Methylphenidate Hydrochloride (methylphenidate hydrochloride 20 mg) Dailymed
Generic: methylphenidate hydrochloride is used for the treatment of Anxiety Disorders Attention Deficit Disorder with Hyperactivity Depressive Disorder Tourette Syndrome Glaucoma Narcolepsy Psychomotor Agitation Tics
IMPRINT: A64
SHAPE: round
COLOR: yellow SCORE: 2
All Imprints
methylphenidate hydrochloride 20 mg - a64 round yellow
methylphenidate hydrochloride 10 mg - a63 round blue
Boxed Warning
Warning: Abuse And Dependence
-
CNS stimulants, including methylphenidate hydrochloride tablets, other methylphenidate-containing products, and amphetamines, have a high potential for abuse and dependence (
5.1 ,9.2 ,9.3 ). -
Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy(
5.1 ,9.2 ).
Go PRO for all pill images
Warning: Abuse And Dependence
CNSstimulants,including methylphenidate hydrochloride tablets, other methylphenidate-containing products, and amphetamines, have a highpotentialforabuseanddependence.Assesstherisk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2, 9.3)].
WARNING: ABUSE AND DEPENDENCE
See full prescribing informationforcomplete boxed warning.
- CNS stimulants, including methylphenidate hydrochloride tablets, other methylphenidate-containing products, and amphetamines, have a high potential for abuse and dependence (
5.1 ,9.2 ,9.3 ).- Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy(
5.1 ,9.2 ).
1 Indications And Usage
Methylphenidate hydrochloride tablets are indicated for the treatment of:
- Attention Deficit Hyperactivity Disorders (ADHD) in pediatric patients 6 years and older and adults
- Narcolepsy
Methylphenidate hydrochloride is a centralnervoussystem(CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorders(ADHD) and Narcolepsy(1).
2 Dosage And Administration
Methylphenidate Hydrochloride Tablets (2.2 ):
- Pediatric Patients 6 Years and Older:Start with 5 mg twice daily (before breakfast and lunch), titrating the dose weekly in 5- to 10-  mg increments. Dosages above 60 mg/dayare not recommended.
- Adults: Average daily dosage is 20 mg to 30 mg,administered 2 or 3 times daily,preferably 30 to 45 minutes before meals. Maximum total daily dosage is 60 mg.
2.1 Pretreatment Screening
Prior to treatingpediatricpatientsandadultswithcentral nervous system(CNS)stimulants,including methylphenidate hydrochloride tablets,assessforthepresenceofcardiacdisease(i.e.,perform a carefulhistory,includingfamilyhistory of suddendeathorventriculararrhythmia, and physicalexamination)[seeWarningsandPrecautions(5.2)].
Assesstheriskofabusepriortoprescribing,and monitorforsignsofabuseanddependencewhileontherapy.Maintaincarefulprescriptionrecords,educatepatientsaboutabuse,monitorforsigns of abuseandoverdose, and periodicallyreevaluate the needformethylphenidate hydrochloride tablets use[seeBoxedWarning,WarningsandPrecautions(5.1),DrugAbuseandDependence(9.2, 9.3)].
2.2 General Dosing Information
Methylphenidate Hydrochloride Tablets
Pediatric Patients 6 years and Older: Start with 5 mg orally twice daily (before breakfast and lunch). Increase dosage gradually, in increments of 5- to 10-mgweekly.Daily dosage above 60 mg is not recommended.
Adults: Average dosage is 20 to 30 mg daily. Administer orally in divided doses 2 or 3 times daily, preferably 30 to 45 minutes before meals. Maximum total daily dosage is 60 mg.Patients who are unable to sleep if medication is taken late in the dayshould take the last dose before 6 p.m. Pharmacological treatment of ADHD may be needed for extended periods. Periodically reevaluate the long-term use of methylphenidate hydrochloride tablets, and adjust dosage as needed.
2.3 Dose Reduction and Discontinuation
If paradoxical worsening of symptoms or other adverse reactions occur, reduce the dosage,or, if necessary,discontinue methylphenidate hydrochloride tablets.Ifimprovement is notobservedafterappropriatedosageadjustmentover a one-monthperiod, the drugshouldbediscontinued.
3 Dosage Forms & Strengths
Tablets
- 5 mg, round, pale yellow to yellow uncoated tablet, debossed with "A62" on one side and plain on other side, may have mottled appearance.
- 10 mg, round, light blue to blue uncoated tablet, debossed with "A63" on one side and functionally scored  on other side, may have mottled appearance.
- 20 mg, round, pale yellow to yellow uncoated tablet, debossed with "A64" on one side and  functionally scored on other side, may have mottled appearance.
- Tablets: 5mg, 10mg, and 20 mg (
3 )
4 Contraindications
- Hypersensitivity to methylphenidate or other components of methylphenidate hydrochloride tablets. Hypersensitivity reactions, such as angioedema and anaphylacticreactions, have been reported in patients treated with methylphenidate[seeAdverse Reactions (6.1)].
- Concomitant treatment with monoamine oxidase inhibitors (MAOIs), or within 14 daysfollowingdiscontinuation of treatment with an MAOI, because of the risk of hypertensive crises [see Drug Interactions (7.1)].
- Known hypersensitivity to methylphenidate or other product components of methylphenidate hydrochloride tablets (
4 ).- Concurrent   treatment with a monoamine oxidase inhibitor (MAOI), or use of an MAOI within the preceding 14 days (
4 ).
