N/A Dailymed
Generic: midazolam injection, 10 mg is used for the treatment of Anxiety Disorders Infant, Premature Pregnancy Psychomotor Agitation Status Epilepticus Glaucoma, Angle-Closure
Boxed Warning
Boxed Warning Section
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Monitor patients for respiratory depression and sedation [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
- The use of benzodiazepines, including midazolam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing Midazolam Injection and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction [see Warnings and Precautions (5.2)].
- The continued use of benzodiazepines may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Although Midazolam Injection is indicated only for intermittent use [see Indications and Usage (1) and Dosage and Administration (2)], if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of Midazolam Injection may precipitate acute withdrawal reactions, which can be life-threatening. For patients using Midazolam Injection more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue Midazolam Injection [see Warnings and Precautions (5.3)].
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Monitor patients for respiratory depression and sedation. (5.1, 7.1)
- The use of benzodiazepines, including midazolam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Before prescribing Midazolam Injection and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction. (5.2)
- Although Midazolam Injection is indicated only for intermittent use (1, 2), if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of Midazolam Injection may precipitate acute withdrawal reactions, which can be lifethreatening. For patients using Midazolam Injection more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue Midazolam Injection. (5.3)
Go PRO for all pill images
Indications & Usage Section
Midazolam Injection is indicated for the treatment of status epilepticus in adults.
Midazolam Injection is a benzodiazepine indicated for the treatment of status epilepticus in adults. (1)
Dosage & Administration Section
Recommended Dose The recommended dose of Midazolam Injection is a single 10 mg dose, administered by intramuscular injection [see Dosage and Administration (2.2)]. Important Administration Instructions
Midazolam Injection should be administered by trained personnel who have had adequate training in the recognition and treatment of status epilepticus and first aid/basic airway management.
Midazolam Injection is for intramuscular use only as a single dose. Inject in the mid-outer thigh (vastus lateralis muscle) using the prefilled autoinjector. The Midazolam autoinjector can inject through clothing. Move all objects from in and around the patient’s clothing that may interfere with the injection. For people who do not have a lot of fat at the mid-outer thigh, bunch up the thigh at the injection site to provide a thicker area for administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit [see Dosage Forms and Strengths (3)]. Refer to the illustrated Midazolam Injection Instructions for Use for autoinjector administration instructions. Monitoring After administration of Midazolam Injection, continuous monitoring of respiratory and cardiac function is recommended until the patient is stabilized. Serious and life-threatening cardiorespiratory adverse reactions, such as hypoventilation, airway obstruction, apnea, and hypotension have been reported with the use of midazolam. Patients should be monitored in a setting that allows for immediate access to resuscitative drugs. Appropriate resuscitation equipment and personnel trained in their use and skilled in airway management should be available [see Warnings and Precautions (5.4), Adverse Reactions (6.1)].
Observation for signs of cardiorespiratory depression is particularly important in patients with chronic obstructive pulmonary disease (COPD), patients 60 or more years of age, and patients who have received concomitant narcotics or other central nervous system (CNS) depressants.
- The recommended dose is a single 10 mg dose, administered by intramuscular injection using the prefilled autoinjector. (2.1)
- Inject in the mid-outer thigh (vastus lateralis muscle). (2.2)
- Continuous monitoring of respiratory and cardiac function is recommended. (2.3)
Dosage Forms & Strengths Section
Injection: 10 mg/0.7 mL of a sterile, clear, colorless to slightly yellow liquid solution in a single-dose prefilled autoinjector.
Injection: 10 mg/0.7 mL in a single-dose pre-filled autoinjector. (3)
Contraindications Section
Midazolam Injection is contraindicated in patients with a known hypersensitivity to midazolam.
Hypersensitivity to midazolam. (4)
Warnings And Precautions Section
Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including Midazolam Injection, and opioids may result in profound sedation, respiratory depression, coma, and death. If a decision is made to use midazolam concomitantly with opioids, monitor patients closely for respiratory depression and sedation [see Drug Interactions (7.1)]. Trained personnel administering Midazolam Injection must have the skills necessary to manage serious cardiorespiratory adverse reactions, including skills in airway management.
