Mometasone Furoate Dailymed
Generic: mometasone is used for the treatment of Dermatomycoses Facial Dermatoses Foot Dermatoses Hand Dermatoses Herpes Simplex Herpes Zoster Inflammation Leg Dermatoses Pruritus Rhinitis, Allergic, Perennial Scalp Dermatoses Tuberculosis, Cutaneous Hypersensitivity
Go PRO for all pill images
Recent Major Changes Section
Indications and Usage (1 ) (removed)           06/2022
Dosage and Administration (2 ) (removed)   06/2022
1 Indications And Usage
Mometasone furoate nasal spray is a corticosteroid indicated for:
- Prophylaxis of Nasal Symptoms of Seasonal Allergic Rhinitis in adult and pediatric patients 12 years of age and older (
1.1 )- Treatment of Chronic Rhinosinusitis with Nasal Polyps in adult patients 18 years of age and older (
1.2 ) 1.1 Prophylaxis of Seasonal Allergic Rhinitis
Mometasone furoate nasal spray 50 mcg is indicated for the prophylaxis of the nasal symptoms of seasonal allergic rhinitis in adult and pediatric patients 12 years and older.
1.2 Treatment of ChronicRhinosinusitis with Nasal Polyps
Mometasone furoate  nasal spray 50 mcg is indicated for the treatment of chronic rhinosinusitis with nasal polyps in adult patients 18 years of age and older.
2 Dosage And Administration
For Nasal Use Only.
Prophylaxis of Seasonal Allergic Rhinitis in Adult and Pediatric Patients 12 Years of Age and Older: 2 sprays in each nostril once daily (2.2 )
- Treatment of Chronic Rhinosinusitis with Nasal Polyps in Adults (18 yrs. and older): 2 sprays in each nostril twice daily. 2 sprays in each nostril once daily may also be effective in some patients (
2.3 )2.1Preparation and Administration
Administer mometasone furoate nasal spray by the nasal route only.
Initial Priming
Prior to initial use of mometasone furoate nasal spray, the pump must be primed by actuating ten times or until a fine spray appears. The pump may be stored unused for up to 1 week without repriming.
Repriming (as needed)
If unused for more than 1 week, reprime by actuating two times, or until a fine spray appears.
2.2 RecommendedDosage for Prophylaxis of Seasonal Allergic Rhinitis
The recommended dosage for prophylaxis treatment of nasal symptoms of seasonal allergic rhinitis in adult and pediatric patients 12 years and older is mometasone furoate 2 sprays (2 sprays deliver a total of 100 mcg of mometasone furoate) in each nostril once daily (total daily dose of 200 mcg).
In patients with a known seasonal allergen that precipitates nasal symptoms of seasonal allergic rhinitis, prophylaxis with 2 sprays (2 sprays deliver a total of 100 mcg of mometasone furoate) in each nostril once daily (total daily dose of 200 mcg) is recommended 2 to 4 weeks prior to the anticipated start of the pollen season.
2.3 RecommendedDosage for Treatment of ChronicRhinosinusitis with Nasal Polyps
The recommended dosage for the treatment of chronic rhinosinusitis with nasal polyps in adults 18 years and older is mometasone furoate 2 sprays (2 sprays deliver a total of 100 mcg of mometasone furoate) in each nostril twice daily (total daily dose of 400 mcg). A dose of 2 sprays (2 sprays deliver a total of 100 mcg of mometasone furoate) in each nostril once daily (total daily dose of 200 mcg) is also effective in some patients.
3 Dosage Forms And Strengths
Nasal spray: 50 mcg of mometasone furoate in each spray.
Nasal spray: 50 mcg of mometasone furoate, USP in each spray (3)  
4 Contraindications
Mometasone furoate nasal spray is contraindicated in patients with known hypersensitivity to mometasone furoate or any of its ingredients [see Warnings and Precautions (5.3), Description (11)].
Patients with known hypersensitivity to mometasone furoate or any of the ingredients of mometasone furoate nasal spray. (4)  
5 Warnings And Precautions
- Epistaxis, nasal ulceration, Candida albicans infection, nasal septal perforation, impaired wound healing. Monitor patients periodically for signs of adverse effects on the nasal mucosa. Avoid use in patients with recent nasal ulcers, nasal surgery, or nasal trauma. (
5.1 )- Glaucoma and cataracts. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use mometasone furoate nasal spray long term. (
5.2 )- Potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. More serious or even fatal course of chickenpox or measles in susceptible patients. Use caution in patients with the above because of the potential for worsening of these infections. (
5.4 )- Hypercorticism and adrenal suppression with higher than recommended dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue mometasone furoate nasal spray slowly. (
5.5 )- Potential reduction in growth velocity in children. Monitor growth routinely in pediatric patients receiving mometasone furoate nasal spray. (
5.6 ,8.4 )5.1 Local NasalAdverseReactions
Epistaxis
Epistaxis was observed more frequently in patients with allergic rhinitis and patients with chronic rhinosinusitis with nasal polyps who received mometasone furoate nasal spray than those who received placebo [see Adverse Reactions (6.1)].
Candida Infection
Localized infections of the nose and pharynx with Candida  albicans has occurred from nasal administration of mometasone furoate. When such an infection develops, use of mometasone furoate nasal spray should be discontinued and appropriate local or systemic therapy instituted, if needed.
Nasal Septum Perforation
Instances of nasal septum perforation occurred in patients following the nasal application of corticosteroids, including mometasone furoate. As with any long-term topical treatment of the nasal cavity, patients using mometasone furoate nasal spray over several months or longer should be examined periodically for possible changes in the nasal mucosa.
Impaired Wound Healing
Because of the inhibitory effect of corticosteroids on wound healing, patients who have experienced recent nasal septum ulcers, nasal surgery, or nasal trauma should not use a nasal corticosteroid until healing has occurred.
5.2 Glaucoma and Cataracts
Glaucoma and cataracts may  be reported with systemic and topical (including nasal, inhaled and ophthalmic) corticosteroid use. Consider referral to an ophthalmologist in patients who develop ocular symptoms or  use mometasone furoate long term [see Adverse Reactions (6)].
5.3 Hypersensitivity Reactions
Hypersensitivity reactions including instances of wheezing may occur after the nasal administration of mometasone furoate. Discontinue mometasone furoate nasal spray if such reactions occur [see Contraindications (4) ].
