Lysodren (mitotane 500 mg) Dailymed
Generic: mitotane is used for the treatment of Cushing Syndrome Adrenocortical Carcinoma
Boxed Warning
Warning: Adrenal Crisis In The Setting Of Shock, Severe Trauma Or Infection
Go PRO for all pill images
Warning: Adrenal Crisis In The Setting Of Shock, Severe Trauma Or Infection
Patients treated with LYSODREN are at increased risk for developing adrenal crisis in the setting of shock, severe trauma or infection that may lead to death.
If shock, severe trauma or infection occurs or develops, temporarily discontinue LYSODREN and administer exogenous steroids. Monitor patients closely for infections and instruct patients to contact their physician immediately if injury, infection, or any other concomitant illness occurs [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK, SEVERE TRAUMA OR INFECTION
See full prescribing information for complete boxed warning.
Patients treated with LYSODREN are at increased risk for developing adrenal crisis in the setting of shock, severe trauma or infection that may lead to death.
If shock, severe trauma or infection occurs or develops, temporarily discontinue LYSODREN and administer exogenous steroids. Monitor patients closely for infections and instruct patients to contact their physician immediately if injury, infection, or any other concomitant illness occurs (2.3 ,5.1 ).
Recent Major Changes Section
BOXED WARNING 01/2024 INDICATIONS AND USAGE ( 1 )01/2024 DOSAGE AND ADMINISTRATION ( 2 )01/2024 CONTRAINDICATIONS ( 4 )01/2024 WARNINGS AND PRECAUTIONS ( 5 )01/2024
1 Indications And Usage
LYSODREN is indicated for the treatment of patients with inoperable, functional or nonfunctional, adrenocortical carcinoma (ACC).
LYSODREN is an adrenal cytotoxic agent indicated for the treatment of patients with inoperable, functional or nonfunctional, adrenocortical carcinoma (ACC). (1 )
2 Dosage And Administration
- The recommended initial dose of LYSODREN is 2000 mg to 6000 mg orally, in three or four divided doses per day with food. (
2.3 )- Titrate LYSODREN dose to achieve a plasma level of 14 to 20 mg/L. (
2.3 )- LYSODREN is lipophilic and accumulates in adipose tissue. (
2.3 )2.1Recommended Evaluation and Testing Before Initiating LYSODREN
Before initiating LYSODREN, evaluate pelvic ultrasound in premenopausal women, liver functions tests and complete blood count [see Warnings and Precautions (5.3, 5.4, 5.5)].
2.2General Precautions
LYSODREN is a hazardous drug. Advise caregivers to wear disposable gloves when handling LYSODREN tablets [see References (15) and Storage and Handling (16)].
2.3Recommended Dosage and Administration
Recommended Dosage
The recommended initial dose of LYSODREN is 2000 mg to 6000 mg orally, in three or four divided doses per day. Monitor mitotane plasma levels and increase the dose based on patient tolerance and clinical response incrementally to achieve a mitotane plasma level of 14 to 20 mg/L, or as tolerated. Consider monitoring mitotane plasma levels every 2 weeks after starting treatment and after each dose adjustment. The target plasma level is usually reached within a period of 3 to 5 months. Monitor mitotane plasma levels periodically (e.g., monthly). Severe neurotoxicity may occur with levels > 20 mg/L.
Dose Adjustments, Monitoring and Discontinuation
In case of mitotane plasma levels above 20 mg/L without toxicities, consider reducing the dose by 50 to 75%. Monitor mitotane plasma levels regularly (e.g., every two months) after discontinuation of treatment. Due to the prolonged half-life, significant levels may persist for weeks after cessation of therapy. LYSODREN is lipophilic and accumulates in adipose tissue. Despite maintaining a constant dose, mitotane levels may suddenly increase. Monitor mitotane plasma levels even when LYSODREN has been withheld as adipose tissue may continue to release mitotane [see Clinical Pharmacology (12.3)]. Due to adipose tissue accumulation of mitotane, close monitoring of mitotane plasma levels is recommended in overweight patients and patients with recent weight loss.
Administration
Swallow LYSODREN tablets whole; do not crush, chew or split. Take LYSODREN with food. Timing of the dose relative to meals must be consistent. Administration with high fat food enhances absorption [See Clinical Pharmacology Section (12.3)]. Do not take tablets showing signs of deterioration. Caregivers should wear disposable gloves when handling the tablets. Avoid exposure to crushed and/or broken tablets. If contact with crushed/broken tablets occurs, wash the contaminated skin immediately and thoroughly. If a patient misses a dose, instruct the patient to take the next dose as scheduled. If a patient vomits after taking a dose, instruct the patient to take the next dose as scheduled. 2.4Dosage Modifications for Adverse Reactions
The recommended dosage reduction for adverse reactions is to decrease the usual daily dose by 500 – 1000 mg.