Abuse, Misuse, and Addiction
The use of benzodiazepines, including Midazolam Injection, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death [see Drug Abuse and Dependence (9.2)]. Before prescribing Midazolam Injection and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction. Use of Midazolam Injection, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of Midazolam Injection along with monitoring for signs and symptoms of abuse, misuse, and addiction. Do not exceed the recommended dosing frequency; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions After Use of Midazolam Injection More Frequently Than Recommended
For patients using Midazolam Injection more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue Midazolam Injection (a patient-specific plan should be used to taper the dose). Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines may lead to clinically significant physical dependence. Although Midazolam Injection is indicated only for intermittent use [see Indications and Usage (1) and Dosage and Administration (2)], if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of Midazolam Injection, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) [see Drug Abuse and Dependence (9.3)].
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months [see Drug Abuse and Dependence (9.3)].
Risks of Cardiorespiratory Adverse Reactions
Serious cardiorespiratory adverse reactions have occurred after administration of midazolam. These have included respiratory depression, airway obstruction, oxygen desaturation, apnea, respiratory arrest and/or cardiac arrest, sometimes resulting in death or permanent neurologic injury. There have also been rare reports of hypotensive episodes requiring treatment during or after diagnostic or surgical manipulations, particularly in patients with hemodynamic instability. Hypotension occurs more frequently in patients premedicated with a narcotic. The danger of hypoventilation, airway obstruction, or apnea is greater in elderly patients and those with chronic disease states or decreased pulmonary reserve [see Use in Specific Populations (8.5, 8.7)]; patients with COPD are highly sensitive to the respiratory depressant effect of midazolam. Midazolam Injection should be administered with caution to patients in shock or coma with depression of vital signs. Trained personnel administering Midazolam Injection must have the skills necessary to manage serious cardiorespiratory adverse reactions, including skills in airway management.
Other Adverse Reactions
Reactions such as agitation, involuntary movements (including tonic/clonic movements and muscle tremor), hyperactivity, and combativeness have been reported with midazolam when used for sedation. These reactions may be caused by inadequate or excessive dosing or improper administration of midazolam; however, consideration should be given to the possibility of cerebral hypoxia or true paradoxical reactions. Agitation also occurred in the randomized controlled clinical study of midazolam injection in patients with status epilepticus [see Adverse Reactions (6.1)].
Risks from Concomitant Use of Central Nervous System Depressants
Concomitant use of barbiturates, alcohol or other central nervous system depressants may increase the risk of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect. Midazolam Injection should be administered with caution to patients in acute alcohol intoxication with depression of vital signs. Narcotic premedication also depresses the ventilatory response to carbon dioxide stimulation. The efficacy and safety of midazolam in clinical use are functions of the dose administered, the clinical status of the individual patient, and the use of concomitant medications capable of depressing the central nervous system (CNS). Anticipated effects range from mild sedation to deep levels of sedation virtually equivalent to a state of general anesthesia where the patient may require external support of vital functions. Trained personnel administering Midazolam Injection must have the skills necessary to manage serious cardiorespiratory adverse reactions, including skills in airway management. For information regarding withdrawal, see Drug Abuse and Dependence (9.3).
Impaired Cognitive Function
Midazolam is associated with a high incidence of partial or complete impairment of recall for several hours following an administered dose. Gross tests of recovery from the effects of midazolam cannot be relied upon to predict reaction time under stress. It is recommended that no patient operate hazardous machinery or a motor vehicle until the effects of the drug, such as drowsiness, have subsided, and as their medical condition permits.
Glaucoma
Benzodiazepines, including Midazolam Injection, can increase intraocular pressure in patients with glaucoma. Measurements of intraocular pressure in patients without eye disease show a moderate lowering following induction with midazolam; patients with glaucoma have not been studied. Patients with open-angle glaucoma may need to have their ophthalmologic status evaluated following treatment with Midazolam Injection. Midazolam Injection is not recommended in patients with narrow-angle glaucoma.