5.4 Immunosuppression and Risk of Infections
Persons who are on drugs which suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in nonimmune children or adults on corticosteroids. In such children or adults who have not had these diseases, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective Prescribing Information for VZIG and IG.) If chickenpox develops, treatment with antiviral agents may be considered.
Corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculous infection of the respiratory tract, or in untreated fungal, bacterial, systemic viral infections, or ocular herpes simplex because of the potential for worsening of these infections.
5.5 Hypercorticism and Adrenal Suppression
Hypercorticism and adrenal suppression may occur when nasal corticosteroids, including mometasone furoate, are used at higher-than-recommended dosages [see Dosage and Administration (2)] or in patients at risk for such effects. If such changes occur, the dosage of mometasone furoate nasal spray should be discontinued slowly, consistent with accepted procedures for discontinuing oral corticosteroid therapy.
5.6 Effect on Growth
Corticosteroids, including mometasone furoate, may cause a reduction in growth velocity when administered to pediatric patients. Routinely, monitor the growth of pediatric patients receiving mometasone furoate nasal spray. To minimize the systemic effects of nasal corticosteroids, including mometasone furoate nasal spray, titrate each patient’s dose to the lowest dosage that effectively controls his/her symptoms [see Use in Specific Populations (8.4)].
6 Adverse Reactions
The following clinically significant adverse reactions are described elsewhere in labeling:
- Epistaxis, Ulcerations, Candida albicans Infection, Impaired Wound Healing [see Warnings and Precautions (5.1)]
- Glaucoma and Cataracts [see Warnings and Precautions (5.2)]
- Immunosuppression and Risk of Infections [see Warnings and Precautions (5.4)]
- Hypercorticism and Adrenal Suppression, including Growth Reduction [see Warnings and Precautions (5.5, 5.6), Use in Specific Populations (8.4)]
The most common adverse reactions (incidence ‚Č• 5%) included headache, viral infection, pharyngitis, epistaxis and cough.¬†(6)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Allergic Rhinitis
Adults and pediatric patients 12 years of age and older
In controlled U.S. and international clinical studies, a total of 3,210 adult and pediatric patients 12 years and older with allergic rhinitis received treatment with mometasone furoate nasal spray at doses of 50 mcg/day to 800 mcg/day. The majority of patients (n = 2,103) were treated with 200 mcg/day. A total of 350 adult and pediatric patients 12 years and older have been treated for one year or longer. Adverse reactions did not differ significantly based on age, sex, or race. Four percent or less of patients in clinical trials discontinued treatment because of adverse events and the discontinuation rate was similar for the vehicle and active comparators.
All adverse reactions (regardless of relationship to treatment) reported by 5% or more of adult and pediatric patients ages 12 years and older who received mometasone furoate nasal spray, 200 mcg/day vs. placebo and that were more common with mometasone furoate nasal spray than placebo, are displayed in Table 1 below.
Table 1: Adult and¬†Pediatric Patients 12 Years and Older ‚Äď Adverse Reactions from Controlled Clinical Trials in Seasonal Allergic and Perennial Allergic Rhinitis (Percent of Patients Reporting)
MOMETASONE FUROATE 200 mcg
(n=2103)
%
VEHICLE
PLACEBO
(n=1671)
%
Headache
26
22
Viral Infection
14
11
Pharyngitis
12
10
Epistaxis/Blood-Tinged Mucus
11
6
Coughing
7
6
Upper Respiratory Tract Infection
6
2
Dysmenorrhea
5
3
Musculoskeletal Pain
5
3
Sinusitis
5
3
Other adverse reactions which occurred in less than 5% but greater than or equal to 2% of adult and pediatric patients (ages 12 years and older) treated with mometasone furoate nasal spray, 200-mcg/day (regardless of relationship to treatment), and more frequently than in the placebo group included: arthralgia, asthma, bronchitis, chest pain, conjunctivitis, diarrhea, dyspepsia, earache, flu-like symptoms, myalgia, nausea, and rhinitis.
Chronic Rhinosinusitis with Nasal Polyps
Adults 18 years of age and older
In controlled clinical studies, the types of adverse reactions observed in patients with chronic rhinosinusitis with nasal polyps were similar to those observed in patients with allergic rhinitis. A total of 594 adult patients (ages 18 to 86 years) received mometasone furoate  nasal spray at doses of 200 mcg once or twice daily for up to 4 months for treatment of chronic rhinosinusitis with nasal polyps. The overall incidence of adverse reactions for patients treated with mometasone furoate  nasal spray was comparable to patients with the placebo except for epistaxis, which was 9% for 200 mcg once daily, 13% for 200 mcg twice daily, and 5% for the placebo.
Nasal ulcers and nasal and oral candidiasis were also reported in patients treated with mometasone furoate  nasal spray primarily in patients treated for longer than 4 weeks.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during the post-marketing period for mometasone furoate  nasal spray: nasal burning and irritation, anaphylaxis and angioedema, disturbances in taste and smell, nasal septal perforation, and vision blurred. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7 Drug Interactions
No formal drug-drug interaction studies have been conducted with mometasone furoate nasal spray.
Inhibitors of Cytochrome P450 3A4:
Studies have shown that mometasone furoate is primarily and extensively metabolized in the liver of all species investigated and undergoes extensive metabolism to multiple metabolites. In vitro studies have confirmed the primary role of cytochrome CYP3A4 in the metabolism of this compound.
Concomitant administration of CYP3A4 inhibitors may  inhibit the  metabolism of, and increase the systemic exposure to, mometasone furoate and potentially increase the risk for systemic corticosteroid side effects. Caution should be exercised when considering the co-administration of mometasone furoate nasal spray with long-term ketoconazole and other known strong CYP3A4 inhibitors (e.g., ritonavir, cobicistat-containing products, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) [see Clinical Pharmacology (12.3)]. Consider the benefit of co-administration versus the potential risk of systemic corticosteroid effects, in which case patients should be monitored for systemic corticosteroid side effects.
