to 16 weeks) placebo-controlled trials of 9 antidepressant drugs (SSRIs and others) in children and adolescents with major depressive disorder (MDD), obsessive compulsive disorder (OCD), or other psychiatric disorders (a total of 24 trials involving over 4400 patients) have revealed a greater risk of adverse events representing suicidal thinking or behavior (suicidality) during the first few months of treatment in those receiving antidepressants.   The average risk of such events in patients receiving antidepressants was 4%, twice the placebo risk of 2%. No suicides occurred in these trials.
Description
Marplan (isocarboxazid), a monoamine oxidase inhibitor, is available for oral administration in 10-mg tablets.  Each tablet also contains lactose, corn starch, povidone, D&C Red No. 27, FD&C Yellow No. 6, and magnesium stearate. Chemically, isocarboxazid is 5-methyl-3-isoxazolecarboxylic acid 2-benzylhydrazide. The structural formula is:
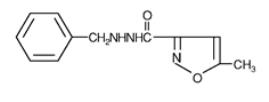
Isocarboxazid is a colorless, crystalline substance with very little taste.
Clinical Pharmacology
Pharmacodynamics
Isocarboxazid is a non-selective hydrazine monoamine oxidase (MAO) inhibitor. In vivo and in vitro studies demonstrated inhibition of MAO in the brain, heart, and liver. The mechanism by which MAO inhibitors act as antidepressants is not fully understood, but it is thought to involve the elevation of brain levels of biogenic amines.  However, MAO is a complex enzyme system, widely distributed throughout the body, and drugs that inhibit MAO in the laboratory are associated with a number of clinical effects.  Thus, it is unknown whether MAO inhibition per se, other pharmacologic actions, or an interaction of both is responsible for the antidepressant effects observed.
Pharmacokinetics
Marplan pharmacokinetic information is not available.
Clinical Efficacy Data
The effectiveness of Marplan was demonstrated in two 6-week placebo-controlled studies conducted in adult outpatients with depressive symptoms that corresponded to the DSM-IV category of major depressive disorder.  The patients often also had signs and symptoms of anxiety (anxious mood, panic, and/or phobic symptoms).  Patients were initiated with a dose of 10 mg bid, with increases every 2 to 4 days, as tolerated, until a therapeutic effect was achieved, up to a maximum dose of 80 mg/day.  Doses were administered on a divided schedule ranging from 2 to 4 times a day.  The mean dose overall for both studies was approximately 40 mg/day, with very few patients receiving doses greater than 60 mg/day.  In both studies at the end of 6 weeks, patients receiving Marplan had significantly greater reduction in signs and symptoms of depression evaluated by the Hamilton Depression Scale, for both the Total Score and the Depressed Mood Score, than patients who received placebo.
Indications And Usage
Marplan is indicated for the treatment of depression. Because of its potentially serious side effects, Marplan is not an antidepressant of first choice in the treatment of newly diagnosed depressed patients.
The efficacy of Marplan in the treatment of depression was established in 6-week controlled trials of depressed outpatients.  These patients had symptoms that corresponded to the DSM-IV category of major depressive disorder; however, they often also had signs and symptoms of anxiety (anxious mood, panic, and/or phobic symptoms) ( see CLINICAL PHARMACOLOGY ).
A major depressive episode (DSM-IV) implies a prominent and relatively persistent (nearly every day for at least 2 weeks) depressed or dysphoric mood that usually interferes with daily functioning, and includes at least five of the following nine symptoms: depressed mood, loss of interest in usual activities, significant change in weight and/or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, and a suicide attempt or suicidal ideation.
The antidepressant effectiveness of Marplan in hospitalized depressed patients, or in endogenomorphically retarded and delusionally depressed patients, has not been adequately studied.
The effectiveness of Marplan in long-term use, that is, for more than 6 weeks, has not been systematically evaluated in controlled trials.  Therefore, the physician who elects to use Marplan for extended periods should periodically evaluate the long-term usefulness of the drug for the individual patient.
Contraindications
Marplan (isocarboxazid) should not be administered in combination with any of the following: MAO inhibitors or dibenzazepine derivatives; sympathomimetics (including amphetamines); some central nervous system depressants (including narcotics and alcohol); antihypertensive, diuretic, antihistaminic, sedative or anesthetic drugs, buproprion HCL, buspirone HCL, dextromethorphan, cheese or other foods with a high tyramine content; or excessive quantities of caffeine.