Neonatal Sedation and Withdrawal Syndrome
Use of Midazolam Injection late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate [see Use in Specific Populations (8.1)]. Monitor neonates exposed to Midazolam Injection during pregnancy or labor for signs of sedation and monitor neonates exposed to Midazolam Injection during pregnancy for signs of withdrawal; manage these infants accordingly.
- Risks of Cardiorespiratory Adverse Reactions: Serious cardiorespiratory adverse reactions have occurred, sometimes resulting in death or permanent neurologic injury, after administration of midazolam. (5.4)
- Other Adverse Reactions: Agitation can occur. (5.5)
- Risks from Concomitant Use of Central Nervous System (CNS) Depressants: May increase risk of hypoventilation, airway obstruction, desaturation, or apnea, and may contribute to profound and/or prolonged drug effect. Practitioners administering Midazolam Injection must have the skills necessary to manage serious cardiorespiratory adverse reactions, including skills in airway management. (5.6)
- Impaired Cognitive Function: Because of partial or complete impairment of recall, patients should not operate hazardous machinery or a motor vehicle until drug effects have subsided. (5.7)
- Glaucoma: Patients with open-angle glaucoma may need to have their ophthalmologic status evaluated following treatment with Midazolam Injection. Midazolam injection is not recommended in patients with narrow-angle glaucoma. (5.8)
- Neonatal Sedation and Withdrawal Syndrome: Midazolam Injection use during pregnancy can result in neonatal sedation and/or neonatal withdrawal.(5.9, 8.1)
Adverse Reactions Section
The following serious adverse reactions are discussed in greater detail in other sections:
- Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
- Abuse, Misuse, and Addiction [see Warnings and Precautions (5.2)]
- Dependence and Withdrawal Reactions After Use of Midazolam Injection More Frequently Than Recommended [see Warnings and Precautions (5.3)]
- Risks of Cardiorespiratory Adverse Reactions [see Warnings and Precautions (5.4)]
- Other Adverse Reactions [see Warnings and Precautions (5.5)]
- Risks from Concomitant Use of Central Nervous System Depressants [see Warnings and Precautions (5.6)]
- Impaired Cognitive Function [see Warnings and Precautions (5.7)]
- Glaucoma [see Warnings and Precautions (5.8)]
- Neonatal Sedation and Withdrawal Syndrome [see Warnings and Precautions (5.9)]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of Midazolam Injection has been established by data from an adequate and well-controlled study of a different midazolam injection in adult patients with status epilepticus [see Clinical Studies (14)]. Below is a display of the adverse reactions of midazolam injection in this adequate and well-controlled study. Adverse Reactions in the Controlled Study of Intramuscular Midazolam in Patients with Status Epilepticus In a double-blind, randomized, active-controlled clinical study, 448 patients were assigned to receive intramuscular (IM) midazolam via an autoinjector, and 445 were assigned to receive intravenous (IV) lorazepam. Approximately 45% of patients were female, and the mean age was 43 years. Patients were administered treatment by a healthcare professional (e.g., paramedic) prior to arrival at a hospital. Table 1 uls the adverse reactions occurring in 2% or more of the IM midazolam-treated patients and at a rate greater than the IV lorazepam-treated patients.