8 Use In Specific Populations
8.1 Pregnancy
Risk Summary
Mometasone is minimally absorbed systemically following nasal use, and maternal use is not expected to result in fetal exposure to the drug. Available data from observational studies of mometasone use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. In animal reproduction studies with pregnant mice, rats, or rabbits (subcutaneous, subcutaneous/topical dermal/oral, and topical dermal/oral, respectively), mometasone furoate caused increased fetal malformations and decreased fetal survival and growth following administration of doses that produced exposures approximately 1/3 to 8 times the maximum recommended human dose (MRHD) on a mcg/m2 or AUC basis [see Data]. However, experience with oral corticosteroids suggests that rodents are more prone to teratogenic effects from corticosteroid exposure than humans.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryofetal development study with pregnant mice dosed throughout the period of organogenesis, mometasone furoate produced cleft palate at a dose less than the maximum recommended daily intranasal dose (MRDID) (on a mcg/m2 basis with maternal subcutaneous doses of 60 mcg/kg and above) and decreased fetal survival at approximately 2 times the MRDID (on a mcg/m2 basis with a maternal subcutaneous dose of 180 mcg/kg). No toxicity was observed with a dose that produced an exposure less than the MRDID (on a mcg/m2 basis with maternal topical dermal doses of 20 mcg/kg and above).
In an embryofetal development study with pregnant rats dosed throughout the period of organogenesis, mometasone furoate produced fetal umbilical hernia at exposures approximately 10 times the MRDID (on a mcg/m2 basis with maternal topical dermal doses of 600 mcg/kg and above) and delays in fetal ossification at a dose approximately 6 times the MRDID (on a mcg/m2 basis with maternal topical dermal doses of 300 mcg/kg and above).
In another reproductive toxicity study, pregnant rats were dosed with mometasone furoate throughout pregnancy or late in gestation. Treated animals had prolonged and difficult labor, fewer live births, lower birth weight, and reduced early pup survival at a dose less than the MRDID (on a mcg/m2 basis with a maternal subcutaneous dose of 15 mcg/kg). There were no findings at a dose less than the MRDID (on a mcg/m2 basis with a maternal subcutaneous dose of 7.5 mcg/kg).
Embryofetal development studies were conducted with pregnant rabbits dosed with mometasone furoate by either the topical dermal route or oral route throughout the period of organogenesis. In the study using the topical dermal route, mometasone furoate caused multiple malformations in fetuses (e.g., flexed front paws, gallbladder agenesis, umbilical hernia, hydrocephaly) at doses approximately 6 times the MRDID (on a mcg/m2 basis with maternal topical dermal doses of 150 mcg/kg and above). In the study using the oral route, mometasone furoate caused increased fetal resorptions and cleft palate and/or head malformations (hydrocephaly and domed head) at a dose approximately 30 times of the MRDID (on a mcg/m2 basis with a maternal oral dose of 700 mcg/kg). At approximately 110 times the MRDID (on a mcg/m2 basis with a maternal oral dose of 2800 mcg/kg), most litters were aborted or resorbed. No effects were observed at a dose approximately 6 times the MRDID (on a mcg/m2 basis with a maternal oral dose of 140 mcg/kg).
8.2Lactation
Risk Summary
There are no available data on the presence of mometasone furoate nasal spray in human milk, the effects on the breastfed child, or the effects on milk production. However, mometasone is minimally absorbed systemically by the mother following nasal use, and breastfeeding is not expected to result in exposure of the infant to mometasone. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for mometasone furoate nasal spray and any potential adverse effects on the breastfed infant from mometasone furoate nasal spray or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of mometasone furoate  nasal spray for prophylaxis of the nasal symptoms of seasonal allergic rhinitis in pediatric patients 12 years of age and older have been established [see Adverse Reactions (6.1) and Clinical Studies (14.1)]. Use of mometasone furoate  nasal spray for this indication is supported by evidence from controlled trials in adult and pediatric patients 12 years of age and older [see Clinical Studies (14.1)]. 
The safety and effectiveness of mometasone furoate nasal spray for the treatment of chronic rhinosinusitis with nasal polyps in pediatric patients less than 18 years of age have not been established. Effectiveness was not demonstrated in one 4-month trial conducted to evaluate the safety and efficacy of mometasone furoate in the treatment of chronic rhinosinusitis with nasal polyps in pediatric patients 6 to 17 years of age. The primary objective of the study was to evaluate safety; efficacy parameters were collected as secondary endpoints. A total of 127 patients with chronic rhinosinusitis with nasal polyps were randomized to placebo or mometasone furoate nasal spray 100 mcg once or twice daily (patients 6 to 11 years of age) or 200 mcg once or twice daily (patients 12 to 17 years of age). The results of this trial did not support the efficacy of mometasone furoate nasal spray in the treatment of chronic rhinosinusitis with nasal polyps in pediatric patients. The adverse reactions reported in this trial were similar to the adverse reactions reported in patients 18 years of age and older with chronic rhinosinusitis with nasal polyps.
Effect on Growth
Controlled clinical studies have shown nasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with nasal corticosteroids, including the impact on final adult height, are unknown. The potential for ‚Äúcatch up‚ÄĚ growth following discontinuation of treatment with nasal corticosteroids has not been adequately studied. The growth of pediatric patients receiving nasal corticosteroids, including mometasone furoate nasal spray, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of safe and effective noncorticosteroid treatment alternatives. To minimize the systemic effects of nasal corticosteroids, including mometasone furoate nasal spray, each patient should be titrated to his/her lowest effective dose.
A clinical study to assess the effect of mometasone furoate nasal spray (100 mcg total daily dose) on growth velocity has been conducted in pediatric patients 3 to 9 years of age with allergic rhinitis. No statistically significant effect on growth velocity was observed for mometasone furoate  nasal spray compared to placebo following one year of treatment. No evidence of clinically relevant HPA axis suppression was observed following a 30-minute cosyntropin infusion.
The potential of mometasone furoate nasal spray to cause growth suppression in susceptible patients or when given at higher doses cannot be ruled out.
8.5 Geriatric Use
A total of 280 patients above 64 years of age with allergic rhinitis or chronic rhinosinusitis with nasal polyps (age range 64 to 86 years) have been treated with mometasone furoate nasal spray for up to 3 or 4 months, respectively. No observed differences in safety and/or effectiveness in geriatric patients compared to younger adult patients.
8.6 Hepatic Impairment
Concentrations of mometasone furoate appear to increase with severity of hepatic impairment [see Clinical Pharmacology (12.3)].