Marplan (isocarboxazid) should not be administered to any patient with a confirmed or suspected cerebrovascular defect or to any patient with cardiovascular disease, hypertension, or history of headache.
Contraindicated Patient Populations
Hypersensitivity
Marplan should not be used in patients with known hypersensitivity to isocarboxazid.
Cerebrovascular Disorders
Marplan should not be administered to any patient with a confirmed or suspected cerebrovascular defect or to any patient with cardiovascular disease or hypertension.
Pheochromocytoma
Marplan should not be used in the presence of pheochromocytoma, as such tumors secrete pressor substances whose metabolism may be inhibited by Marplan.
Liver Disease
Marplan should not be used in patients with a history of liver disease, or in those with abnormal liver function tests.
Renal Impairment
Marplan should not be used in patients with severe impairment of renal function.
Contraindicated MAOI-Other Drug Combinations
Other MAOI Inhibitors or With Dibenzazepine-Related Entities
Marplan should not be administered together with, or in close proximity to, other MAO inhibitors or dibenzazepine-related entities. Hypertensive crises, severe convulsive seizures, coma, or circulatory collapse may occur in patients receiving such combinations.
In patients being transferred to Marplan from another MAO inhibitor or from a dibenzazepine-related entity, a medication-free interval of at least 1 week should be allowed, after which Marplan therapy should be started using half the normal starting dosage for at least the first week of therapy.  Similarly, at least 1 week should elapse between the discontinuation of Marplan and initiation of another MAO inhibitor or dibenzazepine-related entity, or the readministration of Marplan.  The following ul includes some other MAO inhibitors, dibenzazepine-related entities, and tricyclic antidepressants.
Generic Name Trademark (Manufacturer) Other MAO Inhibitors Furazolidone Furoxone¬ģ (Roberts Laboratories) Pargyline HCL Eutonyl¬ģ (Abbott Laboratories) Pargyline HCL and methyclothiazide Eutron¬ģ (Abbott Laboratories) Phenelzine sulfate Nardil¬ģ (Parke-Davis) Procarbazine Matulane¬ģ (Roche Laboratories) Tranylcypromine sulfate Parnate¬ģ (SmithKline Beecham Pharmaceuticals) Dibenzazepine-Related and Other Tricyclics Amitriptyline HCL Elavil¬ģ (Zeneca) Endep¬ģ (Roche Products) Perphenazine and amitriptyline HCL Etrafon¬ģ (Schering) Triavil¬ģ (Merck Sharp & Dohme) Clomipramine hydrochloride Anafranil¬ģ (Novartis) Desipramine HCL Norpramin¬ģ (Hoechst Marion Roussel) Pertofrane¬ģ (Rh√īne-Poulenc Rorer Pharmaceuticals) Imipramine HCL Janimine¬ģ (Abbott Laboratories) Tofranil¬ģ (Novartis) Nortriptyline HCL Aventyl¬ģ (Eli Lilly & Co.) Pamelor¬ģ (Novartis) Protripyline HCL Vivactil¬ģ (Merck Sharp & Dohme) Doxepin HCL Adapin¬ģ (Fisons) Sinequan¬ģ (Pfizer) Carbamazepine Tegretol¬ģ (Novartis) Cyclobenzaprine HCL Flexeril¬ģ (Merck Sharp & Dohme) Amoxapine Asendin¬ģ (Lederle) Maprotiline HCL Ludiomil¬ģ (Novartis) Trimipramine maleate Surmontil¬ģ (Wyeth-Ayerst Laboratories) Bupropion
The concurrent administration of a MAO inhibitor and buproprion hydrochloride (Wellbutrin¬ģ, and Zyban¬ģ, Glaxo Wellcome) is contraindicated. At least 14¬†days should elapse between discontinuation of an MAO inhibitor and initiation of treatment with buproprion hydrochloride.