Table 1 describes the dosage modifications for specific adverse reactions.
Table 1: Recommended Dosage Modifications for LYSODREN for Adverse Reactions Adverse Reaction Severity Severity defined by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0. Dosage Modification Adrenal Crisis and Adrenal Insufficiency [see Warnings and Precautions (5.1)] All Grades
- Withhold LYSODREN during shock, trauma, infection or adrenal insufficiency until recovery to Grade ≤1 or baseline.
- Resume LYSODREN at a reduced dose or permanently discontinue based on severity.
Central Nervous System (CNS) Toxicity [see Warnings and Precautions (5.2)] Grade 2
- Measure mitotane plasma level and modify the dosage according to the following recommendations:
- Mitotane plasma level less than 14 mg/L: reduce the daily dose by 1000 mg.- Mitotane plasma level in the therapeutic window (14 to 20 mg/L): reduce the daily dose by 1500 mg.- Mitotane plasma level above 20 mg/L: withhold LYSODREN until recovery to grade ≤1 or baseline. Seven to ten (7-10) days after symptoms resolve, resume LYSODREN at reduced dose.Grade 3 or 4
- Measure mitotane plasma level and modify the dosage according to the following recommendations:
- Withhold LYSODREN until recovery to grade ≤ 1 or baseline. Seven to ten (7-10) days after symptoms resolve, resume LYSODREN at a reduced dose or permanently discontinue based on severity.Gastrointestinal (GI) toxicity Grade 3 or 4
- Measure mitotane plasma level and modify the dosage according to the following recommendations:
- Mitotane plasma level less than 14 mg/L: reduce the daily dose by 1000 mg.- Mitotane plasma level in the therapeutic window (14 to 20 mg/L): reduce the daily dose by 1500 mg.- Mitotane plasma level above 20 mg/L: withhold LYSODREN until recovery to grade ≤1 or baseline.- After symptoms resolve, resume LYSODREN at a reduced dose or permanently discontinue based on severity.Hepatotoxicity [see Warnings and Precautions (5.4)] Grade 3 or 4
- Measure mitotane plasma level and modify the dosage according to the following recommendations:
- Withhold LYSODREN until recovery to Grade ≤1 or baseline.- Resume at a reduced dose or permanently discontinue based on severity.Hematologic Toxicity [see Warnings and Precautions (5.5)] Grade 2
- Measure mitotane plasma level and modify the dosage according to the following recommendations:
- Withhold LYSODREN until recovery to Grade ≤1 or baseline.- Resume at the same dose.Grade 3 or 4
- Measure mitotane plasma level and modify the dosage according to the following recommendations:
- Withhold LYSODREN until recovery to Grade ≤1 or baseline.- Resume at a reduced dose or permanently discontinue based on severity.Other Adverse Reactions [see Adverse Reactions (6.1)] Grade 2
- Measure mitotane plasma level and modify the dosage according to the following recommendations:
- Withhold LYSODREN until recovery to Grade ≤1 or baseline.- Resume at the same dose.Grade 3 or 4
- Measure mitotane plasma level and modify the dosage according to the following recommendations:
- Withhold LYSODREN until recovery to Grade ≤1 or baseline.- Resume at a reduced dose or permanently discontinue based on severity.
3 Dosage Forms And Strengths
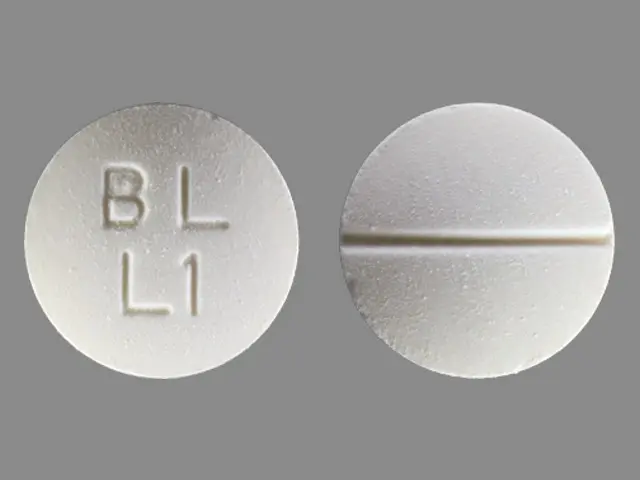
Tablets: 500 mg white, round, biconvex, scored tablets, bisected on one side and impressed with "BL" over "L1" on the other side.
Tablets: 500 mg, scored. (3 )
4 Contraindications
None.