10 Overdosage
There are no data available on the effects of acute or chronic overdosage with mometasone furoate nasal spray. Because of low systemic bioavailability, and an absence of acute drug-related systemic findings in clinical studies, overdose is unlikely to require any therapy other than observation. Chronic overdosage with any corticosteroid may result in signs or symptoms of hypercorticism [see Warnings and Precautions (5.5)].
11 Description
Mometasone furoate anhydrous, USP, the active component of mometasone furoate nasal spray, 50 mcg, is an anti-inflammatory corticosteroid having the chemical name, 9,21-Dichloro-11√ü,17-dihydroxy-16őĪ-methylpregna-1,4-diene-3,20-dione 17-(2-furoate), and the following chemical structure:
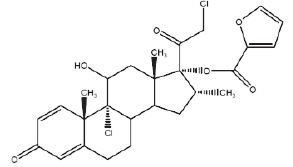
Mometasone furoate anhydrous, USP is a white to off-white powder, with an empirical formula of C27H30Cl2O6, and a molecular weight of 521.43 g/mol. It is practically insoluble in water; slightly soluble in methanol, ethanol, and isopropanol; soluble in acetone and chloroform; and freely soluble in tetrahydrofuran. Its partition coefficient between octanol and water is greater than 5,000.
Mometasone furoate nasal spray 50 mcg is a metered-dose, manual pump spray unit containing an aqueous suspension of mometasone furoate, USP in an aqueous medium containing benzalkonium chloride, citric acid, glycerin, microcrystalline cellulose and carboxymethylcellulose sodium, polysorbate 80 and sodium citrate.  The pH is between 4.3 and 4.9.
12 Clinical Pharmacology
12.1 Mechanism of Action
Mometasone furoate nasal spray is a corticosteroid demonstrating potent anti-inflammatory properties. The precise mechanism of corticosteroid action on allergic rhinitis is not known. Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation.
In two clinical studies utilizing nasal antigen challenge, mometasone furoate nasal spray decreased some markers of the early- and late-phase allergic response. These observations included decreases (vs. placebo) in histamine and eosinophil cationic protein levels, and reductions (vs. baseline) in eosinophils, neutrophils, and epithelial cell adhesion proteins. The clinical significance of these findings is not known.
The effect of mometasone furoate  nasal spray on nasal mucosa following 12 months of treatment was examined in 46 patients with allergic rhinitis. There was no evidence of atrophy and there was a marked reduction in intraepithelial eosinophilia and inflammatory cell infiltration (e.g., eosinophils, lymphocytes, monocytes, neutrophils, and plasma cells).
12.2 Pharmacodynamics
Adrenal Function in Adults: Four clinical pharmacology studies have been conducted in humans to assess the effect of mometasone furoate nasal spray at various doses on adrenal function. In one study, daily doses of 200 and 400 mcg of mometasone furoate nasal spray and 10 mg of prednisone were compared to placebo in 64 patients (22 to 44 years of age) with allergic rhinitis. Adrenal function before and after 36 consecutive days of treatment was assessed by measuring plasma cortisol levels following a 6-hour Cortrosyn (ACTH) infusion and by measuring 24-hour urinary free cortisol levels. Mometasone furoate nasal spray, at both the 200- and 400-mcg dose, was not associated with a statistically significant decrease in mean plasma cortisol levels post-Cortrosyn infusion or a statistically significant decrease in the 24-hour urinary free cortisol levels compared to placebo. A statistically significant decrease in the mean plasma cortisol levels post-Cortrosyn infusion and 24-hour urinary free cortisol levels was detected in the prednisone treatment group compared to placebo.
A second study assessed adrenal response to mometasone furoate nasal spray (400 and 1,600 mcg/day), prednisone (10 mg/day), and placebo, administered for 29 days in 48 male volunteers (21 to 40 years of age). The 24-hour plasma cortisol area under the curve (AUC0-24), during and after an 8-hour Cortrosyn infusion and 24-hour urinary free cortisol levels were determined at baseline and after 29 days of treatment. No statistically significant differences in adrenal function were observed with mometasone furoate nasal spray compared to placebo.
A third study evaluated single, rising doses of mometasone furoate nasal spray (1,000, 2,000, and 4,000 mcg/day), orally administered mometasone furoate (2,000, 4,000, and 8,000 mcg/day), orally administered dexamethasone (200, 400, and 800 mcg/day), and placebo (administered at the end of each series of doses) in 24 male volunteers (22 to 39 years of age). Dose administrations were separated by at least 72 hours. Determination of serial plasma cortisol levels at 8 AM and for the 24-hour period following each treatment were used to calculate the plasma cortisol area under the curve (AUC0-24). In addition, 24-hour urinary free cortisol levels were collected prior to initial treatment administration and during the period immediately following each dose. No statistically significant decreases in the plasma cortisol AUC, 8 AM cortisol levels, or 24-hour urinary free cortisol levels were observed in volunteers treated with either mometasone furoate nasal spray or oral mometasone, as compared with placebo treatment. Conversely, nearly all volunteers treated with the three doses of dexamethasone demonstrated abnormal 8 AM cortisol levels (defined as a cortisol level <10 mcg/dL), reduced 24-hour plasma AUC values, and decreased 24-hour urinary free cortisol levels, as compared to placebo treatment.
In a fourth study, adrenal function was assessed in 213 patients (18 to 81 years of age) with chronic rhinosinusitis with nasal polyps before and after 4 months of treatment with either mometasone furoate nasal spray, (200 mcg once or twice daily) or placebo by measuring 24-hour urinary free cortisol levels. Mometasone furoate nasal spray, at both doses (200 and 400 mcg/day), was not associated with statistically significant decreases in the 24-hour urinary free cortisol levels compared to placebo.