Selective Serotonin Re
Marplan should not be administered in combination with any SSRI.¬† There have been reports of serious, sometimes fatal, reactions (including hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation and confusion progressing to delirium and coma) in patients receiving fluoxetine (Prozac¬ģ, Lilly) in combination with a monoamine oxidase inhibitor (MAOI), and in patients who have recently discontinued fluoxetine and are then started on a MAOI. Some cases presented with features resembling neuroleptic malignant syndrome. ¬†Fluoxetine and other SSRIs should therefore not be used in combination with Marplan, or within 14 days of discontinuing therapy with Marplan. ¬†As fluoxetine and its major metabolite have very long elimination half-lives, at least 5 weeks should be allowed after stopping fluoxetine before starting Marplan. At least 2¬†weeks should be allowed after stopping sertraline (Zoloft¬ģ, Pfizer) or paroxetine (Paxil¬ģ, SmithKline Beecham Pharmaceuticals) before starting Marplan. ¬†In addition, there should be an interval of least 10 days between discontinuation of Marplan and initiation of fluoxetine or other SSRIs.
Buspirone
Marplan should not be used in combination with buspirone HCL (Buspar¬ģ, Bristol Myers Squibb); several cases of elevated blood pressure have been reported in patients taking MAO inhibitors who were then given buspirone HCL. ¬†At least 10¬†days should elapse between the discontinuation of Marplan and the institution of buspirone HCL. ¬†Serious reactions may also occur when MAO inhibitors are given with serotoninergic drugs (e.g., dexfenfluramine, fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram, venlafaxine).
Sympathomimetics
Marplan should not be administered in combination with sympathomimetics, including amphetamines, or with over-the-counter drugs such as cold, hay fever, or weight-reducing preparations that contain vasoconstrictors.
During Marplan therapy, it appears that some patients are particularly vulnerable to the effects of sympathomimetics when the activity of metabolizing enzymes is inhibited. Use of sympathomimetics and compounds such as guanethidine, methyldopa, methylphenidate, reserpine, epinephrine, norepinephrine, phenylalanine, dopamine, levodopa, tyrosine, and tryptophan with Marplan may precipitate hypertension, headache, and related symptoms. The combination of MAO inhibitors and tryptophan has been reported to cause behavioral and neurologic symptoms, including disorientation, confusion, amnesia, delirium, agitation, hypomanic signs, ataxia, myoclonus, hyperreflexia, shivering, ocular oscillations, and Babinski signs.
Meperidine
Meperidine should not be used concomitantly with MAO inhibitors or within 2- or 3-weeks following MAO therapy. Serious reactions have been precipitated with concomitant use, including coma, severe hypertension or hypotension, severe respiratory depression, convulsions, malignant hyperpyrexia, excitation, peripheral vascular collapse, and death.  It is thought that these reactions may be mediated by accumulation of 5-HT (serotonin) consequent to MAO inhibition.
Dextromethorphan
Marplan should not be used in combination with dextromethorphan.  The combination of MAO inhibitors and dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior.
Cheese or Other Foods With a High Tyramine Content
Hypertensive crises have sometimes occurred during Marplan therapy after ingestion of foods with a high tyramine content.  In general, patients should avoid protein foods in which aging or protein breakdown is used to increase flavor.  In particular, patients should be instructed not to take foods such as cheese (particularly strong or aged varieties), sour cream, Chianti wine, sherry, beer (including non-alcoholic beer), liqueurs, pickled herring, anchovies, caviar, liver, canned figs, raisins, bananas or avocados (particularly if overripe), chocolate, soy sauce, sauerkraut, the pods of broad beans (fava beans), yeast extracts, yogurt, meat extracts, meat prepared with tenderizers, or dry sausage.
Anesthetic Agents
Patients taking Marplan should not undergo elective surgery requiring general anesthesia. Also, they should not be given cocaine or local anesthesia containing sympathomimetic vasoconstrictors.  The possible combined hypotensive effects of Marplan and spinal anesthesia should be kept in mind. Marplan should be discontinued at least 10 days before elective surgery.
CNS Depressants
Marplan should not be used in combination with some central nervous system depressants, such as narcotics, barbiturates, or alcohol.
Antihypertensives
Marplan should not be used in combination with antihypertensive agents, including thiazide diuretics. A marked potentiating effect on these drugs has been reported, resulting in hypotension.
Caffeine
Excessive use of caffeine in any form should be avoided in patients receiving Marplan.
Warnings To Physicians
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs.  Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment.  Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials of nine antidepressant drugs (SSRIs) and others) in children and adolescents with MDD, Obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients.  There was considerable variation in risk among drugs, but a tendency toward an increase in the younger patients `for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications.  These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
Table 1 Age Range Drug-Placebo Difference in   Number of Cases of Suicidality   Per 1000 Patients Treated Increases Compared to Placebo <18 14 additional cases 18 to 24 5 additional cases Decreases Compared to Placebo 25 to 64 1 fewer case >65 6 fewer cases
No suicides occurred in any of the pediatric trials.  There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases .
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a casual link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset or were not part of the patient’s presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.  Prescriptions for MARPLAN should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder
A major depressive episode may be the initial presentation of bipolar disorder.  It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of these symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.   It should be noted that MARPLAN is not approved for use in treating bipolar depression.
WARNINGS:
Marplan can cause serious side effects.  It is not recommended as initial therapy but should be reserved for patients who have not responded satisfactorily to other antidepressants.
Hypertensive Crises
The most important reaction associated with MAO inhibitors is the occurrence of hypertensive crises, which have sometimes been fatal, resulting from the co-administration of MAOIs and certain drugs and foods( see CONTRAINDICATIONS ).
These crises are characterized by some or all of the following symptoms: occipital headache which may radiate frontally, palpitation, neck stiffness or soreness, nausea or vomiting, sweating (sometimes with fever and sometimes with cold, clammy skin), and photophobia. Either tachycardia or bradycardia may be present, and associated constricting chest pain and dilated pupils may occur.   Intracranial bleeding, sometimes fatal, has been reported in association with the increase in blood pressure.
Blood pressure should be followed closely in patients taking Marplan to detect any pressor response.
Therapy should be discontinued immediately if palpitations or frequent headaches occur during Marplan therapy as these symptoms may be prodromal of a hypertensive crisis.
If a hypertensive crisis occurs, Marplan should be discontinued, and therapy to lower blood pressure should be instituted immediately. Although there has been no systematic study of treatment of hypertensive crisis, phentolamine (available as Regitine¬ģ, Novartis) has been used and is recommended at a dosage of 5¬†mg IV. Care should be taken to administer the drug slowly in order to avoid producing an excessive hypotensive effect. ¬†Fever should be managed by means of external cooling. ¬†Other symptomatic and supportive measures may be desirable in particular cases. ¬†Parenteral reserpine should not be used.
Warnings to the Patient
Patients should be instructed to report promptly the occurrence of headache or other unusual symptoms, i.e., palpitation and/or tachycardia, a sense of constriction in the throat or chest, sweating, dizziness, neck stiffness, nausea, or vomiting.  Patients should be warned against eating the foods uled under CONTRAINDICATIONS  while on Marplan therapy and should also be told not to drink alcoholic beverages. The patient should also be warned about the possibility of hypotension and faintness, as well as drowsiness sufficient to impair performance of potentially hazardous tasks, such as driving a car or operating machinery.
Patients should also be cautioned not to take concomitant medications, whether prescription or over-the-counter drugs such as cold, hay fever, or weight-reducing preparations, without the advice of a physician. They should be advised not to consume excessive amounts of caffeine in any form. Likewise, they should inform their physicians and their dentist about the use of Marplan.
Limited Experience With Marplan at Higher Doses
Because of the limited experience with systematically monitored patients receiving Marplan at the higher end of the currently recommended dose range of up to 60 mg/day, caution is indicated in patients for whom a dose of 40 mg/day is exceeded ( see ADVERSE REACTIONS ).
Precautions
Information for Patients
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with Marplan and should counsel them in its appropriate use. ¬†A patient Medication Guide about ‚ÄúAntidepressant Medications, Depression and Other Serious Mental Illness, and Suicidal Thoughts and Actions‚ÄĚ is available for Marplan.¬† The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. ¬†Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have.¬† The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking Marplan.
Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down.   Families and caregivers of patients should be advised to observe for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt.   Such symptoms should be reported to the patient’s prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient’s presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Pediatric Use   -  Safety and effectiveness in the pediatric population have not been established (see BOX WARNING and WARNINGS-Clinical Worsening and Suicide Risk ).
Anyone considering the use of Marplan in a child or adolescent must balance the potential risks with the clinical need.
General
Hypotension
Hypotension has been observed during Marplan therapy.  Symptoms of postural hypotension are seen most commonly, but not exclusively, in patients with preexistent hypertension; blood pressure usually returns rapidly to pretreatment levels upon discontinuation of the drug.  Dosage increases should be made more gradually in patients showing a tendency toward hypotension at the beginning of therapy.  Postural hypotension may be relieved by having the patient lie down until blood pressure returns to normal. When Marplan is combined with phenothiazine derivatives or other compounds known to cause hypotension, the possibility of additive hypotensive effects should be considered.