None. (4 )
5 Warnings And Precautions
- Adrenal Insufficiency and Adrenal Crisis: Temporarily withhold LYSODREN during shock, trauma, infection or adrenal insufficiency. Steroid replacement may be necessary. (
5.1 )- Central Nervous System (CNS) Toxicity: Monitor behavioral and neurologic assessments and mitotane plasma levels at regular intervals. Mitotane plasma levels exceeding 20 mg/L are associated with a greater incidence of toxicity. Advise patients not to drive or operate hazardous machinery if experiencing CNS adverse reactions. (
5.2 )- Ovarian Macrocysts in Premenopausal Women: Monitor pelvic ultrasound at baseline and at regular intervals. Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity. (
5.3 )- Hepatotoxicity: Monitor liver functions tests prior to starting LYSODREN, during dose titration and as clinically indicated. Withhold, reduce the dose or permanently discontinue based on severity. (
5.4 )- Hematologic Toxicity: Monitor complete blood counts prior to starting LYSODREN, during dose titration and as clinically indicated. Withhold, reduce the dose or permanently discontinue based on severity. (
5.5 )- Prolonged Bleeding Time: Prolonged bleeding time has occurred in patients treated with mitotane and this should be taken into account when surgery is considered. (
5.6 )- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective, nonhormonal contraception. (
5.8 )5.1Adrenal Insufficiency and Adrenal Crisis
Adrenal Insufficiency
LYSODREN can cause adrenal insufficiency or worsen existing adrenal insufficiency in patients with adrenocortical carcinoma.
Monitor for both glucocorticoid and mineralocorticoid insufficiency and replace systemic corticosteroids accordingly. Due to increased steroid clearance and increase of steroid-binding protein, high-dose replacement therapy may be required and free cortisol and corticotropin (ACTH) should be monitored to adapt the systemic corticosteroids.
Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity [see Dosage and Administration (2.4)].
Adrenal Crisis in the Setting of Shock, Severe Trauma or Infection
LYSODREN can cause adrenal suppression and adrenal crisis in the setting of shock, severe trauma or infection.
Advise patients of the signs and symptoms of adrenal suppression and to contact their healthcare provider immediately if shock, trauma, infection, or adrenal suppression occurs. Withhold LYSODREN before planned surgeries.
Temporarily withhold LYSODREN during shock, trauma, infection or adrenal suppression [see Dosage and Administration (2.4)].
Provide supportive care and administer systemic corticosteroids until recovery.
5.2Central Nervous System Toxicity
LYSODREN can cause central nervous system toxicity, including sedation, lethargy, and vertigo [see Adverse Reactions (6.1)].
Monitor behavioral and neurologic assessments and mitotane plasma levels at regular intervals. Mitotane plasma levels exceeding 20 mg/L are associated with a greater incidence of toxicity.
In cases of cognitive dysfunction, thyroid function should be evaluated as mitotane may induce hypothyroidism.
LYSODREN can impair the ability to drive and operate machinery. Advise patients not to drive or operate hazardous machinery if they are experiencing CNS adverse reactions. Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity [see Dosage and Administration (2.4)].
5.3Ovarian Macrocysts in Premenopausal Women
LYSODREN can cause non-malignant, multiple and bilateral ovarian macrocysts in premenopausal women.
Ovarian macrocysts can be symptomatic (e.g., pelvic pain or discomfort, or menstrual irregularities) or asymptomatic.
Complications from these cysts, including adnexal torsion and hemorrhagic cyst rupture, have occurred.
Advise female patients to contact their healthcare provider immediately for gynecological symptoms such as vaginal bleeding and/or pelvic pain [see Adverse Reactions (6.1)].
Monitor pelvic imaging in premenopausal females at baseline and in regular intervals during treatment with LYSODREN.
Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity [see Dosage and Administration (2.4)].
5.4Hepatotoxicity
LYSODREN can cause hepatoxicity, including liver injury or failure.
Monitor liver function tests prior to starting treatment with LYSODREN, during dose titration, and periodically during treatment as clinically indicated.
Isolated gamma-glutamyl transferase (GGT) elevation may occur.
Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity of hepatoxicity [see Dosage and Administration (2.4)].
5.5Hematologic toxicity
LYSODREN can cause leukopenia, anemia and thrombocytopenia [see Adverse Reactions (6)]. Monitor complete blood counts including neutrophil count prior to starting treatment with LYSODREN, during dose titration, and periodically during treatment as clinically indicated. Withhold, reduce the dose, or permanently discontinue LYSODREN based on severity of cytopenia [see Dosage and Administration (2.4)].
5.6Prolonged Bleeding Time
LYSODREN can cause platelet function disorders due to abnormal adenosine diphosphate (ADP)-induced platelet aggregation. Some patients may have a prolonged bleeding time, while others may have a normal bleeding time.
Routine in vitro bleeding time is not suitable to detect this platelet defect and to assess bleeding risk.
Perform ADP-inducted platelet aggregometry testing prior to surgery or dental procedures to determine mitotane-induced bleeding risk. For patients with prolonged bleeding time, withhold or reduce the dose of LYSODREN as clinically indicated.