Three clinical pharmacology studies have been conducted in pediatric patients to assess the effect of mometasone furoate nasal spray on the adrenal function at daily doses of 50, 100, and 200 mcg vs. placebo. In one study, adrenal function before and after 7 consecutive days of treatment was assessed in 48 pediatric patients with allergic rhinitis (ages 6 to 11 years) by measuring morning plasma cortisol and 24-hour urinary free cortisol levels. Mometasone furoate nasal spray, at all three doses, was not associated with a statistically significant decrease in mean plasma cortisol levels or a statistically significant decrease in the 24-hour urinary free cortisol levels compared to placebo. In the second study, adrenal function before and after 14 consecutive days of treatment was assessed in 48 pediatric patients (ages 3 to 5 years) with allergic rhinitis by measuring plasma cortisol levels following a 30-minute Cortrosyn infusion. Mometasone furoate nasal spray, 50 mcg, at all three doses (50, 100, and 200 mcg/day), was not associated with a statistically significant decrease in mean plasma cortisol levels post-Cortrosyn infusion compared to placebo. All patients had a normal response to Cortrosyn. In the third study, adrenal function before and after up to 42 consecutive days of once-daily treatment was assessed in 52 patients with allergic rhinitis (ages 2 to 5 years), 28 of whom received mometasone furoate nasal spray, 50 mcg per nostril (total daily dose 100 mcg), by measuring morning plasma cortisol and 24-hour urinary free cortisol levels. Mometasone furoate nasal spray was not associated with a statistically significant decrease in mean plasma cortisol levels or a statistically significant decrease in the 24-hour urinary free cortisol levels compared to placebo.
12.3 Pharmacokinetics
Absorption:
Mometasone furoate administered as a nasal spray suspension has very low bioavailability (<1%) in plasma using a sensitive assay with a lower quantitation limit (LOQ) of 0.25 pcg/mL.
Distribution:
The in vitro protein binding for mometasone furoate was reported to be 98% to 99% in concentration range of 5 ng/mL to 500 ng/mL.
Elimination:
Following intravenous administration, the effective plasma elimination half-life of mometasone furoate is 5.8 hours. Any absorbed drug is excreted as metabolites mostly via the bile, and to a limited extent, into the urine.
Metabolism:
Studies have shown that any portion of a mometasone furoate dose which is swallowed and absorbed undergoes extensive metabolism to multiple metabolites. There are no major metabolites detectable in plasma. Upon in vitro incubation, one of the minor metabolites formed is 6ß-hydroxy-mometasone furoate. In human liver microsomes, the formation of the metabolite is regulated by cytochrome P-450 3A4 (CYP3A4).
Specific Populations:
Patients with Hepatic Impairment: Administration of a single inhaled dose of 400 mcg mometasone furoate to subjects with mild (n = 4), moderate (n = 4), and severe (n = 4) hepatic impairment resulted in only 1 or 2 subjects in each group having detectable peak plasma concentrations of mometasone furoate (ranging from 50 to 105 pcg/mL). The observed peak plasma concentrations appear to increase with severity of hepatic impairment, however, the numbers of detectable levels were few.
Patients with Renal Impairment: The effects of renal impairment on mometasone furoate pharmacokinetics have not been adequately investigated.
Pediatric Patients: Mometasone furoate pharmacokinetics have not been investigated in the pediatric population [see Use in Specific Populations (8.4)].
Male and Female Patients: The effects of gender on mometasone furoate pharmacokinetics have not been adequately investigated.
Racial or Ethnic Groups: The effects of race on mometasone furoate pharmacokinetics have not been adequately investigated.
Drug Interaction Studies:
Inhibitors of Cytochrome P450 3A4: In a drug interaction study, an inhaled dose of mometasone furoate 400 mcg was given to 24 healthy subjects twice daily for 9 days and ketoconazole 200 mg (as well as placebo) were given twice daily concomitantly on Days 4 to 9. Mometasone furoate plasma concentrations were <150 pcg/mL on Day 3 prior to co-administration of ketoconazole or placebo. Following concomitant administration of ketoconazole, 4 out of 12 subjects in the ketoconazole treatment group (n=12) had peak plasma concentrations of mometasone furoate >200 pcg/mL on Day 9 (211 to 324 pcg/mL).
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study in Sprague Dawley rats, mometasone furoate demonstrated no statistically significant increase in the incidence of tumors at inhalation doses up to 67 mcg/kg (approximately 1 and 2 times the maximum recommended daily nasal dose [MRDID] in adults [400 mcg] and children [100 mcg], respectively, on a mcg/m2 basis). In a 19-month carcinogenicity study in Swiss CD-1 mice, mometasone furoate demonstrated no statistically significant increase in the incidence of tumors at inhalation doses up to 160 mcg/kg (approximately 2 times the MRDID in adults and children, respectively, on a mcg/m2 basis).
Mometasone furoate increased chromosomal aberrations in an in vitro Chinese hamster ovary-cell assay, but did not increase chromosomal aberrations in an in vitro Chinese hamster lung cell assay. Mometasone furoate was not mutagenic in the Ames test or mouse-lymphoma assay, and was not clastogenic in an in vivo mouse micronucleus assay and a rat bone marrow chromosomal aberration assay or a mouse male germ-cell chromosomal aberration assay. Mometasone furoate also did not induce unscheduled DNA synthesis in vivo in rat hepatocytes.
In reproductive studies in rats, impairment of fertility was not produced by subcutaneous doses up to 15 mcg/kg (less than the MRDID in adults on a mcg/m2 basis).
13.2 Animal Toxicology and/or Pharmacology
Reproduction Toxicology Studies
In mice, mometasone furoate caused cleft palate at subcutaneous doses of 60 mcg/kg and above (less than the MRDID in adults on a mcg/m2 basis). Fetal survival was reduced at 180 mcg/kg (approximately 2 times the MRDID in adults on a mcg/m2 basis). No toxicity was observed at 20 mcg/kg (less than the MRDID in adults on a mcg/m2 basis).
In rats, mometasone furoate produced umbilical hernia at topical dermal doses of 600 mcg/kg and above (approximately 10 times the MRDID in adults on a mcg/m2 basis). A dose of 300 mcg/kg (approximately 6 times the MRDID in adults on a mcg/m2 basis) produced delays in ossification, but no malformations. In rabbits, mometasone furoate caused multiple malformations (e.g., flexed front paws, gallbladder agenesis, umbilical hernia, hydrocephaly) at topical dermal doses of 150 mcg/kg and above (approximately 6 times the MRDID in adults on a mcg/m2 basis). In an oral study, mometasone furoate increased resorptions and caused cleft palate and/or head malformations (hydrocephaly or domed head) at 700 mcg/kg (approximately 30 times the MRDID in adults on a mcg/m2 basis). At 2,800 mcg/kg (approximately 110 times the MRDID in adults on a mcg/m2 basis), most litters were aborted or resorbed. No toxicity was observed at 140 mcg/kg (approximately 6 times the MRDID in adults on a mcg/m2 basis).