Table 1 Adverse Reactions in 2% or More of IM Midazolam-Treated Patients and More Frequent than in IV Lorazepam-Treated Patients in Out of Hospital Treatment of Status Epilepticus
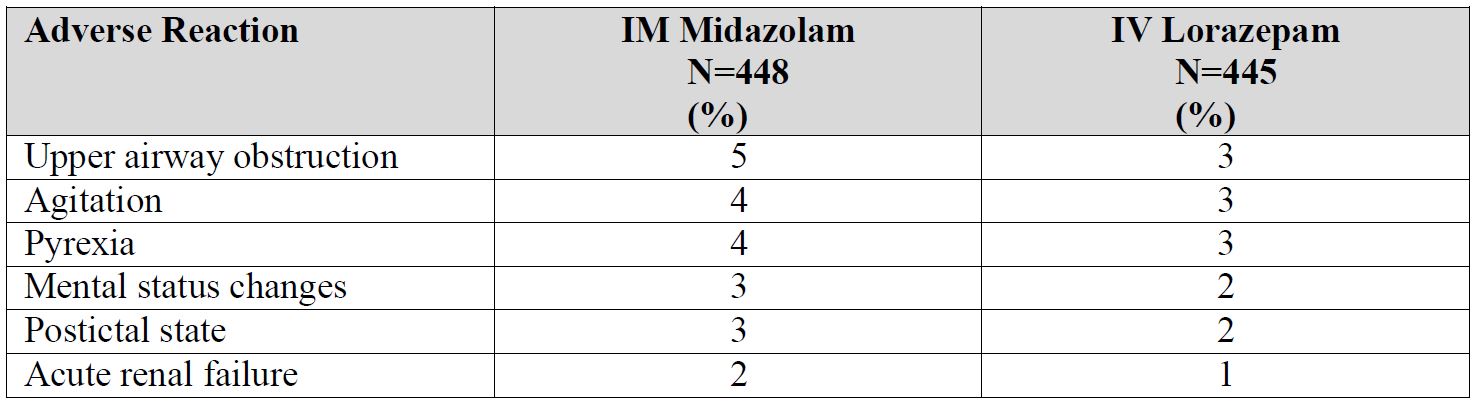
Adverse Reactions in Other Midazolam Studies Midazolam Injection is only indicated for status epilepticus, but for uses other than that for which Midazolam Injection is indicated, fluctuations in vital signs were the most frequently seen findings following parenteral administration of midazolam in adults, and included decreased tidal volume and/or respiratory rate decrease [11% of patients following intramuscular administration], as well as variations in blood pressure and pulse rate. The majority of serious adverse effects, particularly those associated with oxygenation and ventilation, have been reported when midazolam was administered with other medications capable of depressing the CNS. The incidence of such events was higher in patients undergoing procedures involving the airway without the protective effect of an endotracheal tube (e.g., upper endoscopy and dental procedures). The following additional adverse reactions were reported after intramuscular administration in adults: Headache (1.3%), and local effects at the IM injection site including pain (3.7%), induration (0.5%), redness (0.5%), and muscle stiffness (0.3%).
The most common adverse reactions (incidence >3%) in clinical trials in patients with status epilepticus were upper airway obstruction, agitation, and pyrexia. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Rafa Laboratories, Ltd. at 1-386-418-7911 or FDA at 1-800-FDA-1088 or www.fda.gov.medwatch.
Drug Interactions Section
Effect of Concomitant Use of Benzodiazepines and Opioids
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids. Monitor patients closely for respiratory depression and sedation.
Other CNS Depressants and Alcohol
The sedative effect of Midazolam Injection is accentuated by concomitantly administered medication that depresses the central nervous system, particularly opioids (e.g., morphine, meperidine, and fentanyl), secobarbital, and droperidol, and also by alcohol [see Warnings and Precautions (5.1, 5.4, 5.6)].
Cytochrome P450-3A4 Inhibitors
Caution is advised when Midazolam Injection is administered concomitantly with drugs that are known to inhibit the P450-3A4 enzyme system (e.g., cimetidine, erythromycin, diltiazem, verapamil, ketoconazole, and itraconazole). These drug interactions may result in prolonged sedation caused by a decrease in plasma clearance of midazolam [see Clinical Pharmacology (12.3)].
- Benzodiazepines and Opioids: Risk of respiratory depression is increased. (7.1)
- Other CNS Depressants and Alcohol: Sedative effect of midazolam is increased. (7.2)
- Cytochrome P450-3A4 Inhibitors: May result in prolonged sedation due to decreased plasma clearance of midazolam. (7.3)
Use In Specific Populations Section
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as Midazolam Injection, during pregnancy. Healthcare providers are encouraged to recommend that pregnant women who are taking Midazolam Injection during pregnancy enroll in the North American Antiepileptic Drug (NAAED) pregnancy registry by calling 1-888-233-2334 or visiting http://www.aedpregnancyregistry.org/.