Lower Seizure Threshold
Because Marplan lowers the convulsive threshold in some animal experiments, suitable precautions should be taken if epileptic patients are treated. Marplan appears to have varying effects in epileptic patients; while some have a decrease in frequency of seizures, others have more seizures.
Drugs that lower the seizure threshold, including MAO inhibitors, should not be used with Amipaque¬ģ (metrizamide, Sanofi Winthrop Pharmaceuticals). ¬†As with other MAO inhibitors, Marplan should be discontinued at least 48¬†hours before myelography and should not be resumed for at least 24¬†hours postprocedure.
Hepatotoxicity
There is a low incidence of altered liver function or jaundice in patients treated with Marplan.  In the past, it was difficult to differentiate most cases of drug-induced hepatocellular jaundice from viral hepatitis although this is no longer true.  Periodic liver chemistry tests should be performed during Marplan therapy; use of the drug should be discontinued at the first sign of hepatic dysfunction or jaundice.
Suicide
In depressed patients, the possibility of suicide should always be considered and adequate precautions taken. Exclusive reliance on drug therapy to prevent suicidal attempts is unwarranted, as there may be a delay in the onset of therapeutic effect or an increase in anxiety or agitation.   Also, some patients fail to respond to drug therapy or may respond only temporarily. The strictest supervision, and preferably hospitalization, are required.
Use in Patients With Concomitant Illness
MAO inhibitors can suppress anginal pain that would otherwise serve as a warning of myocardial ischemia.
In patients with impaired renal function, Marplan should be used cautiously to prevent accumulation.
Some MAO inhibitors have contributed to hypoglycemic episodes in diabetic patients receiving insulin or glycemic agents. Marplan should therefore be used with caution in diabetics using these drugs.
Marplan may aggravate coexisting symptoms in depression, such as anxiety and agitation.
Use Marplan with caution in hyperthyroid patients because of their increased sensitivity to pressor amines.
Marplan should be used cautiously in hyperactive or agitated patients, as well as in schizophrenic patients, because it may cause excessive stimulation.  Activation of mania/hypomania has been reported in a small proportion of patients with major affective disorder who were treated with marketed antidepressants.
Drug Interactions
See CONTRAINDICATIONS , WARNINGS , and PRECAUTIONS  sections for information on drug interactions.
Marplan should be administered with caution to patients receiving Antabuse¬ģ (disulfiram, Wyeth-Ayerst Laboratories).¬†¬†¬†In a single study, rats given high intraperitoneal doses of an MAO inhibitor plus disulfiram experienced severe toxicity, including convulsions and death.
Concomitant use of Marplan and other psychotropic agents is generally not recommended because of possible potentiating effects. This is especially true in patients who may subject themselves to an overdosage of drugs.   If combination therapy is needed, careful consideration should be given to the pharmacology of all agents to be used. The monoamine oxidase inhibitory effects of Marplan may persist for a substantial period after discontinuation of the drug, and this should be borne in mind when another drug is prescribed following Marplan. To avoid potentiation, the physician wishing to terminate treatment with Marplan and begin therapy with another agent should allow for an interval of 10 days.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies to evaluate carcinogenic potential have not been conducted with this drug, and there is no information concerning mutagenesis or impairment of fertility.
Pregnancy
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressant, including MARPLAN, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or visiting online at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants
The potential reproductive toxicity of isocarboxazid has not been adequately evaluated in animals. It is also not known whether isocarboxazid can cause embryo/fetal harm when administered to a pregnant woman or can affect reproductive capacity.  Marplan should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Levels of excretion of isocarboxazid and/or its metabolites in human milk have not been determined, and effects on the nursing infant are unknown. Marplan should be used in women who are nursing only if clearly needed.
Pediatric Use
Marplan is not recommended for use in patients under 16 years of age, as safety and effectiveness in pediatric populations have not been demonstrated.
Adverse Reactions
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials
Systematically collected data are available from only 86 patients exposed to Marplan, of whom only 52 received doses of ‚Č•50 mg/day, including only 11 who were dosed at ‚Č•60 mg/day. Because of the limited experience with systematically monitored patients receiving Marplan at the higher end of the currently recommended dose range of up to 60¬†mg/day, caution is indicated in patients for whom a dose of 40¬†mg/day is exceeded ( see WARNINGS ).