5.7Hormone binding protein
Mitotane has been shown to increase plasma levels of hormone binding proteins (e.g., sex hormone-binding globulin (SHBG) and corticosteroid-binding globulin (CBG)). This should be taken into account when interpreting the results of hormonal assays and may result in gynecomastia.
5.8Embryo-Fetal Toxicity
LYSODREN can cause fetal harm when administered to a pregnant woman. Abnormal pregnancy outcomes, such as preterm births and early pregnancy loss, can occur in patients exposed to mitotane during pregnancy. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective nonhormonal contraception, during treatment with LYSODREN and after discontinuation of treatment for as long as mitotane plasma levels are detectable, since LYSODREN can render some hormonal contraceptives ineffective [see Drug Interactions (7.2), Use in Specific Populations (8.1, 8.3)].
6 Adverse Reactions
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Adrenal Insufficiency and Adrenal Crisis [see Warnings and Precautions (5.1)]
- Central Nervous System Toxicity [see Warnings and Precautions (5.2)]
- Ovarian Macrocysts in Premenopausal Women [see Warnings and Precautions (5.3)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
- Hematologic Toxicity [see Warnings and Precautions (5.5)]
- Prolonged Bleeding Time [see Warnings and Precautions (5.6)]
- Hormone Binding Protein [see Warnings and Precautions (5.7)]
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.8)]
Most common adverse reactions include: anorexia, epigastric discomfort, nausea, vomiting, diarrhea, depression, dizziness, vertigo, rash, hypercholesterolemia, hypertriglyceridemia, hypothyroidism, and decreased blood free testosterone in males. (6 )
To report SUSPECTED ADVERSE REACTIONS, contact Direct Success Inc.at 1-844-597-6373 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Reported adverse reactions include:
- Metabolism and nutrition disorders: Anorexia
- Gastrointestinal disorders: Epigastric discomfort, nausea, vomiting, diarrhea, mucosal inflammation, dyspepsia
- Nervous system disorders: Depression, dizziness, vertigo, confusion, headache, ataxia, mental impairment, weakness, dysarthria, paresthesia, polyneuropathy, movement disorder, balance disorder, dysgeusia
- Skin and subcutaneous tissue disorders: Rash, pruritus, hypersensitivity reactions
- Blood and lymphatic system disorders: Leukopenia, anemia, thrombocytopenia, prolonged bleeding time, hematuria, hemorrhagic cystitis
- Endocrine: Growth retardation, hypothyroidism
- Eye disorders: Maculopathy, visual blurring, diplopia, lens opacity, retinopathy
- Hepatobiliary disorders: Hepatitis, elevation of liver enzymes, liver injury (hepatocellular/cholestatic/mixed)
- Reproductive system and breast disorders: Gynecomastia, hypogonadism (in males)
- Investigations: Hypercholesterolemia, hypertriglyceridemia, decreased plasma androstenedione, decreased plasma testosterone in females, increased sex hormone binding globulin in females and males, decreased blood free testosterone in males, hypouricemia
- Musculoskeletal disorders: Muscular weakness, generalized aching
- General disorders: Fever
- Renal and urinary disorders: Albuminuria/proteinuria
- Vascular disorders: Hypertension, orthostatic hypotension, flushing
- Infections: Opportunistic infection
- Respiratory, thoracic and mediastinal disorders: Dyspnea
- Cardiac: QT prolongation
7 Drug Interactions
- Spironolactone: Avoid concomitant use with LYSODREN. (
7.1 )- Certain CYP3A Substrates: Avoid concomitant use with LYSODREN. (
7.2 )- Hormonal contraceptives: Avoid concomitant use with LYSODREN. (
7.2 )- Warfarin: Avoid concomitant use with LYSODREN. (
7.2 )7.1Effects of Other Drugs on LYSODREN
Spironolactone
Spironolactone may block the action of mitotane. Avoid concomitant use of mitotane with spironolactone [see Clinical Pharmacology (12.3)].
7.2Effects of LYSODREN on Other Drugs
Certain CYP3A substrates
Mitotane is a strong CYP3A inducer. Concomitant use of LYSODREN may decrease the levels of CYP3A substrates, which may reduce the activity of these substrates [see Clinical Pharmacology (12.3)].
Avoid concomitant use of LYSODREN with other CYP3A substrates, where minimal level changes may lead to serious therapeutic failures. If concomitant use cannot be avoided, modify the dosage of the CYP3A substrate in accordance with the approved product labeling.
Hormonal Contraceptives
Avoid concomitant use of LYSODREN with hormonal contraceptives [see Warnings and Precautions (5.8), Use in Specific Populations (8.3)].
Warfarin
Mitotane may induce the metabolism of warfarin, which may reduce its level and its efficacy [see Clinical Pharmacology (12.3)].