Risk Summary
Infants born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal [see Warnings and Precautions (5.9) and Clinical Considerations]. Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects (see Data). Administration of midazolam to rats and rabbits during the period of organogenesis or to rats during late pregnancy and throughout lactation at doses greater than those used clinically did not result in adverse effects on development (see Animal Data). Data for other benzodiazepines suggest the possibility of increased neuronal cell death and long-term effects on neurobehavioral and immunological function based on findings in animals following prenatal or early postnatal exposure at clinically relevant doses. The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia, and sedation in neonates. Monitor neonates exposed to Midazolam Injection during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to Midazolam Injection during pregnancy for signs of withdrawal. Manage these neonates accordingly [see Warnings and Precautions (5.9)].
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Animal Data
When midazolam (0, 0.2, 1, or 4 mg/kg/day) was administered intravenously to pregnant rats during the period of organogenesis, no adverse effects on embryofetal development were observed. The highest dose tested, which was associated with minimal evidence of maternal toxicity, is approximately 4 times the recommended human dose (RHD) of 10 mg based on body surface area (mg/m 2). When midazolam (0, 0.2, 0.6, and 2 mg/kg/day) was administered intravenously to rabbits during the period of organogenesis, no adverse effects on embryofetal development were reported. The high dose, which was not associated with evidence of maternal toxicity, is approximately 4 times the RHD on a mg/m 2 basis. When midazolam (0, 0.2, 1, or 4 mg/kg/day) was administered intravenously to female rats during late gestation and throughout lactation, no clear adverse effects were noted in the offspring. The high dose, which was not associated with evidence of maternal toxicity, is approximately 4 times the RHD on a mg/m 2 basis.
In published animal studies, administration of benzodiazepines or other drugs that enhance GABAergic inhibition to neonatal rats has been reported to result in widespread apoptotic neurodegeneration in the developing brain at plasma concentrations relevant for seizure control in humans. The window of vulnerability to these changes in rats (postnatal days 0-14) includes a period of brain development that takes place during the third trimester of pregnancy in humans.
Lactation
Risk Summary
Midazolam is excreted in human milk. There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. There are no data on the effects of midazolam on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Midazolam Injection and any potential adverse effects on the breastfed child from Midazolam Injection or from the underlying maternal condition.
Clinical Considerations
Infants exposed to Midazolam Injection through breast milk should be monitored for sedation, poor feeding and poor weight gain.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Benzodiazepines are not recognized as a treatment for status epilepticus in neonates and should not be used in this population.
Geriatric Use
Of the total number of patients from the intent-to-treat (ITT) population in the clinical trial of a different midazolam injection, 14.9 percent were 65 years of age and over, while 8.3 percent were 75 years of age and over. Geriatric patients may have altered drug distribution; diminished hepatic and/or renal function; longer elimination half-lives for midazolam and its metabolites, and subjects over 70 years of age may be particularly sensitive [see Clinical Pharmacology (12.3)]. Administration of IM midazolam to elderly patients has been associated with rare reports of death under circumstances compatible with cardiorespiratory depression [see Warnings and Precautions (5.4)]. In most of these cases, the patients also received other CNS depressants capable of depressing respiration, especially narcotics [see Warnings and Precautions (5.1, 5.6)]. Close monitoring of geriatric patients is recommended.
Renal Impairment
Patients with renal impairment may have a slower elimination of midazolam and its metabolites, which may result in prolonged drug exposure [see Clinical Pharmacology (12.3)].
Congestive Heart Failure
Patients with congestive heart failure eliminate midazolam more slowly, which may result in prolonged drug exposure [see Clinical Pharmacology (12.3)].
Pregnancy: Based on animal data, may cause fetal harm. (8.1) See 17 for PATIENT COUNSELING INFORMATION.
Boxed Warning Section
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Monitor patients for respiratory depression and sedation [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
- The use of benzodiazepines, including midazolam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing Midazolam Injection and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction [see Warnings and Precautions (5.2)].
- The continued use of benzodiazepines may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Although Midazolam Injection is indicated only for intermittent use [see Indications and Usage (1) and Dosage and Administration (2)], if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of Midazolam Injection may precipitate acute withdrawal reactions, which can be life-threatening. For patients using Midazolam Injection more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue Midazolam Injection [see Warnings and Precautions (5.3)].