5 Warnings And Precautions
- Serious CardiovascularEvents:Sudden death has been reported in association with CNS-stimulant treatment at usual doses in pediatric patients with structural cardiac abnormalities or other serious heart problems.In adults, sudden death, stroke, and myocardialinfarction have been reported. Avoid use in patients with known structuralcardiacabnormalities,cardiomyopathy, serious heart rhythm arrhythmias, or coronary artery disease (
5.2 ).- Blood
Pressure and    Heart RateIncreases:Monitorblood pressure and pulse. Consider the benefits and risk in patients for whom an increase in blood pressure or heartrate would be problematic( 5.3 ).- Psychiatric Adverse Reactions: Use of stimulantsmay cause psychotic or manicsymptoms in patients with no prior history or exacerbation of symptoms in patients with preexisting psychiatric illness. Evaluate for preexistingpsychotic or bipolar disorder prior to methylphenidate hydrochloride use(
5.4 ).- Priapism:Cases of painful and prolonged penile erections, and priapism have been reported with methylphenidateproducts.Immediatemedical attention should be sought if signs or symptoms of prolonged penile erections or priapism areobserved(
5.5 ).- Peripheral
Vasculopathy,  I ncluding Raynaud’s Phenomenon:Stimulants  used to treat ADHD are associated with peripheralvasculopathy,includingRaynaud’sphenomenon.Carefulobservation for digital changes is necessaryduringtreatment with ADHD stimulants( 5.6 ).- Long-Term
Suppression    of Growth: Monitor height and weight at appropriateintervals in pediatric patients ( 5.7 ).5.1 Potential for Abuse and Dependence
CNS stimulants,including methylphenidate hydrochloride tablets, other methylphenidate-containingproducts, and amphetamines, have a high potential for abuse and dependence. Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy[seeBoxed Warning, Drug Abuse and Dependence (9.2, 9.3)].
5.2 Serious Cardiovascular Reactions
Sudden death, stroke, and myocardial infarction have been reported in adults with CNS stimulanttreatment at recommended doses. Sudden death has been reported in pediatric patients with structural cardiac abnormalities and other serious heart problems taking CNS stimulants at recommended doses for ADHD. Avoid use in patients with known serious structural cardiac abnormalities,cardiomyopathy, serious heart rhythm abnormalities,coronary artery disease, and other serious heart problems. Further evaluate patients who develop exertional chest pain, unexplained syncope, or arrhythmias during methylphenidate hydrochloride treatment.
5.3 Blood Pressure and Heart Rate Increases
CNS stimulants cause an increase in blood pressure (mean increaseapproximately 2 to 4 mmHg) and heart rate (mean increase approximately 3 to 6 beats per minute). Individuals may have larger increases. Monitor all patients for hypertension and tachycardia.
5.4 Psychiatric Adverse Reactions
Exacerbation of Preexisting Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a preexisting psychoticdisorder.
Induction of a Manic Episode in Patients with Bipolar Disorder
CNS stimulants may induce a manic or mixedmood episode in patients. Prior to initiating treatment, screen patients for risk factors for developing a manic episode (e.g., comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, or depression).
New Psychotic or Manic Symptoms
CNS stimulants, at recommended doses, may cause psychotic or manic symptoms (e.g., hallucinations, delusional thinking, or mania) in patients without a prior history of psychotic illness or mania. If such symptoms occur, consider discontinuing methylphenidate hydrochloride. In a pooled analysis of multipleshort-term, placebo-controlled studies of CNS stimulants,psychotic or manicsymptoms occurred in approximately 0.1% of CNSstimulant-treated patients, compared to 0 in placebo-treated patients.
5.5 Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate products in both pediatric and adult patients. Priapism was not reported with drug initiation but developed after sometime on the drug, often subsequent to an increase in dose. Priapism has also appeared during a period of drug withdrawal (drug holidays or duringdiscontinuation).Patients who developabnormally sustained or frequent and painful erections should seek immediate medical attention.
5.6 Peripheral Vasculopathy, Including Raynauds Phenomenon
CNS stimulants, including methylphenidate hydrochloride, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Signs and symptoms are usuallyintermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud’s phenomenon, were observed in postmarketing reports at different times and at therapeutic doses in all age groups throughoutthe course of treatment. Signs and symptomsgenerallyimprove after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
5.7 Long-Term Suppression of Growth
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
Careful follow-up of weight and height in pediatric patients ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturaulic subgroups of newly methylphenidate-treated and non-medication treated patients over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated pediatric patients (i.e., treatment for 7 days per week throughoutthe year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth reboundduringthis period of development.
Closely monitor growth (weight and height) in pediatric patients treated with CNS stimulants, including methylphenidate hydrochloride. Patients who are not growing or gainingheight or weight as expected may need to have their treatment interrupted.