Avoid concomitant use of LYSODREN with warfarin. If concomitant use cannot be avoided, monitor INR more frequently and adjust warfarin dose as recommended in accordance with the recommendations in the warfarin Prescribing Information.
8 Use In Specific Populations
- Lactation: Advise not to breastfeed. (
8.2 )- H epatic Impairment: LYSODREN is not recommended for patients with severe hepatic impairment. In patients with mild to moderate hepatic impairment, monitor mitotane plasma levels frequently and modify the dosage as needed. (
8.6 )- Renal Impairment: LYSODREN is not recommended for patients with severe renal impairment. In patients with mild or moderate renal impairment, monitor mitotane plasma levels frequently and modify the dosage as needed. (
8.7 )8.1 Pregnancy
Risk Summary
LYSODREN can cause fetal harm. Limited postmarketing cases report preterm births and early pregnancy loss in women treated with LYSODREN during pregnancy. Animal reproduction studies have not been conducted with mitotane.
Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
Mitotane is excreted in human milk; however, the effect of LYSODREN on the breastfed child, or on milk production is unknown. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with LYSODREN and after discontinuation of treatment for as long as mitotane plasma levels are detectable.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating LYSODREN [see Use in Specific Populations (8.1)].
Contraception
LYSODREN can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Females
Advise females of reproductive potential to use effective nonhormonal contraception during treatment with LYSODREN and after discontinuation of therapy for as long as mitotane plasma levels are detectable [see Clinical Pharmacology (12.3)]. LYSODREN can render hormonal contraceptives ineffective [see Drug Interaction (7.2)].
8.4 Pediatric Use
Effectiveness in pediatric patients has not been established.
Based on published case reports, mitotane may negatively impact neuro-psychological development (e.g., motor and speech delay, memory impairment) in children and adolescents. In cases of cognitive dysfunction, thyroid function should be evaluated as mitotane may induce hypothyroidism. Other effects of mitotane observed in pediatric patients that are cited in medical literature or in a pharmacovigilance database include growth delay and estrogenic-like effects such as uterine bleeding, breast development in females and gynecomastia in males.
8.5 Geriatric Use
Clinical studies of LYSODREN did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the older and younger patients. In general, dose selection for an older patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Mitotane is metabolized through the liver and mitotane plasma levels may increase if liver function is impaired.
Because of the increased risk of adverse reactions in patients with mild or moderate hepatic impairment, monitor mitotane plasma levels more frequently and modify the dosage as needed [see Dosage and Administration (2.3)]. LYSODREN is not recommended for use in patients with severe hepatic impairment [see Warnings and Precautions (5.4)].
8.7 Renal Impairment
Mitotane is eliminated through the kidney and mitotane plasma levels may increase if renal function is impaired.
Because of the increased risk of adverse reactions in patients with mild and moderate renal impairment, monitor mitotane plasma levels more frequently and modify the dosage as needed [see Dosage and Administration (2.4)]. LYSODREN is not recommended for use in patients with severe renal impairment.
10 Overdosage
LYSODREN overdosage (plasma levels are above 20 mg/L) can cause central nervous system toxicity, including sedation, lethargy, and vertigo, as well as muscular weakness and gait disturbance. Withhold LYSODREN as clinically indicated for signs or symptoms of toxicity.
LYSODREN is lipophilic and has a prolonged half-life; therefore, it may take weeks for plasma levels to decrease. LYSODREN is not likely to be dialyzable. Increase the frequency of mitotane plasma level monitoring, as clinically indicated.
11 Description
LYSODREN (mitotane) is an oral adrenal cytotoxic agent. The chemical name is (±)-1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl) ethane (also known as o,p′-DDD). The chemical structure is:

Mitotane is a white granular solid composed of clear colorless crystals. It is tasteless and has a slight pleasant aromatic odor. It is soluble in ethanol and has a molecular weight of 320.05.
Inactive ingredients in LYSODREN are: microcrystalline cellulose, polyethylene glycol 3350, silicon dioxide, and starch.
12 Clinical Pharmacology
12.1 Mechanism of Action
Mitotane is an adrenal cytotoxic agent with an unknown mechanism of action. Mitotane modifies the peripheral metabolism of steroids and directly suppresses the adrenal cortex. A reduction in 17-hydroxycorticosteroids in the absence of decreased corticosteroid levels and increased formation of 6-β-hydroxycortisol have been reported.
12.2 Pharmacodynamics
Mitotane exposure-response relationships and the time course of pharmacodynamic response have not been fully characterized.
12.3 Pharmacokinetics
Absorption
Following oral administration of LYSODREN, 40% of the dose is absorbed.
Effect of Food
The effect of food on the absorption of mitotane (LYSODREN) is not known.
High fat food: Data with various mitotane formulations suggest that administration with high fat food enhances absorption of mitotane.