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
See full prescribing information for complete boxed warning.
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Monitor patients for respiratory depression and sedation. (5.1, 7.1)
- The use of benzodiazepines, including midazolam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Before prescribing Midazolam Injection and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction. (5.2)
- Although Midazolam Injection is indicated only for intermittent use (1, 2), if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of Midazolam Injection may precipitate acute withdrawal reactions, which can be lifethreatening. For patients using Midazolam Injection more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue Midazolam Injection. (5.3)
Overdosage Section
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, exclient, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal [see Warnings and Precautions (5.2)]. Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway maintenance. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information. Consider contacting a poison center (1-800-221-2222) or a medical toxicologist for additional overdosage management recommendations.
Description Section
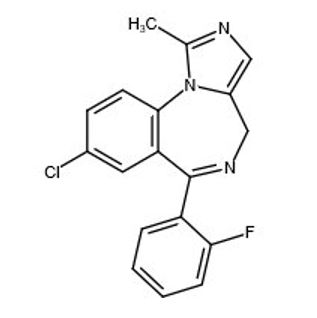
Midazolam is a white to light yellow crystalline compound, insoluble in water. The hydrochloride salt of midazolam, which is formed in situ, is soluble in aqueous solutions. Chemically, midazolam is 8-chloro-6- (2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine. Midazolam has the empirical formula C 18H 13ClFN 3, a calculated molecular weight of 325.77 and the following structural formula:
Midazolam Injection contains a sterile, nonpyrogenic solution for intramuscular injection in a single-dose prefilled autoinjector. Each 0.7 mL contains 10 mg midazolam (equivalent to 11.12 mg midazolam hydrochloride) compounded with 0.7% sodium chloride and 1.1% hydrochloric acid. The pH is adjusted to approximately 3 with hydrochloric acid.
Nonclinical Toxicology Section
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Midazolam maleate was administered in the diet to mice and rats for 2 years at doses of 0, 1, 9, or 80 mg/kg/day. In female mice in the highest dose group there was a marked increase in the incidence of hepatic tumors. In highdose male rats there was a small but statistically significant increase in benign thyroid follicular cell tumors. The highest dose not associated with increased tumor incidences in mice and rats (9 mg/kg/day) is approximately 4 and 9 times, respectively, the recommended human dose (RHD) of 10 mg based on body surface area (mg/m2). The pathogenesis of induction of these tumors is not known. These tumors were found after chronic administration, whereas human use will ordinarily be of single or several doses.
Mutagenesis
Midazolam was negative for genotoxicity in in vitro (Ames, mammalian cell clastogenicity) and in vivo (mouse bone marrow micronucleus) assays.
Impairment of Fertility
When midazolam (0, 1, 4, or 16 mg/kg) was orally administered to male and female rats prior to and during mating and continuing in females throughout gestation and lactation, no adverse effects on male or female fertility were noted. Midazolam plasma exposures (AUC) at the highest dose tested were approximately 6 times that in humans at the RHD.
How Supplied Section
How Supplied
Midazolam Injection is a clear, colorless to slightly yellow sterile solution available in single-dose prefilled autoinjectors containing 10 mg/0.7 mL. Midazolam Injection is supplied in the following packaging configurations:
- Carton of 480 prefilled autoinjectors: NDC 71053-595-01
Storage and Handling
Store at 20 ºC to 25 ºC (68 ºF to 77 ºF); excursions permitted between 15 ºC and 30 ºC (59 ºF and 86 ºF) [See USP Controlled Room Temperature]. DO NOT FREEZE.