6 Adverse Reactions
The following are discussed in more detail in other sections of the labeling:
- Abuse and Dependence [see Boxed Warning, Warningsand Precautions (5.1), Drug Abuse and Dependence (9.2, 9.3 )]
- Knownhypersensitivity    to methylphenidate or other ingredients of methylphenidate hydrochloride tablets [see Contraindications(4)]
- Hypertensive crisis with Concomitant Use of Monoamine Oxidase Inhibitors[seeContraindications (4), Drug Interactions (7.1)]
- Serious Cardiovascular Reactions [see Warningsand Precautions (5.2)]
- Blood Pressure and Heart Rate Increases [see Warnings and Precautions (5.3)]
- Psychiatric Adverse Reactions [seeWarningsandPrecautions(5.4)]
- Priapism [seeWarningsand Precautions (5.5)]
- Peripheral Vasculopathy, IncludingRaynaud’sPhenomenon[seeWarningsand Precautions (5.6)]
- Long-Term Suppression of Growth [see Warnings and Precautions (5.7)]
The following adverse reactions associated with the use of methylphenidate hydrochloride tablets, and othermethylphenidate products were identified in clinical trials,spontaneous reports, and literature. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Adverse Reactions Reported With methylphenidate hydrochloride
Infections andInfestations:nasopharyngitis
Blood and the Lymphatic System Disorders:leukopenia, thrombocytopenia,anemia
Immune System Disorders: hypersensitivity reactions, includingangioedema, and anaphylaxis
Metabolism and Nutrition Disorders: decreased appetite, reduced weight gain, and suppression of growth during prolongeduse in pediatric patients
Psychiatric Disorders: insomnia,anxiety, restlessness, agitation, psychosis(sometimes with visual and tactile hallucinations), depressed mood
Nervous System Disorders:headache, dizziness, tremor, dyskinesia, including choreoatheetoid movements, drowsiness, convulsions, cerebrovascular disorders (including vasculitis, cerebral hemorrhages and cerebrovascular accidents), serotonin syndrome in combination with serotonergic drugs
EyeDisorders: blurred vision, difficulties in visual accommodation
Cardiac Disorders: tachycardia,palpitations, increased blood pressure, arrhythmias, angina pectoris
Respiratory, Thoracic, and Mediastinal Disorders: cough
Gastrointestinal Disorders: dry mouth, nausea, vomiting,abdominal pain, dyspepsia
Hepatobiliary    Disorders: abnormal   liver function, ranging from transaminase elevation to severe hepatic injury
Skin andSubcutaneousTissue Disorders: hyperhidrosis, pruritus, urticaria, exfoliative dermatitis, scalp hair loss, erythemamultiforme rash, thrombocytopenic purpura
Musculoskeletal and Connective Tissue Disorders: arthralgia, muscle cramps, rhabdomyolysis
Investigations: weight loss (adult ADHD patients)
Additional Adverse Reactions Reported with Other Methylphenidate-ContainingProducts
The ul below shows adverse reactions not uled for methylphenidate hydrochloride tablets that have been reported with other methylphenidate-containingproducts.
Blood and Lymphatic Disorders: pancytopenia
Immune System Disorders: hypersensitivity reactions, such as auricular swelling, bullous conditions, eruptions, exanthemas
Psychiatric Disorders: affect lability, mania,disorientation, and libido changes
Nervous System Disorders:migraine
EyeDisorders: diplopia, mydriasis
Cardiac Disorders: sudden cardiac death, myocardial infarction, bradycardia, extrasystole
Vascular Disorders: peripheral coldness, Raynaud'sphenomenon
Respiratory, Thoracic, and Mediastinal Disorders: pharyngolaryngeal   pain, dyspnea
Gastrointestinal Disorders: diarrhea, constipation
Skin andSubcutaneousTissue Disorders: angioneuroticedema,erythema, fixed drug eruption
Musculoskeletal, Connective Tissue, and Bone Disorders: myalgia,muscle    twitching
Renal and Urinary Disorders: hematuria
Reproductive System and Breast Disorders: gynecomastia
GeneralDisorders: fatigue, hyperpyrexia
Urogenital Disorders: priapism
Commonadverse reactions: tachycardia, palpitations, headache, insomnia, anxiety, hyperhidrosis, weight loss, decreased appetite, dry mouth, nausea, and abdominal pain (6 ). To report SUSPECTED ADVERSE REACTIONS, contact Ascend Laboratories, LLC at 1-877-ASC-RX01 (877-272-7901) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
7 Drug Interactions
- Antihypertensive  D rugs:Monitorbloodpressure.Adjust dosage of antihypertensive drug as needed (
7 .1).- Halogenated Anesthetics: Avoid use of methylphenidate hydrochloride on the day of surgery if halogenated anesthetics will be used (
7 .1).7.1 Clinically Important Drug Interactions With methylphenidate hydrochloride
Table 1 presents clinically important drug interactions with methylphenidate hydrochloride.
Table 1: Clinically ImportantDrugInteractionswith  methylphenidate hydrochloride
Monoamine Oxidase Inhibitors (MAOI) Clinical Impact Concomitant   use of MAOIs and CNS stimulants, including methylphenidate hydrochloride can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure [see   Contraindications (4)]. Intervention Concomitant   use of methylphenidate hydrochloride with MAOIs or within 14 days after discontinuing MAOI treatment is contraindicated. Examples selegiline, tranylcypromine, isocarboxazid, phenelzine, linezolid, methylene blue Antihypertensive Drugs Clinical Impact Methylphenidate hydrochloride may decrease the effectiveness of drugs used to treat hypertension [see Warnings and Precautions(5.3)]. Intervention Monitor blood pressure and adjust the dosage of the antihypertensive drug as needed. Examples Potassium-sparing and thiazide diuretics, calcium channel blockers, angiotensin-converting-enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta blockers, centrally acting alpha-2 receptor agonists Halogenated Anesthetics Clinical Impact Concomitant   use of halogenated anesthetics and methylphenidate hydrochloride may increase the risk of sudden blood pressure and heart rate increase during surgery. Intervention Avoid use of methylphenidate hydrochloride in patients being treated with anesthetics on the day of surgery. Examples halothane, isoflurane, enflurane, desflurane, sevoflurane
Risperidone Clinical Impact Combined use of methylphenidate with risperidone when there is a change, whether an increase or decrease, in dosage of either or both medications, may increase the risk of extrapyramidal symptoms (EPS) Intervention Monitor for signs of EPS
8 Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry  
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including methylphenidate hydrochloride, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for ADHD Medications at 1-866-961-2388 or visit https://womensmentalhealth.org/adhd-medications/.
Risk Summary  
Published studies and postmarketing reports on methylphenidate use during pregnancy have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There may be risks to the fetus associated with the use of CNS stimulants use during pregnancy (see Clinical Considerations).