When rats received subcutaneous doses of mometasone furoate throughout pregnancy or during the later stages of pregnancy, 15 mcg/kg (less than the MRDID in adults on a mcg/m2 basis) caused prolonged and difficult labor and reduced the number of live births, birth weight, and early pup survival. Similar effects were not observed at 7.5 mcg/kg (less than the MRDID in adults on a mcg/m2 basis).
14 Clinical Studies
14.1 Prophylaxisof Seasonal Allergic Rhinitis in Adult andPediatricPatients 12 Years of Age and Older
The efficacy and safety of mometasone furoate nasal spray in the prophylaxis and treatment of seasonal allergic rhinitis and the treatment of perennial allergic rhinitis have been evaluated in 18 controlled trials, and one uncontrolled clinical trial, in approximately 3,000 adults (ages 17 to 85 years) and pediatric patients (ages 12 to 16 years). Of the total number of patients, there were 1,757 males and 1,453 females, including a total of 283 adolescents (182 boys and 101 girls) with seasonal allergic or perennial allergic rhinitis. Patients were treated with mometasone furoate nasal spray at doses ranging from 50 to 800 mcg/day. The majority of patients were treated with 200 mcg/day. The allergic rhinitis trials evaluated the total nasal symptom scores that included stuffiness, rhinorrhea, itching, and sneezing. Patients treated with mometasone furoate nasal spray, 200 mcg/day had a statistically significant decrease in total nasal symptom scores compared to placebo-treated patients. No additional benefit was observed for mometasone furoate doses greater than 200 mcg/day. A total of 350 patients have been treated with mometasone furoate nasal spray for 1 year or longer.
Prophylaxis of seasonal allergic rhinitis for patients 12 years of age and older with mometasone furoate nasal spray given at a dose of 200 mcg/day, was evaluated in two clinical studies in 284 patients. These studies were designed such that patients received 4 weeks of prophylaxis with mometasone furoate nasal spray prior to the anticipated onset of the pollen season; however, some patients received only 2 to 3 weeks of prophylaxis. Patients receiving 2 to 4 weeks of prophylaxis with mometasone furoate nasal spray demonstrated a statistically significantly smaller mean increase in total nasal symptom scores with onset of the pollen season as compared to placebo patients.
14.2 Chronic Rhinosinusitis with Nasal Polyps in Adults 18 Years of Age and Older
Two studies were performed to evaluate the efficacy and safety of mometasone furoate nasal spray in the treatment of chronic rhinosinusitis with nasal polyps. These studies involved 664 patients with chronic rhinosinusitis with nasal polyps, 441 of whom received mometasone furoate nasal spray. These studies were randomized, double-blind, placebo-controlled, parallel-group, multicenter studies in patients 18 to 86 years of age with bilateral nasal polyps. Patients were randomized to receive mometasone furoate nasal spray 200 mcg once daily, 200 mcg twice daily or placebo for a period of 4 months. The co-primary efficacy endpoints were 1) change from baseline in nasal congestion/obstruction averaged over the first month of treatment; and 2) change from baseline to last assessment in bilateral polyp grade during the entire 4 months of treatment as assessed by endoscopy. Efficacy was demonstrated in both studies at a dose of 200 mcg twice daily and in one study at a dose of 200 mcg once a day (see Table 2 below).
Table 2: Effect of Mometasone Furoate Nasal Spray in Two Randomized, Placebo-Controlled Trials in Patients with Chronic Rhinosinusitis with Nasal Polyps
Mometasone Furoate 200 mcg qd
Mometasone Furoate 200 mcg bid
Placebo
P-value for Mometasone Furoate 200 mcg qd vs. placebo
P-value for Mometasone Furoate 200 mcg bid vs. placebo
Study 1
N=115
N=122
N=117
Baseline bilateral polyp grade*
4.21
4.27
4.25
Mean change from baseline in bilateral polyps grade
-1.15
-0.96
-0.50
<0.001
0.01
Baseline nasal congestion †
2.29
2.35
2.28
Mean change from baseline in nasal congestion
-0.47
-0.61
-0.24
0.001
<0.001
Study 2
N=102
N=102
N=106
Baseline bilateral polyp grade*
4.00
4.10
4.17
Mean change from baseline in bilateral polyps grade
-0.78
-0.96
-0.62
0.33
0.04
Baseline nasal congestion†
2.23
2.20
2.18
Mean change from baseline in nasal congestion
-0.42
-0.66
-0.23
0.01
<0.001
* polyps in each nasal fossa were graded by the investigator based on endoscopic visualization, using a scale of 0 to 3 where 0=no polyps; 1=polyps in the middle meatus, not reaching below the inferior border of the middle turbinate; 2=polyps reaching below the inferior border of the middle turbinate but not the inferior border of the inferior turbinate; 3=polyps reaching to or below the border of the inferior turbinate, or polyps medial to the middle turbinate (score reflects sum of left and right nasal fossa grades).
† nasal congestion/obstruction was scored daily by the patient using a 0 to 3 categorical scale where 0=no symptoms, 1=mild symptoms, 2=moderate symptoms and 3=severe symptoms.
There were no clinically relevant differences in the effectiveness of mometasone furoate nasal spray, in the studies evaluating treatment of chronic rhinosinusitis with nasal polyps across subgroups of patients defined by gender, age, or race.
16 How Supplied/storage And Handling
Mometasone furoate nasal spray, 50 mcg is supplied in a white, high-density, polyethylene bottle fitted with a white metered-dose, manual spray pump, and turquoise cap. It contains 17 g of product formulation, 120 sprays, each delivering 50 mcg of mometasone furoate, USP per actuation.
NDC 65162-891-29
Store at 20¬į to 25¬įC (68¬į to 77¬įF); excursions permitted between 15¬į to 30¬įC (59¬į to 86¬įF) [see USP Controlled Room Temperature]. Protect from light.
When mometasone furoate nasal spray, 50 mcg is removed from its cardboard container, prolonged exposure of the product to direct light should be avoided. Brief exposure to light, as with normal use, is acceptable.
SHAKE WELL BEFORE EACH USE.
Keep out of reach of children.