Clinical Studies Section
The effectiveness of Midazolam Injection administered via this autoinjector for the treatment of status epilepticus in adults is based upon an adequate and well-controlled study of a different midazolam injection, which is described below, and a bioavailability study comparing intramuscular administration of 10 mg midazolam using this autoinjector versus midazolam vial in healthy adults. The safety and effectiveness of midazolam injection for the treatment of status epilepticus was established in a multi-center, randomized, double-blind (double-dummy), active-control trial comparing midazolam administered intramuscularly (IM) via a different autoinjector to lorazepam administered intravenously (IV). Patients meeting the diagnosis of status epilepticus, with continuing convulsive seizure activity after the arrival of paramedics, were eligible for enrollment. The ITT population consisted of 893 patients who were randomized to receive either IM midazolam (n=448) or IV lorazepam (n=445). Following randomization, each patient received study treatments administered by a healthcare professional (e.g., paramedic) prior to arrival at a hospital. According to the double-dummy design, adult patients received 10 mg IM midazolam followed by IV placebo or received IM placebo followed by 4 mg IV lorazepam. The primary efficacy endpoint was the termination of convulsive seizure activity (without the need for rescue medication) prior to arrival at the emergency department (ED) as determined by the ED attending physician. A statistically significantly higher percentage of midazolam-treated patients met the primary efficacy endpoint, as shown in Table 2.
Table 2: Primary Efficacy Analysis Results: Seizure Termination (without rescue medication) IM Midazolam (n=448) IV Lorazepam Treatment success (%) 73.4 63.4 p-value a 0.002
a Fischer's exact test
Drug Abuse And Dependence Section
Controlled Substance
Midazolam Injection contains midazolam, a Schedule IV controlled substance.
Abuse
Midazolam is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders [see Warnings and Precautions (5.2)].The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
Midazolam was actively self-administered in primate models used to assess the positive reinforcing effects of psychoactive drugs. Midazolam produced physical dependence of a mild to moderate intensity in cynomolgus monkeys after 5 to 10 weeks of administration.
Available data concerning the drug abuse and dependence potential of midazolam suggest that its abuse potential is at least equivalent to that of diazepam.
Dependence
Physical Dependence After Use of Midazolam Injection More Frequently Than Recommended
Midazolam Injection may produce physical dependence if used more frequently than recommended. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Although Midazolam Injection is indicated only for intermittent use [see Indications and Usage (1) and Dosage and Administration (2)], if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use [see Warnings and Precautions (5.3)].
For patients using Midazolam Injection more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue Midazolam Injection [see Warnings and Precautions (5.3)].
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to Midazolam Injection may develop after use more frequently than recommended. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of benzodiazepines may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
Clinical Pharmacology Section
Mechanism of Action
The exact mechanism of action for midazolam in the treatment of status epilepticus is not fully understood, but is thought to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABAA receptor.
Pharmacodynamics
The effects of midazolam on the CNS are dependent on the dose administered, the route of administration, and the presence or absence of other medications.
Pharmacokinetics
The pharmacokinetics of midazolam were evaluated in a single dose-escalation trial in healthy subjects; following IM injection of midazolam at doses ranging from fixed total amounts of 5 mg to 30 mg (one half to three times the recommended dose) or body weight based dose of 0.10 mg/kg to 0.49 mg/kg, the overall median time to maximum plasma concentration (Tmax) was observed at approximately 0.5 hours after administration. The rate and extent of systemic exposure, as assessed by maximum plasma concentration (Cmax) and area under the plasma drug concentration-time curve from time 0 to infinity (AUC0-∞), tended to increase proportionally with increasing dose from 5 mg to 30 mg.
Absorption
Following IM administration of a single 10 mg midazolam dose to healthy subjects via this autoinjector, midazolam was absorbed with median Tmax (range) of 0.5 (0.17 to 1.03) hours; midazolam mean (±SD) Cmax and AUC 0-∞ were 127 (±47.3) ng/mL and 454 (±134) ng∙hr/mL, respectively.
Distribution
The mean (±SD) apparent volume of distribution (Vz/F) of midazolam following a single IM dose of 10 mg midazolam via this autoinjector was 169 (±61.7) L in healthy subjects. In humans, midazolam has been shown to cross the placenta and enter into fetal circulation, and has been detected in human milk and CSF [see Use in Specific Populations (8.1, 8.2)]. In adults, midazolam is approximately 97% bound to plasma protein, principally albumin. The 1-hydroxy metabolite is approximately 89% bound to plasma protein
Elimination
Elimination of the parent drug takes place via hepatic metabolism of midazolam to hydroxylated metabolites that are conjugated and excreted in the urine.