No effects on morphological development were observed in embryo-fetal development studies with oral administration of methylphenidate to pregnant rats and rabbits during organogenesis at doses up to 10 and 15 times, respectively, the maximum recommended human dose (MRHD) of 60 mg/day given to adolescents on a mg/m2 basis. However, spina bifida was observed in rabbits at a dose 52 times the MRHD given to adolescents. A decrease in pup body weight was observed in a pre- and post-natal development study with oral administration of methylphenidate to rats throughout pregnancy and lactation at doses 6 times the MRHD given to adolescents (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations  
Fetal/Neonatal Adverse Reactions  
CNS stimulants, such as methylphenidate hydrochloride, can cause vasoconstriction and thereby decrease placental perfusion. No fetal and/or neonatal adverse reactions have been reported with the use of therapeutic doses of methylphenidate during pregnancy; however, premature delivery and low birth weight infants have been reported in amphetamine-dependent mothers.
Data  
Animal Data
In embryo-fetal development studies conducted in rats and rabbits, methylphenidate was administered orally at doses of up to 75 and 200 mg/kg/day, respectively, during the period of organogenesis. Malformations (increased incidence of fetal spina bifida) were observed in rabbits at the highest dose, which is approximately 52 times the MRHD of 60 mg/day given to adolescents on a mg/m2 basis. The no effect level for embryo-fetal development in rabbits was 60 mg/kg/day (15times the MRHD given to adolescents on a mg/m2 basis). There was no evidence of morphological development effects in rats, although increased incidences of fetal skeletal variations were seen at the highest dose level (10 times the MRHD of 60 mg/day given to adolescents on a mg/m2 basis), which was also maternally toxic. The no effect level for embryo-fetal development in rats was 25 mg/kg/day (3 times the MRHD on a mg/m2 basis). When methylphenidate was administered to rats throughout pregnancy and lactation at doses of up to 45 mg/kg/day, offspring body weight gain was decreased at the highest dose (6 times the MRHD of 60 mg/day given to adolescents on a mg/m2 basis), but no other effects on postnatal development were observed. The no effect level for pre- and postnatal development in rats was 15 mg/kg/day (~2 times the MRHD given to adolescents on a mg/m2 basis).
8.2 Lactation
Risk Summary  
Limited published literature, based on milk sampling from seven mothers reports that methylphenidate is present in human milk, which resulted in infant doses of 0.16% to 0.7% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.1 and 2.7. There are no reports of adverse effects on the breastfed infant and no effects on milk production. Long-term neurodevelopmental effects on infants from stimulant exposure are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for methylphenidate hydrochloride and any potential adverse effects on the breastfed infant from methylphenidate hydrochloride or from the underlying maternal condition.
Clinical Considerations  
Monitor breastfeeding infants for adverse reactions, such as agitation, insomnia, anorexia, and reduced weight gain.
8.4 Pediatric Use
The safety and effectiveness of methylphenidate hydrochloride for the treatment of ADHD have been established in pediatric patients 6 to 17 years.
The safety and effectiveness of methylphenidate hydrochloride in pediatric patients less than 6 years have not been established.
The long-term efficacy of methylphenidate hydrochloride in pediatric patients has not been established.
 
Long-Term Suppression of Growth  
Growth should be monitored during treatment with stimulants, including methylphenidate hydrochloride. Pediatric patients who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.7)].
 
Juvenile Animal Toxicity Data  
Rats treated with methylphenidate early in the postnatal period through sexual maturation demonstrated a decrease in spontaneous locomotor activity in adulthood. A deficit in acquisition of a specific learning task was observed in females only. The doses at which these findings were observed are at least 4 times the MRHD of 60 mg/day given to children on a mg/m2 basis.
In a study conducted in young rats, methylphenidate was administered orally at doses of up to 100 mg/kg/day for 9 weeks, starting early in the postnatal period (postnatal Day 7) and continuing through sexual maturity (postnatal Week 10). When these animals were tested as adults (postnatal Weeks 13 to 14), decreased spontaneous locomotor activity was observed in males and females previously treated with 50 mg/kg/day (approximately 4 times the MRHD of 60 mg/day given to children on a mg/m2 basis) or greater, and a deficit in the acquisition of a specific learning task was seen in females exposed to the highest dose (8 times the MRHD given to children on a mg/m2 basis). The no effect level for juvenile neurobehavioral development in rats was 5 mg/kg/day (approximately 0.5 times the MRHD given to children on a mg/m2 basis). The clinical significance of the long-term behavioral effects observed in rats is unknown.
8.5 Geriatric Use
Methylphenidate hydrochloride has not been studied in the geriatricpopulation.
9 Drug Abuse And Dependence
9.1 Controlled Substance
Methylphenidate hydrochloride tablets contain methylphenidate hydrochloride, a Schedule II controlledsubstance.
9.2 Abuse
CNS stimulants,including methylphenidate hydrochloride, have a high potential for abuse. Abuse is characterized by impaired control over drug use despiteharm, and craving.
Signs and symptoms of CNS stimulant abuse include increased heart rate, respiratory rate, blood pressure, and/or sweating, dilated pupils, hyperactivity, restlessness,insomnia, decreased appetite, loss of coordination, tremors, flushed skin, vomiting, and/or abdominal pain. Anxiety,psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed. Abusers of CNS stimulantsmay chew, snort, inject, or use other unapproved routes of administration which may result in overdose and death [see Overdosage (10)].
To reduce the abuse of CNS stimulants, including methylphenidate hydrochloride, assess the risk of abuse prior to prescribing. After prescribing, keep careful prescription records, educate patients and their families about abuse and on proper storage and disposal of CNS stimulants[see How Supplied/Storage and Handling (16)], monitor for signs of abuse while on therapy, and reevaluate the need for methylphenidate hydrochloride use.
9.3 Dependence
Tolerance
Tolerance (a state of adaptation in which exposure to a drug results in a reduction of the   drug's desired and/or   undesired effects over time) can occur during chronic therapy with CNS stimulants, including methylphenidate hydrochloride.