17 Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Local Nasal Adverse Reactions
Patients should be informed that treatment with mometasone furoate nasal spray may be associated with adverse reactions which include epistaxis (nose bleed) and nasal septum perforation. Candida infection may also occur. Because of the inhibitory effect of corticosteroids on wound healing, patients who have experienced recent nasal septum ulcers, nasal surgery, or nasal trauma should not use a nasal corticosteroid until healing has occurred [see Warnings and Precautions (5.1)]. Patients should be cautioned not to spray mometasone furoate nasal spray directly onto the nasal septum.
Glaucoma and Cataracts
Advise patients that long-term use of nasal and inhaled corticosteroids may increase the risk of some eye problems (glaucoma or cataracts); regular eye examinations should be considered. Patients should be cautioned not to spray mometasone furoate nasal spray into the eyes [see Warnings and Precautions (5.2)].
Immunosuppression and Risk of Infections
Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles, and patients should also be advised that if they are exposed, medical advice should be sought without delay [see Warnings and Precautions (5.4)].
Use Regularly for Best Effect
Patients should use mometasone furoate nasal spray on a regular basis for optimal effect. Improvement in nasal symptoms of allergic rhinitis has been shown to occur within 1 to 2 days after initiation of dosing. Maximum benefit is usually achieved within 1 to 2 weeks after initiation of dosing. Patients should not increase the prescribed dosage but should contact their physician if symptoms do not improve, or if the condition worsens. Administration to young children should be aided by an adult.
If a patient missed a dose, the patient should be advised to administer the dose as soon as they remember. The patient should not use more than the recommended dose for the day.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ  08807
Rev. 08-2022-03
Patient Information
Mometasone Furoate (moe met’ a sone fure’ oh ate) Nasal Spray, 50 mcg
FOR NASAL USE ONLY
Read the Patient Information that comes with mometasone furoate before you start using it and each time you get a refill. There may be new information. This Patient Information does not take the place of talking to your healthcare provider about your medical condition or treatment. If you have any questions about mometasone furoate, ask your healthcare provider.
What is mometasone furoate?
Mometasone furoate nasal spray is a man-made (synthetic) corticosteroid medicine that is used to:
- prevent nasal symptoms of seasonal allergic rhinitis in people 12 years of age and older.
- treat chronic rhinosinusitis with nasal polyps in people 18 years and older.
It is not known if mometasone furoate nasal spray is safe and effective in children under:
- 12 years of age to prevent nasal symptoms of seasonal allergic rhinitis.
- 18 years of age to treat chronic rhinosinusitis with nasal polyps.
Who should not use mometasone furoate?
Do not use mometasone furoate nasal spray if you are allergic to mometasone furoate or any of the ingredients in mometasone furoate nasal spray. See the end of this leaflet for a complete ul of ingredients in mometasone furoate nasal spray.
What should I tell my healthcare provider before and during treatment with mometasone furoate?
Before you take mometasone furoate, tell your healthcare provider about all of your medical conditions, including if you:
- have had recent nasal sores, nasal surgery, or nasal injury.
- have eye or vision problems, such as cataracts, glaucoma (increased pressure in your eye), and blurred vision, or other changes in your vision.
- have tuberculosis or any untreated fungal, bacterial, viral infections, or eye infections caused by herpes.
- have been near someone who has chickenpox or measles.
- are not feeling well or have any other symptoms that you do not understand.
- are pregnant or planning to become pregnant. It is not known if mometasone furoate will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding or planning to breastfeed. It is not known whether mometasone furoate passes into your breast milk.
Tell your healthcare provider about all the medicines you take including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
- certain medicines for HIV (such as ritonavir, atazanavir, indinavir, nelfinavir, and saquinavir)
- cobicistat-containing products
- certain antifungals (such as ketoconazole and itraconazole)
- certain antibiotics (such as clarithromycin and telithromycin)
- certain antidepressants (such as nefazodone)
If you take these medicines with mometasone furoate, your healthcare provider should monitor you for side effects.
Mometasone furoate may affect the way other medicines work, and other medicines may affect how mometasone furoate works.
Know the medicines you take. Keep a ul of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use mometasone furoate?
- Use mometasone furoate exactly as prescribed by your healthcare provider.
- This medicine is for use in the nose only. Do not spray it into your mouth or eyes.
- An adult should supervise a child using this medicine.
- For best results, you should keep using mometasone furoate regularly each day without missing a dose. If you do miss a dose of mometasone furoate, take it as soon as you remember. However, do not take more than the daily dose prescribed by your healthcare provider.
- Do not use mometasone furoate more often than prescribed. Ask your healthcare provider if you have any questions.
- For detailed instructions on how to use mometasone furoate nasal spray, see the ‚ÄúPatient Instructions for Use‚ÄĚ at the end of this leaflet.
- See your healthcare provider regularly to check your symptoms while taking mometasone furoate and to check for side effects.
What should I avoid while taking mometasone furoate?
If you are taking other corticosteroid medicines for allergy, by mouth or injection, your healthcare provider may advise you to stop taking them after you begin using mometasone furoate.
What are the possible side effects of mometasone furoate?
Mometasone furoate may cause serious side effects, including:
- thrush (candida), a fungal infection in your nose and throat. Tell your healthcare provider if you have any redness or white colored patches in your nose or throat.
- hole in the cartilage of the nose (nasal septal perforation). A whistling sound when you breathe may be a symptom of nasal septum perforation.
- slow wound healing. Do not use mometasone furoate until your nose has healed if you have a sore in your nose, if you have surgery on your nose, or if your nose has been injured.
- eye problems, including glaucoma, cataracts, and blurred vision. You should have regular eye exams.
- allergic reactions. Allergic reactions including wheezing may happen after using mometasone furoate. If wheezing happens stop using mometasone furoate. Tell your healthcare provider or get medical help right away.
- immune system problems that may increase your risk of infections. You are more likely to get infections if you take medicines that weaken your immune system. Avoid contact with people who have contagious diseases such as chicken pox or measles while using mometasone furoate. Symptoms of infection may include: fever, pain, aches, chills, feeling tired, nausea and vomiting. Tell your healthcare provider about any signs of infection while you are using mometasone furoate.
- adrenal insufficiency. Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones. Symptoms of adrenal insufficiency can include: tiredness, weakness, nausea and vomiting, and low blood pressure.
- slowed growth in children. Your child’s growth should be checked regularly while using mometasone furoate.