Distribution
Mitotane is found in most tissues of the body; however, fat is the primary site of distribution.
Elimination
Following discontinuation of mitotane, the plasma terminal half-life ranges from 18 to 159 days (median 53 days).
Metabolism
Mitotane is converted to a water-soluble metabolite. Mitotane metabolism includes hepatic and extrahepatic metabolism. Mitotane is metabolized to 1,1-(o,p'-dichlorodiphenyl) acetic acid (o,p'-DDA), as the major circulating metabolite.
Excretion
No unchanged mitotane is found in urine or bile. Approximately 10% of the administered dose is recovered in the urine as a water-soluble metabolite. A variable amount of metabolite (1%-17%) is excreted in the bile.
Specific Populations
Patients with Hepatic or Renal Impairment
The pharmacokinetics of mitotane have not been studied in patients with hepatic impairment or patients with renal impairment.
Drug Interaction Studies
Effects of Mitotane on Other Drugs
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenicity and mutagenicity of mitotane are unknown. Fertility studies have not been conducted with mitotane.
15 References
- OSHA Hazardous Drugs. http://www.osha.gov/hazardous-drugs/
16 How Supplied/storage And Handling
LYSODREN tablets are supplied as 500 mg white, round, biconvex, scored tablets, bisected on one side and impressed with "BL" over "L1" on the other side.
100 tablets per bottle: NDC 76336-080-60
STORAGE AND HANDLING SECTION
Store bottles at 25°C (77°F); excursions permitted between 15°C and 30°C (59°F-86°F).
Mitotane is a hazardous drug. Follow applicable special handling and disposal procedures [see References (15)].
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Adrenal Insufficiency and Adrenal Crisis
- Advise patients of the risk of adrenal suppression that may require steroid treatment and interruption or discontinuation of LYSODREN [see Warnings and Precautions (5.1)].
- Advise patients to discontinue LYSODREN in the case of shock, severe trauma or infection and contact their healthcare provider immediately [see Warnings and Precautions (5.1)].
- Advise patients to inform their healthcare provider of any planned surgeries [see Warnings and Precautions (5.1)].
Central Nervous System Toxicity
- Advise patients to contact their healthcare provider if they experience new or worsening symptoms of central nervous system (CNS) toxicity including sedation, lethargy and vertigo [see Warnings and Precautions (5.2)].
- Instruct patients not to drive or operate hazardous machinery if they are experiencing CNS adverse reactions [see Warnings and Precautions (5.2)].
Ovarian Macrocysts in Premenopausal Women
- Advise premenopausal women that their healthcare provider will monitor them with routine imaging and to contact their healthcare provider if they experience gynecological symptoms such as vaginal bleeding and/or pelvic pain [see Warnings and Precautions (5.3)].
Hepatotoxicity
- Advise patients that their healthcare provider will monitor them with laboratory tests to monitor liver function and to immediately contact their healthcare provider to report signs and symptoms of hepatotoxicity [see Warnings and Precautions (5.4)].
Hematologic Toxicity
- Advise patients to immediately contact their healthcare provider for fever, other signs of infection, unusual bruising, bleeding, tiredness or pallor [see Warnings and Precautions (5.5) ].
Prolonged Bleeding Time
- Advise patients of the possibility of prolonged bleeding time while taking LYSODREN and of the need to withhold LYSODREN if surgery or dental procedures are needed [see Warnings and Precautions (5.6)].
- Advise patients to inform their healthcare provider of any planned surgeries or dental procedures [see Warnings and Precautions (5.6)].
- Advise patients to contact their healthcare provider for bruising or bleeding [see Warnings and Precautions (5.6)].
Embryo-Fetal Toxicity
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1)].
Females of Reproductive Potential
- Advise females of reproductive potential to use effective nonhormonal contraception during treatment with LYSODREN and after discontinuation of treatment for as long as mitotane plasma levels are detectable, since LYSODREN can render some hormonal contraceptives ineffective [see Drug Interactions (7.2), Use in Specific Populations (8.3)].
Lactation
- Advise females not to breastfeed during treatment with LYSODREN [see Use in Specific Populations (8.2)].
Drug Interactions
- Advise patients and their caregivers to inform their healthcare providers of all concomitant medications, herbal and dietary supplements [see Drug Interactions (7.1, 7.2)].
Address medical inquiries to:Direct Success Inc. 1710 Hwy 34 Farmingdale, NJ 07727 1-844-597-6373 Fax: 1-855-674-6767
Manufactured by: Latina Pharma S.p.A.Via Murillo, 7 04013 Sermoneta (Latina)Italy
For: HRA Pharma Rare Diseases
Spl Medguide Section
MEDICATION GUIDE LYSODREN® (LY-SO-DREN) (mitotane) tablets, for oral use This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 10/2024 What is the most important information I should know about LYSODREN? LYSODREN can cause serious side effects including: Adrenal Insufficiency and Adrenal Crisis.