Dependence
Physical dependence (which is manifested by a withdrawal syndrome produced by abrupt cessation, rapid dose reduction, or administration of an antagonist)may occur in patients treated with CNS stimulants, including methylphenidate hydrochloride. Withdrawal symptoms after abrupt   cessation   following   prolonged high-dosage administration of CNS stimulants include dysphoric mood; fatigue; vivid,  unpleasant dreams; insomnia or hypersomnia; increased appetite; and psychomotor retardation or agitation.
10 Overdosage
Human Experience Signs and symptoms of acute overdosage, resulting principally from overstimulation of the central nervous system and from excessive sympathomimetic effects, may include the following: nausea, vomiting, diarrhea, restlessness, anxiety, agitation, tremors, hyperreflexia, muscle twitching, convulsions (which may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, hypotension, tachypnea, mydriasis, dryness of mucous membranes, and rhabdomyolysis. Overdose Management Consult with a Certified Poison Control Center (1-800-222-1222) for the latest recommendations.
11 Description
Methylphenidate hydrochloride tablets USP contains methylphenidate hydrochloride, a CNS stimulant. It is available as tablets of 5mg, 10mg, and 20 mg strength for oral administration. Methylphenidate hydrochloride is methyl őĪ-phenyl-2-piperidineacetate hydrochloride, and its structural formula is: ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬† ¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†¬†
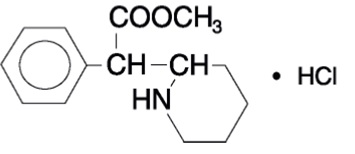
Methylphenidate hydrochloride USP is a white, odorless, fine crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77 g/mol. Methylphenidate hydrochloride tablets USP contains the following inactive ingredients: Colloidal Silicon Dioxide, D&C Yellow #10 Aluminium lake, FD&C Blue #1/Brilliant Blue FCF Aluminium lake (10-mg tablets), Lactose Monohydrate, Magnesium Stearate, Polyethylene glycol, Pregelatinized starch (5-mg and 20-mg tablets), Sucrose.
12 Clinical Pharmacology
12.1 Mechanism of Action
Methylphenidatehydrochloride is a CNS stimulant. The mode of therapeutic action in ADHD and narcolepsy is not known.
12.2 Pharmacodynamics
Methylphenidate is a racemic mixturecomprised of the d- and l-threo  enantiomers. The d-threo enantiomer is more pharmacologically active than the l-threo enantiomer.Methylphenidateblocksthe reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space.
Cardiac Electrophysiology
A formal QT study has not been conducted in patients taking methylphenidate.
The effect of dexmethylphenidate, the pharmacologically active d-enantiomer of methylphenidate, on the QT interval was evaluated in a double-blind, placebo- and open-label active(moxifloxacin)-controlled study following single doses of dexmethlyphenidate XR 40 mg(maximum recommended adult total daily dosage) in 75 healthyvolunteers.
Electrocardiograms were collected up to 12 hourspostdose. Frederica’s method for heart rate correction was employed to derive the corrected QT interval (QTcF). The maximum mean prolongation of QTcF intervals was less than 5 ms, and the upperlimit of the 90% confidence interval was below 10 ms for all time-matchedcomparisons versus placebo. This was below the threshold of clinical concern and there was no evident exposure response relationship.
12.3 Pharmacokinetics
Absorption
Methylphenidate in the extended-release tablets is more slowly but as extensively absorbed as in the regular tablets. Relative bioavailability of the extended-release tablet compared to the methylphenidate hydrochloride tablet, measured by the urinary excretion of methylphenidate major metabolite (őĪ-phenyl-2-piperidine acetic acid) was 105% (49% to 168%) in children and 101% (85% to 152%) in adults. The time to peak rate in children was 1.9 hours (0.3 to 4.4 hours) for the methylphenidate hydrochloride tablets and 4.7 hours(1.3 to 8.2hours) for the methylphenidate hydrochloride extended-release tablets. An average of 67% of extended-release tablet dose was excreted in children as compared to 86% in adults.
Effect of Food
After a high-fat meal, both area under the curve (AUC) (by 25 %) and Cmax (by 27 %) are higher. Time to Cmax (Tmax) is faster after a high-fat meal (median Tmax: 2.5 hours) ascompared to without food (median Tmax: 3 hours).
Distribution
Binding toplasma proteins is low (10% to 33%). The volume of distribution was 2.65 ¬Ī 1.11L/kg for d-methylphenidate and 1.80 ¬Ī 0.91L/kg for l- methylphenidate.
Elimination
The systemic clearance is 0.40 ¬Ī 0.12 L/h/kg for d-methylphenidate and 0.73 ¬Ī 0.28L/h/kg for l-methylphenidate.
Metabolism
Methylphenidate is metabolizedprimarily by de-esterification to alpha-phenyl-piperidine acetic acid (ritalinic acid), which has little or no pharmacologic activity.
Excretion
After oral administration, 78% to 97% of the dose is excreted in the urine and 1% to 3% in feces in the form of metabolites within 48 to 96 hours. Most of the dose is excreted in the urine as alpha-phenyl-2-piperidine acetic acid (60% to 86%). The cumulative urinary excretion of alpha-phenyl-2-piperidine acetic acid are not significantly different for methylphenidate hydrochloride extended-release tablets.
Studies in SpecificPopulations
Male    and Female Patients
In a clinical study involving adult subjects who received methylphenidate hydrochloride extended-release tablets, plasma concentrations of methylphenidate’s major metabolite appeared to be greater in females than in males. No gender differences were observed for methylphenidate plasma concentration in the same subjects.
Racial or Ethnic Groups
There is insufficient experience with the use ofmethylphenidate to detectethnic variations in pharmacokinetics.