The table that follows enumerates the incidence, rounded to the nearest percent, of treatment emergent adverse events that occurred among 86 depressed patients who received Marplan at doses ranging from 20 to 80 mg/day in placebo-controlled trials of 6 weeks in duration. Events included are those occurring in 1% or more of patients treated with Marplan and for which the incidence in patients treated with Marplan was greater than the incidence in placebo-treated patients.
The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in clinical trials.  Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and non-drug factors to the adverse event incidence rate in the population studied.
The commonly observed adverse event that occurred in Marplan patients with an incidence of 5% or greater and at least twice the incidence in placebo patients were nausea, dry mouth, and dizziness (see Table).
In three clinical trials for which the data were pooled, 4 of 85 (5%) patients who received placebo, 10 of 86 (12%) who received <50¬†mg of Marplan per day, and 1 of 52 (2%) who received ‚Č•50 mg of Marplan per day prematurely discontinued treatment. ¬†The most common reasons for discontinuation were dizziness, orthostatic hypotension, syncope, and dry mouth.
Treatment-Emergent Adverse Events Incidence in Placebo-Controlled Clinical Trials¬†with Marplan Doses of 40 to 80 mg/day1 ¬†¬† BODY SYSTEM/ ADVERSE EVENT PLACEBO (N=85) MARPLAN <50 mg (N=86) MARPLAN ‚Č• 50 mg (N=52) 2 MISCELLANEOUS ¬†¬†Drowsy 0 4% 0% ¬†¬†Anxiety 1 2% 0% ¬†¬†Chills 0% 2% 0% ¬†¬†Forgetful 1% 2% 2% ¬†¬†Hyperactive 0% 2% 0% ¬†¬†Lethargy 0% 2% 2% ¬†¬†Sedation 1% 2% 0% ¬†¬†Syncope 0% 2% 0% INTEGUMENTARY ¬†¬†Sweating 0% 2% 2% MUSCULOSKELETAL ¬†¬†Heavy feeling 0% 2% 0% CARDIOVASCULAR ¬†¬†Orthostatic hypotension 1% 4% 4% ¬†¬†Palpitations 1% 2% 0% GASTROINTESTINAL ¬†¬†Dry mouth 4% 9% 6% ¬†¬†Constipation 6% 7% 4% ¬†¬†Nausea 2% 6% 4% ¬†¬†Diarrhea 1% 2% 0% UROGENITAL ¬†¬†Impotence 0% 2% 0% ¬†¬†Urinary frequency 1% 2% 0% ¬†¬†Urinary hesitancy 0% 1% 4% CENTRAL NERVOUS SYSTEM ¬†¬†Headache 13% 15% 6% ¬†¬†Insomnia 4% 4% 6% ¬†¬†Sleep disturbance 0% 5% 2% ¬†¬†Tremor 0% 4% 4% ¬†¬†Myoclonic jerks 0% 2% 0% ¬†¬†Paresthesia 1% 2% 0% SPECIAL SENSES ¬†¬†Dizziness 14% 29% 15%
1Events reported by at least 1% of patients treated with Marplan are presented, except for those that had an incidence on placebo greater than or equal to that on Marplan.
2All patients also received Marplan at doses <50 mg.
Other Events Observed During the Post
Isolated cases of akathisia, ataxia, black tongue, coma, dysuria, euphoria, hematologic changes, incontinence, neuritis, photosensitivity, sexual disturbances, spider telangiectases, and urinary retention have been reported. These side effects sometimes necessitate discontinuation of therapy.  In rare instances, hallucinations have been reported with high dosages, but they have disappeared upon reduction of dosage or discontinuation of therapy.  Toxic amblyopia was reported in one psychiatric patient who had received isocarboxazid for about a year; no causal relationship to isocarboxazid was established.  Impaired water excretion compatible with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) has been reported.
To report SUSPECTED ADVERSE REACTIONS, contact Validus Pharmaceuticals LLC at
1-866-982-5438 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Abuse And Dependence
Controlled Substance Class
Marplan is not a controlled substance.
Physical and Psychological Dependence
Marplan has not been systematically studied in animals or humans for its potential for abuse, tolerance, or physical dependence.  There have been reports of drug dependency in patients using doses of Marplan significantly in excess of the therapeutic range.  Some of these patients had a history of previous substance abuse.  The following withdrawal symptoms have been reported: restlessness, anxiety, depression, confusion, hallucinations, headache, weakness, and diarrhea. Consequently, physicians should carefully evaluate Marplan patients for history of drug abuse and follow such patients closely, observing them for signs of misuse or abuse (e.g., development of tolerance, incrementations of dose, drug-seeking behavior).