- Adrenal Insufficiency. LYSODREN can cause your adrenal glands to stop making enough corticosteroid hormones (adrenal insufficiency) or make this problem worse in people with cancer of the adrenal glands (adrenocortical carcinoma). Your healthcare provider may temporarily stop, reduce your dose, or permanently stop treatment with LYSODREN if you develop adrenal insufficiency during treatment.
- Adrenal Crisis. LYSODREN can cause your adrenal glands to suddenly stop making enough corticosteroid hormones (adrenal crisis). You are at increased risk for developing adrenal crisis if you experience shock, severe injury, or infection during treatment with LYSODREN. Adrenal crisis may lead to death. Tell your healthcare provider right away if you get an injury, infection, or other illness during treatment with LYSODREN. Your healthcare provider will temporarily stop LYSODREN if shock, severe injury, or infections happen during treatment. Tell your healthcare provider if you have any planned surgery.
Your healthcare provider will check your levels of corticosteroid hormones during treatment and may give you corticosteroid medicine if you develop adrenal gland problems. Tell your healthcare provider right away if you develop any signs or symptoms of adrenal gland problems, including:
- severe weakness
- confusion
- pain in the lower back and legs
- stomach (abdominal) pain
- nausea, vomiting, or diarrhea
- feeling lightheaded or dizzy
- passing out
- feeling very tired
- decreased appetite
- weight loss
- areas of darkened skin
- craving salt
- low blood sugar
- feeling irritable or depressed
- hair loss
See " What are the possible side effects of LYSODREN?" for more information about side effects. What is LYSODREN? LYSODREN is a prescription medicine used to treat people with cancer of the adrenal glands (adrenocortical carcinoma) that is functional (when the adrenal glands make more corticosteroid hormone than normal) or nonfunctional (when the adrenal glands make less corticosteroid hormone than normal) and the cancer cannot be removed by surgery. Effectiveness in pediatric patients has not been established. Before taking LYSODREN, tell your healthcare provider about all of your medical conditions, including if you:
- have an injury, infection or illness
- plan to have surgery. Tell your healthcare providers you are taking LYSODREN. You may need to stop taking LYSODREN before any surgery and dental procedures.
- have cysts on your ovaries
- have liver or kidney problems
- have bleeding problems
- are pregnant or plan to become pregnant. LYSODREN can harm your unborn baby. Females who can become pregnant:
- Your healthcare provider will do a pregnancy test before you start treatment with LYSODREN.
- Use effective birth control (contraception) that does not contain hormones (nonhormonal) such as condoms or diaphragms and spermicide during treatment with LYSODREN and for as long as your healthcare provider tells you to after you stop treatment.
- Talk to your healthcare provider about nonhormonal birth control methods that may be right for you.
- Birth control methods that contain hormones such as birth control pills, injections, or patches may not work as well during and after treatment with LYSODREN.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with LYSODREN.
- are breastfeeding or plan to breastfeed. LYSODREN can pass into your breast milk and may harm your baby. Do not breastfeed during treatment and after you stop taking LYSODREN until your healthcare provider tells you it is okay. Talk to your healthcare provider about the best way to feed your baby during and after treatment with LYSODREN.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking LYSODREN with certain other medicines may affect the way LYSODREN and the other medicines work and may increase your risk of side effects. Especially tell your healthcare provider if you take:
- spironolactone
- midazolam or other CYP3A substrates
- hormonal birth control
- warfarin
Ask your healthcare provider about any other medicine that may not be uled above. Know the medicines you take. Keep a ul of them to show your healthcare provider and pharmacist when you get a new medicine. How should I take LYSODREN?
- Take LYSODREN exactly as your healthcare provider tells you to take it.
- Do not change your dose unless your healthcare provider tells you to.
- Take your daily dose of LYSODREN in 3 or 4 divided doses each day.
- Swallow LYSODREN tablets whole. Do not crush, chew or split the tablets.
- Do not take any LYSODREN tablets that are broken or crushed.
- Take LYSODREN with food, preferably a high-fat meal or snack. Talk to your healthcare provider about examples of foods you should eat.
- Caregivers should wear disposable gloves when handling LYSODREN tablets. You should avoid contact with crushed or broken tablets. If skin contact with crushed or broken tablets happens, thoroughly wash the skin area right away with soap and water.
- If you miss a dose of LYSODREN, skip the missed dose and take the next dose at your regularly scheduled time. Do not take 2 doses at the same time to make up for a missed dose.
- If you vomit after taking LYSODREN, take the next dose at your regularly scheduled time.
- If you take too much LYSODREN, call your healthcare provider right away or go to the nearest emergency room.