Patientswith   Renal Impairment
Methylphenidate has not been studied in renally-impaired patients. Renal impairment is expected to have minimal effect on the pharmacokinetics of methylphenidate since less than 1% of a radiolabeled dose is excreted in the urine as unchanged compound,and the major metabolite (ritalinic acid), has little or no pharmacologic activity.
Patients with Hepatic Impairment
Methylphenidate has not been studied in patients with hepatic impairment.Hepaticimpairment is expected to have minimal effect on the pharmacokinetics of methylphenidate since it is metabolizedprimarily to ritalinic acid by nonmicrosomalhydrolytic esterases that are widely distributedthroughoutthebody.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
In a lifetime carcinogenicity study carriedout in B6C3F1mice, methylphenidate caused an increase in hepatocellular adenomas, and in males only, an increase in hepatoblastomas at a daily dose of approximately 60 mg/kg/day.Thisdose is approximately 2 times the MRHD of 60mg/day given to children on mg/m2 basis.
Hepatoblastoma is a relatively rare rodentmalignanttumor type. There was no increase in totalmalignant hepatic tumors.Themouse strain used is sensitive to the development of hepatictumors and thesignificance of these results tohumansis unknown.
Methylphenidatedidnotcause any increase in tumors in a lifetimecarcinogenicity study carriedout in F344 rats; the highest dose used wasapproximately 45 mg/kg/day,which is approximately 4 times the MRHD (children) on a mg/m2 basis.
In a 24-week carcinogenicity study in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. Male and femalemice were fed diets containing the same concentration of methylphenidate as in the lifetime carcinogenicity study; the high-dose groups were exposed to 60 to 74mg/kg/day of methylphenidate.
Mutagenesis
Methylphenidate was not mutagenic in the in vitro Ames reverse mutation assay, in the in vitromouse lymphoma cell forward mutation assay, or in the in vitro chromosomal aberration assay using human lymphocytes. Sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response, in an in vitro assay in cultured Chinese Hamster Ovary cells. Methylphenidate was negative invivo  in males and females in the mouse bone marrow micronucleus assay.
ImpairmentofFertility
No human data on the effect of methylphenidate on fertility are available. Methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week continuous breeding study. The study was conducted at doses up to 160 mg/kg/day,approximately 10 times the MRHD  of  60mg/day  given  to adolescents on a mg/m2 basis.
16 How Supplied/storage And Handling
Methylphenidate Hydrochloride Tablets, USP
- 5 mg tablets: Round, pale yellow to yellow uncoated tablet, debossed with "A62" on one side and plain on other side, may have mottled appearance.
                 NDC 67877-616-01
                 Bottles of 100 Tablets
                 NDC 67877-616-33
                 Carton of 10 Tablets (1 X 10 Unit-Dose)
- 10 mg tablets: Round, light blue to blue uncoated tablet, debossed with "A63" on one side and  functionally scored on other side, may have mottled appearance.
                  NDC 67877-617-01
                  Bottles of 100 Tablets
                 NDC 67877-617-33
                 Carton of 10 Tablets (1 X 10 Unit-Dose)
- 20 mg tablets: Round, pale yellow to yellow uncoated tablet, debossed with "A64" on one side and functionally scored on other side, may have mottled appearance.
                 NDC 67877-618-01
                 Bottles of 100 Tablets
                NDC 67877-618-33
¬† ¬† ¬† ¬† ¬† ¬† ¬† ¬† Carton of 10 Tablets (1 X 10 Unit-Dose) Store at 20¬įC to 25¬įC (68¬įF to 77¬įF); excursions permitted to¬†15¬įC to 30¬įC (59¬įF to 86¬įF)¬†[see USP controlled room temperature]. Protect from light.
Dispense in tight, light-resistant container (USP).
Disposal
Comply with local laws and regulations on drugdisposal of CNS stimulants. Dispose of remaining, unused, or expired methylphenidate hydrochloride tabletsby a medicine takeback program or by an authorized collector registered with the Drug EnforcementAdministration. If no take-back program or authorized collector is available, mix methylphenidate hydrochloride tablets with an undesirable, nontoxic substance to make it less appealing to children and pets. Place the mixture in a container, such as a sealed plastic bag and discard methylphenidate hydrochloride tablets in the householdtrash.
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Controlled Substance Status/High Potential for Abuse and Dependence
Advise patients that methylphenidate hydrochloride tablets are controlled substances, and they can be abused and lead to dependence. Instruct patients that theyshould not give methylphenidate hydrochloride tablets to anyone else. Advise patients to store methylphenidate hydrochloride tablets in a safe place, preferably locked, to prevent abuse. Advise patients to comply with laws and regulations on drug disposal. Advise patients to dispose of remaining, unused, or expired methylphenidate hydrochloride tablets by a medicine take-back program if available [see Boxed Warning, Warnings and Precautions (5.1), Drug Abuse and Dependence (9.1, 9.2, 9.3), How Supplied/Storage   and   Handling(16)].
Serious Cardiovascular Risks
Advise patients that there is a potential serious cardiovascular risk, including sudden death, myocardial infarction, stroke, and hypertension with methylphenidate hydrochloride tablets use. Instruct patients to contact a healthcare provider immediately if they develop symptoms, such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease [see Warnings and Precautions (5.2)].
Blood Pressure and Heart Rate Increases
Instruct patients that methylphenidate hydrochloride tablets can cause elevations of their blood pressure and pulse rate [see Warnings and Precautions (5.3)].
Psychiatric Risks
Advise patients that methylphenidate hydrochloride tablets, at recommended doses, can cause psychotic or manic   symptoms, even in patients without prior   history of psychotic   symptoms or mania[see Warnings and Precautions (5.4)].
Priapism
Advise patients of the possibility of painful or prolonged penile erections (priapism). Instruct them to seek immediate medical attention in the event of priapism[see Warnings   and Precautions (5.5)].