The most common side effects of mometasone furoate include:
- headache
- viral infection
- sore throat
- nosebleeds
- cough
These are not all the possible side effects of mometasone furoate. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store mometasone furoate nasal spray?
- Store mometasone furoate nasal spray at room temperature between 59¬įF to 86¬įF (15¬įC to 30¬įC).
- Protect from light.
- Avoid prolonged exposure of mometasone furoate container to direct light.
- Shake well before each use.
- Keep mometasone furoate and all medicines out of the reach of children.
General information about the safe and effective use of mometasone furoate.
Medicines are sometimes prescribed for conditions that are not uled in a Patient Information leaflet. Do not use mometasone furoate for a condition for which it was not prescribed. Do not give mometasone furoate to other people even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about mometasone furoate that is written for health professionals.
What are the ingredients in mometasone furoate nasal spray?
Active ingredient: mometasone furoate anhydrous, USP
Inactive ingredients: benzalkonium chloride, citric acid, glycerin, microcrystalline cellulose and carboxymethylcellulose sodium, polysorbate 80 and sodium citrate.
For more information, go to www.amneal.com or call 1-877-835-5472.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 08-2022-03
Patient Instructions For Use
Mometasone Furoate (moe met’ a sone fure’ oh ate) Nasal Spray, 50 mcg
For use in your nose only.
Read the Patient Instructions for Use carefully before you start to use your mometasone furoate nasal spray. If you have any questions, ask your healthcare provider.
Shake the bottle well before each use.
- Remove the plastic cap (see Figure 1).
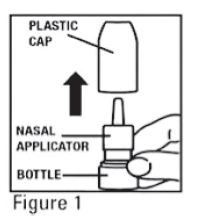
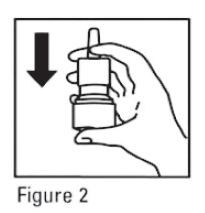
- Before you use mometasone furoate for the first time, prime the pump by pressing downward on the shoulders of the white nasal applicator using your index finger and middle finger while holding the base of the bottle with your thumb (see Figure 2). Do Not pierce the nasal applicator. Press down and release the pump 10 times or until a fine spray appears. Do Not spray into eyes. The pump is now ready to use. The pump may be stored unused for up to 1 week without repriming. If unused for more than 1 week, reprime by spraying 2 times or until a fine spray appears.
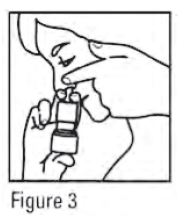
- Gently blow your nose to clear the nostrils. Close 1 nostril. Tilt your head forward slightly, keep the bottle upright, carefully insert the nasal applicator into the other nostril (see Figure 3). Do Not spray directly onto the nasal septum (the wall between the two nostrils).
- For each spray, hold the spray bottle upright and press firmly downward 1 time on the shoulders of the white nasal applicator using your index and middle fingers while supporting the base of the bottle with your thumb. Breathe gently inward through the nostril (see Figure 4).
Note: It is important to keep the mometasone furoate unit in an upright orientation (as seen in Figure 4). Failure to do so may result in an incomplete or non-existent spray.
- Then breathe out through the mouth.
- Repeat in the other nostril.
- Wipe the nasal applicator with a clean tissue and replace the plastic cap.
Each bottle of mometasone furoate nasal spray contains enough medicine for you to spray medicine from the bottle 120 times. Do not use the bottle of mometasone furoate nasal spray after 120 sprays. Additional sprays after the 120 sprays may not contain the right amount of medicine, you should keep track of the number of sprays used from each bottle of mometasone furoate nasal spray, and throw away the bottle even if it has medicine still left in. Do not count any sprays used for priming the device. Talk with your healthcare provider before your supply runs out to see if you should get a refill of your medicine.
Pediatric Use: Administration to children should be supervised by an adult. Steps 1 through 7 from the Patient Instructions for Use should be followed.
Cleaning: Do not try to unblock the nasal applicator with a sharp object. Please see Patient Instructions for Cleaning Applicator.
Patient Instructions for Cleaning Applicator
- To clean the nasal applicator, remove the plastic cap (see Figure 5).
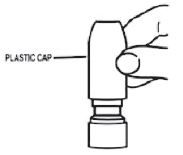
                  Figure 5
- Pull gently upward on the white nasal applicator to remove (see Figure 6).
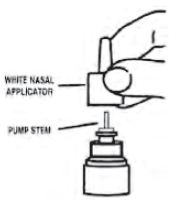
                     Figure 6
- Soak the nasal applicator in cold tap water and rinse both ends of the nasal applicator under cold tap water and dry (see Figure 7). Do not try to unblock the nasal applicator by inserting a pin or other sharp object as this will damage the applicator and cause you not to get the right dose of medicine.
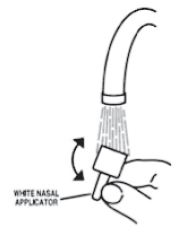
Figure 7
4. Rinse the plastic cap under cold water and dry (see Figure 8).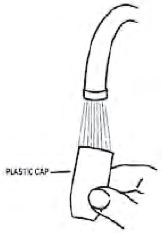
Figure 8
 
5. Put the nasal applicator back together making sure the pump stem is reinserted into the applicator’s center hole (see Figure 9).
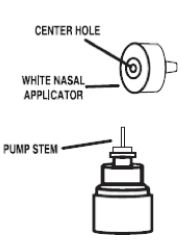
Figure 9
6. Reprime the pump by pressing downward on the shoulders of the white nasal applicator using your index and middle fingers while holding the base of the bottle with your thumb. Press down and release the pump 2 times or until a fine spray appears. Do Not spray into eyes. The pump is now ready to use. The pump may be stored unused for up to 1 week without repriming. If unused for more than 1 week, reprime by spraying 2 times or until a fine spray appears (see Figure 10).
Figure 10
7. Replace the plastic cap (see Figure 11).Figure 11
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ  08807
Rev. 08-2022-03
Principal Display Panel
NDC 65162-891-29
Mometasone Furoate Nasal Spray Label
Rx Only
Amneal Pharmaceuticals LLC

NDC 65162-891-29
Mometasone Furoate Nasal Spray Carton
Rx Only
Amneal Pharmaceuticals LLC
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