What should I avoid while taking LYSODREN? Do not drive or operate machinery until you know how LYSODREN affects you. LYSODREN may cause sleepiness, decreased energy, and dizziness, which may affect your ability to drive and operate machinery. What are the possible side effects of LYSODREN? LYSODREN can cause serious side effects including:
- See "What is the most important information I should know about LYSODREN?"
- Neurological problems (central nervous system toxicity). LYSODREN can cause decreased awareness and alertness. See " What should I avoid while taking LYSODREN? " Tell your healthcare provider if you develop any of the following signs and symptoms during treatment or if they get worse:
- slow thinking
- slow movement or decreased coordination
- confusion
- difficulty concentrating
- memory loss
- trouble talking
- sleepiness
- dizziness
- feelings of pins and needles in your hands and feet
- feeling very tired
Your healthcare provider may test your blood to make sure your thyroid is producing enough thyroid hormone and to check the mitotane level if you develop any of these signs and symptoms during treatment.
- Ovarian cysts in premenopausal women. LYSODREN can cause noncancerous large cysts (macrocysts) on the ovaries of women who have not gone through menopause or just starting menopause (premenopausal). The cysts may cause pain or discomfort in your pelvic area and abnormal periods (menstruation), or they may not cause any symptoms at all. If you are premenopausal, your healthcare provider will do an ultrasound of your ovaries before starting LYSODREN and as needed during treatment. Tell your healthcare provider right away if you develop vaginal bleeding or pelvic pain.
- Liver problems. LYSODREN can cause liver problems, including liver injury or liver failure. Tell your healthcare provider right away if you develop any signs and symptoms of liver problems during LYSODREN treatment, including:
- yellowing of your skin or the white part of your eyes
- dark colored (tea colored) urine
- pain in your right upper stomach-area (abdomen)
- itching
- loss of appetite
- nausea, vomiting or diarrhea
- bleeding or bruising
- tiredness
- Low blood cell counts. LYSODREN can cause decreased blood cell counts including decreases in white blood cells, platelets, and red blood cells. This may increase your risk of infection, bleeding, and anemia. Tell your healthcare provider right away if you develop any signs or symptoms of low blood cell counts, including:
- fever
- trouble breathing
- unusual bruising or bleeding
- dizziness or lightheadedness
- tiredness
- pale skin or lightness of skin color
- Prolonged bleeding time. LYSODREN can cause bleeding that lasts longer than usual. If you need to have surgery or dental procedures during treatment with LYSODREN, your healthcare provider will do blood tests to check for prolonged bleeding risks. Tell your healthcare provider right away if you develop signs or symptoms of prolonged bleeding during treatment with LYSODREN, including:
- unusual bleeding or bleeding that will not stop
- bruising
- lightheadedness
- vomiting blood or your vomit looks like coffee grinds
- blood in your stool or black stool that looks like tar
- pink or brown urine
- coughing up blood or blood clots
- menstrual bleeding that is heavier than normal
- nose bleeds that happen often
- Changes in hormone levels. LYSODREN may cause changes in certain hormone levels in your blood. If you are male, your breasts may swell or become larger during treatment.
Your healthcare provider will do blood tests before you start treatment with LYSODREN, during your treatment, and after you stop treatment with LYSODREN to check mitotane levels in your body and to check for side effects. Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with LYSODREN if you develop certain side effects. The most common side effects of LYSODREN include:
- eating disorders (anorexia)
- upper stomach area (abdominal) discomfort
- nausea
- vomiting
- diarrhea
- depression
- dizziness or lightheadedness
- rash
- high cholesterol
- increase of triglycerides (fat in blood)
- decreased thyroid hormones
- decreased testosterone (in males)
These are not all of the possible side effects of LYSODREN. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Direct Success Inc. at 1-844-597-6373. How should I store LYSODREN? Store LYSODREN at 77°F (25°C). Keep LYSODREN and all medicines out of the reach of children. General information about the safe and effective use of LYSODREN. Medicines are sometimes prescribed for purposes other than those uled in a Medication Guide. Do not use LYSODREN for a condition for which it was not prescribed. Do not give LYSODREN to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about LYSODREN that is written for health professionals. What are the ingredients in LYSODREN? Active ingredient: mitotane Inactive ingredients: microcrystalline cellulose, polyethylene glycol 3350, silicon dioxide, and starch. Manufactured by: Latina Pharma S.p.A., Via Murillo, 7, 04013 Sermoneta (Latina), Italy; Manufactured for HRA Pharma Rare Diseases. For more information, go to www.lysodren.com or call 1-844-597-6373.
Principal Display Panel - 100 Tablet Bottle Carton
NDC 76336-080-60
LYSODREN (mitotane) tablets, for oral use
EACH TABLET CONTAINS 500 mg
100 Tablets Rx only
HRA Pharma Rare Diseases

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site