Circulation Problems in Fingers and Toes [Peripheral Vasculopathy, Including Raynaud’s Phenomenon]
Instruct patients about the risk of peripheral vasculopathy, including Raynaud’s phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful,and/or   may change color from pale, to blue, to red. Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
Instruct patients to call their physician   immediately with any   signs of unexplained wounds appearing on fingers or toes while taking methylphenidate hydrochloride tablets. Further clinical evaluation (e.g., rheumatology    referral) may be appropriate for certain patients [see Warnings   and Precautions (5.6)].
Suppression of Growth
Advise patients that methylphenidate hydrochloride tablets may cause slowing of growth and weight loss [see   Warnings and Precautions ( 5.7 )].
Pregnancy Registry 
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in patients exposed to ADHD medications, including methylphenidate hydrochloride tablets , during pregnancy [see Use in Specific Populations ( 8.1 )].
Manufactured by:
Alkem Laboratories
1733 Gilsinn Lane, Fenton, MO 63026
Distributed by:
Ascend Laboratories, LLC
  Parsippany, NJ 07054
Medication Guide
Methylphenidate Hydrochloride Tablets, USP CII
[(meth′′ il fen′ i date hye′′ droe klor′ ide)]
What is themost important information I should knowabout methylphenidate hydrochloride tablets?
Methylphenidate hydrochloride tablets is a federal controlled substance (CII) because it canbe abused or lead to dependence.Keep methylphenidate hydrochloride tablets in a safe place to prevent misuse and abuse. Selling or giving away methylphenidate hydrochloride tablets may harm others and is against the law.
Tell your doctor if you or your child have abused orbeendependent on alcohol, prescription medicines, or street drugs.
The following have been reported with use of methylphenidate hydrochloride and other stimulantmedicines:
1.Heart-related problems :
- sudden death in patients who have heart problems or heart defects
- stroke and heart attack in adults
- increased blood pressure and heart rate
Tell your doctor if you or your child haveany heart problems,heartdefects, highblood pressure, or a family history of theseproblems.
Your doctor should check you or yourchild carefully for heart problems before starting methylphenidate hydrochloride tablets.
Your doctor should check you or your child’s blood pressureand heart rateregularly during treatment with methylphenidate hydrochloride tablets.
Call your doctor right away if you or your child has any signs of heart problems, such as chest pain, shortness of breath, or fainting while taking methylphenidate hydrochloride tablets.
2. Mental (psychiatric) problems :
All Patients
- new
or worse behavior    and thought problems - new
or worse bipolar illness - new
or    worse aggressivebehavior orhostility - new
psychotic symptoms (such as hearing voices, believing things that arenot true, aresuspicious)or newmanic symptoms
Tell your doctor about any mental problems you or your child have, or about a family history of suicide, bipolar illness, ordepression.
Call your doctor right awayifyou oryour child have any neworworsening mental symptoms or problemswhile taking methylphenidate hydrochloride tablets, especially seeing or hearing things thatare not real, believing things that are not real, or aresuspicious.
What is methylphenidate hydrochloride tablets?
- Methylphenidate hydrochloride tablets is a central nervous system (CNS) stimulant prescription medicine. It is used for the treatment of Attention DeficitHyperactivityDisorder (ADHD).Methylphenidate hydrochloride tablets may help increase attention and decreaseimpulsiveness and hyperactivity in patientswith ADHD.
- Methylphenidate hydrochloride tablets should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
- Methylphenidate hydrochloride tablets is also used in the treatment of a sleep disordercalled narcolepsy.
It is not known if  m ethylphenidate hydrochloride tablets is safe and effective in children under 6 yearsof age.
Who should not take methylphenidate hydrochloride tablets?
Methylphenidate hydrochloride tablets should not be taken if you or your child:
- are allergictomethylphenidate hydrochloride, orany of theingredients in methylphenidate hydrochloride tablets. See the end of this Medication Guide for a complete ul of ingredients in methylphenidate hydrochloride tablets.
- are taking or have taken within the past 14 days an anti-depression medicine called a monoamine oxidase inhibitor (MAOI).
Methylphenidate hydrochloride tablets maynot be right for you oryour child. Before starting methylphenidate hydrochloride tablets, tell your oryourchild’s doctor about all health conditions (or a family history of), including:
- heart problems, heart defects, high blood pressure
- mental problems, including psychosis, mania, bipolarillness, or depression
- circulation problems in fingers or toes
- if you are pregnant or plan to becomepregnant. It is not known if methylphenidate hydrochloride tablets will harm your unborn baby.
o  There is a pregnancy registry for females who are exposed to ADHD medications, including methylphenidate hydrochloride tablets, during pregnancy. The purpose of the registry is to collect information about the health of females exposed to methylphenidate hydrochloride tablets and their baby. If you or your child becomes pregnant during treatment with methylphenidate hydrochloride tablets, talk to your healthcare provider about registering with the National Pregnancy Registry of ADHD Medications at 1-866-961-2388 or visit online at https://womensmentalhealth.org/adhd-medications/.
- if you are breastfeedingor plan to breastfeed.Methylphenidate passesintoyourbreastmilk. Talk to your healthcare provider about the best way to feed your baby during treatment with methylphenidate hydrochloride tablets.
Tell your doctor about all of the medicines thatyou or your child takes, including prescription and over-the-countermedicines, vitamins, and herbal supplements. Methylphenidate hydrochloride tablets and some medicines may interact with each other and causeserious side effects. Sometimes the doses of other medicines will need to be adjusted while taking methylphenidate hydrochloride tablets.
Your doctorwill decide whethermethylphenidate hydrochloride tablets can be taken with other medicines.