Overdosage
The lethal dose of Marplan in humans is not known. ¬†There has been one report of a fatality in a patient who ingested 400¬†mg of Marplan together with an unspecified amount of another drug. Symptoms: Major overdosage may be evidenced by tachycardia, hypotension, coma, convulsions, respiratory depression, sluggish reflexes, pyrexia, and diaphoresis; these signs may persist for 8 to 14¬†days. Treatment: General supportive measures should be used, along with immediate gastric lavage or emetics.¬† If the latter are given, the danger of aspiration must be borne in mind. An adequate airway should be maintained, with supplemental oxygen if necessary.¬†¬†¬†The mechanism by which amine-oxidase inhibitors produce hypotension is not fully understood, but there is evidence that these agents block the vascular bed response. ¬†Thus it is suggested that plasma may be of value in the management of this hypotension. ¬†Administration of pressor amines such as Levophed¬ģ (levarterenol bitartrate) may be of limited value (note that their effects may be potentiated by Marplan). Continue treatment for several days until homeostasis is restored. ¬†Liver function studies are recommended during the 4 to 6¬†weeks after recovery, as well as the time of overdosage.
In managing overdosage, consider the possibility of multiple drug involvement.  The physician should consider contacting a poison control center on the treatment of any overdose.
Dosage And Administration
For maximum therapeutic effect, the dosage of Marplan must be individually adjusted on the basis of careful observation of the patient.  Dosage should be started with one tablet (10 mg) of Marplan twice daily.  If tolerated, dosage may be increased by increments of one tablet (10 mg) every 2 to 4 days to achieve a dosage of four tablets daily (40 mg) by the end of the first week of treatment. Dosage can then be increased by increments of up to 20 mg/week, if needed and tolerated, to a maximum recommended dosage of 60 mg/day. Daily dosage should be divided into two to four dosages.  After maximum clinical response is achieved, an attempt should be made to reduce the dosage slowly over a period of several weeks without jeopardizing the therapeutic response.  Beneficial effect may not be seen in some patients for 3 to 6 weeks.  If no response is obtained by then, continued administration is unlikely to help.
Because of the limited experience with systematically monitored patients receiving Marplan at the higher end of the currently recommended dose range of up to 60 mg/day, caution is indicated in patients for whom a dose of 40 mg/day is exceeded (see ADVERSE REACTIONS ).
How Supplied
Marplan tablets 10 mg are peach colored, round and scored with "MARPLAN 10" imprinted on one side and scored on the other.  Supplied in bottles of 100 (NDC 30698-032-01).
Storage: Store at 77¬ļF (25¬ļC); excursions permitted to 59¬ļ to 86¬ļF (15¬ļ to 30¬ļC) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in USP.
R x   O nly

Manufactured for and Distributed by: Validus Pharmaceuticals LLCParsippany, NJ 07054info@validuspharma.comwww.validuspharma.com1-866-982-5438
© 2023 Validus Pharmaceuticals LLC    All rights reserved
60009-09        April 2023
Spl Medguide Section
Medication Guide
Marplan ¬ģ ¬† Tablets
(isocarboxazid)
Antidepressant Medicines, Depression and other Serious Mental Illnesses,
and Suicidal Thoughts or Actions
Read the Medication Guide that comes with your or your family member’s antidepressant medicine.  This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines.  Talk to your or your family member’s, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
1.   Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment .
2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness  (also called manic-depressive illness) or suicidal thoughts or actions.
3.   How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.  This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled.  Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a ul of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
- Tell your healthcare provider if you are pregnant or plan to become pregnant during treatment with Marplan. ‚óč If you become pregnant during treatment with Marplan, talk to your healthcare ¬†¬†¬†¬†provider about registering with the National Pregnancy Registry for Antidepressants. You can register by calling 1-844-405-6185 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured for and Distributed by :   Validus Pharmaceuticals LLCParsippany, NJ 07054 info@validuspharma.com www.validuspharma.com 1-866-982-5438
© 2023 Validus Pharmaceuticals LLC    All rights reserved
60143-03   April 2023
Principal Display Panel
NDC 30698-032-01MARPLAN¬ģ (isocarboxazid) 10 mg100 TabletsRx Only
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