Especiallytell your doctor if you oryour child takes:
- anti-depression medicines, including MAOIs
- blood pressure medicines (anti-hypertensive)
Know the medicines that you or yourchild takes. Keep a ul of your medicines with you to showyour doctor and pharmacist.
- You should not take methylphenidate hydrochloride tablets on the day of your operation if a certain type of anestheticis used. This isbecause there is a chance of a sudden rise in blood pressure and heart rate during the operation.
Do not start any newmedicine while taking methylphenidate hydrochloride tablets without talking to your doctor first.
Howshould methylphenidate hydrochloride tablets be taken?
- Take methylphenidate hydrochloride tablets exactly as prescribed. Your doctormay adjust thedose until it is right for you or your child.
- Methylphenidate hydrochloride tablets is usually taken 2 to 3 times a day.
- Take methylphenidate hydrochloride tablets 30 to 45 minutes before a meal.
- From time-to-time, your doctor may stop methylphenidate hydrochloride tablets treatment for a while to checkADHD symptoms.
- Your doctor may do regular checks of the blood, heart, and bloodpressure while takingmethylphenidate hydrochloride tablets.
- Childrenshould have their height and weight checked often whiletakingmethylphenidate hydrochloride tablets. Methylphenidate hydrochloride tablets treatment may be stopped if a problem is found during these check-ups.
- In case of poisoning, call your poison control center at 1-800-222-1222 right away, or goto the nearest hospitalemergencyroom.
What are the possible side effects of methylphenidate hydrochloride tablets?Methylphenidate hydrochloride tablets may cause serious side effects, including:
- see ‚ÄúWhat is the most important information I should knowabout methylphenidate hydrochloride tablets?‚ÄĚ for information on reported heart and mental problems.
- painful and
prolonged erections (priapism) have occurredwith methylphenidate. Ifyou or your child developspriapism, seekmedical help right away. Because of the potential for lasting damage, priapismshould be evaluated by a doctor immediately. - circulation problems in fingers and toes (peripheral vasculopathy, including Raynaud’s phenomenon):
o fingers or toesmay feel numb, cool, painful
o fingers or toesmay change color from pale, to blue, to red
Tell your doctor if you or your child have, numbness, pain, skin color change, or sensitivityto temperature inthe fingers or toes.
- Call your doctor right away if you have or your child has any signs of unexplained wounds appearing on fingersor toes while taking methylphenidate hydrochloride tablets.
- Slowing of growth
(height and weight) in children
Common side effects include:
- fast heart beat
- abnormal heartbeat (palpitations)
- headache
- trouble sleeping  
- nervousness
- sweating a lot     
- decreased appetite          
- dry mouth           
- nausea
- stomach pain
Call your doctor for medical advice about side effects.You may report side effects to FDA at1-800-FDA-1088.
Howshould I store methylphenidate hydrochloride tablets?
- Store¬†methylphenidate hydrochloride tablets in a safe place and in a tightly closed container at room temperature between 68¬įF to 77¬įF (20¬įC to 25¬įC).
- Protect from light.
- Dispose of remaining, unused, or expired methylphenidate hydrochloride tablets by a medicine take-back program at authorized collection sites, such as retail pharmacies, hospital or clinicpharmacies, and law enforcement locations. If no take-back program or authorizedcollector isavailable, mix methylphenidate hydrochloride tablets with an undesirable, nontoxic substance,such as dirt, cat litter, or used coffee grounds to make it less appealing to children and pets. Place the mixture in a container, such as a sealed plastic bag and throw away (discard) methylphenidate hydrochloride tablets in the household trash.
- Keep
methylphenidate hydrochloride tablets and all medicines out of the reach of children.
General information about the safe and effective use ofmethylphenidate hydrochloride tablets.
Medicines are sometimesprescribed for purposes other than those uled in a Medication Guide. You can ask your pharmacist or doctor for information about methylphenidate hydrochloride tablets that is written for healthcareprofessionals. Do not use methylphenidate hydrochloride tablets for a condition for which it was not prescribed. Do not give methylphenidate hydrochloride tablets to other people,even if they have the samesymptoms thatyou have. Itmay harm them and it is against the law.
What are theingredientsin methylphenidate hydrochloride tablets?
Active ingredient: Methylphenidate HCl
Inactive ingredients: Colloidal Silicon Dioxide, D&C Yellow #10 Aluminium lake, FD&C Blue #1/Brilliant Blue FCF Aluminium lake (10-mg tablets), Lactose Monohydrate, Magnesium Stearate, Polyethylene glycol, Pregelatinized starch (5-mg and 20-mg tablets), Sucrose.
To report SUSPECTED ADVERSE REACTIONS, contact Ascend Laboratories, LLC at 1-877-ASC-RX01 (877-272-7901) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
 
Manufactured by:
    Alkem Laboratories
    1733 Gilsinn Lane, Fenton, MO 63026
Distributed by:
Ascend Laboratories, LLC 
Parsippany, NJ 07054
Revised: August, 2021
This Medication Guide has been approved by the U.S. Food and Drug Administration.
    L916 (150009) VER 01
Package Label.principal Display Panel
NDC 67877-616-01Methylphenidate Hydrochloride Tablets, USP 5 mg Rx only             100 Tablets
NDC 67877-616-33 Methylphenidate Hydrochloride Tablets, USP 5 mg Rx only             10 Tablets
NDC 67877-617-01Methylphenidate Hydrochloride Tablets, USP 10 mg Rx only             100 Tablets

NDC 67877-617-33 Methylphenidate Hydrochloride Tablets, USP 10 mg Rx only             10 Tablets

  NDC 67877-618-01Methylphenidate Hydrochloride Tablets, USP 20 mg Rx only     100 Tablets 
        NDC 67877-618-33 Methylphenidate Hydrochloride Tablets, USP 20 mg Rx only             10 Tablets

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