Metabolism
In vitro studies with human liver microsomes indicate that the biotransformation of midazolam is mediated by the cytochrome P450-3A4 (CYP3A4). This enzyme is present in gastrointestinal tract mucosa, as well as in the liver. The 1-hydroxy-midazolam (also termed alpha-hydroxymidazolam) metabolite comprises 60% to 70% of the biotransformation products of midazolam, while 4-hydroxy-midazolam constitutes 5% or less. Small amounts of a dihydroxy derivative have also been detected, but not quantified. The principal urinary excretion products are glucuronide conjugates of the hydroxylated derivatives. Studies of the intravenous administration of 1-hydroxy-midazolam in humans suggest that 1-hydroxymidazolam is at least as potent as the parent compound, and may contribute to the net pharmacologic activity of midazolam. In vitro studies have demonstrated that the affinities of 1- and 4-hydroxy-midazolam for the benzodiazepine receptor are approximately 20% and 7%, respectively, relative to midazolam.
Excretion
Following IM administration of 10 mg midazolam via this autoinjector, mean (±SD) terminal elimination halflife was 5.16 (±1.77) hours and mean (±SD) apparent total body clearance (CL/F) of midazolam was and 23.8 (±6.77) L/hr. The principal urinary excretion product is 1-hydroxy-midazolam in the form of a glucuronide conjugate; smaller amounts of the glucuronide conjugates of 4-hydroxy- and dihydroxy-midazolam are detected as well. The amount of midazolam excreted unchanged in the urine after a single IV dose is less than 0.5%. Following a single IV infusion in 5 healthy volunteers, 45% to 57% of the dose was excreted in the urine as the 1-hydroxymethyl midazolam conjugate.
Specific Populations
Changes in the pharmacokinetic profile of midazolam due to drug interactions, physiological variables, etc., may result in changes in the plasma concentration-time profile and pharmacological response to midazolam in these patients. For example, patients with acute renal failure (ARF) appear to have a longer elimination half-life for midazolam [see Use in Specific Populations (8.6)]. In other groups, the relationship between prolonged half-life and duration of effect has not been established.
Obesity
In a study comparing normal (n=20) and obese patients (n=20), the mean half-life was greater in the obese group (5.9 versus 2.3 hours). This was due to an increase of approximately 50% in the volume of distribution (Vd) corrected for total body weight. The clearance was not significantly different between groups.
Geriatric Patients
In three parallel-group studies, the pharmacokinetics of midazolam administered IV or IM were compared in young (mean age 29 years, n=52) and healthy elderly subjects (mean age 73 years, n=53). Plasma half-life was approximately 2-fold higher in the elderly. The mean Vd based on total body weight increased consistently between 15% and 100% in the elderly. The mean CL (total clearance) decreased approximately 25% in the elderly in two studies, and was similar to that of the younger patients in the other [see Use in Specific Populations (8.5)].
Male and Female Patients
No meaningful differences in midazolam exposures (C max and AUC) were observed between male and female adults after IM administration.
Patients with Congestive Heart Failure
In patients suffering from congestive heart failure, a 2-fold increase in the elimination half-life, a 25% decrease in the plasma clearance, and a 40% increase in the volume of distribution of midazolam were observed.
Patients with Renal Impairment
Patients with renal impairment may have longer elimination half-lives for midazolam and its metabolites [see Use in Specific Populations (8.6)]. Midazolam and 1-hydroxy-midazolam pharmacokinetics were compared between 6 intensive care unit (ICU) patients who developed acute renal failure (ARF) and a control group of subjects with normal renal function. Midazolam was administered as an IV infusion (5 to 15 mg/hour). Midazolam clearance was reduced (1.9 versus 2.8 mL/min/kg), and the half-life was prolonged (7.6 hours versus 13 hours) in the ARF patients. The renal clearance of the 1-hydroxy-midazolam glucuronide was prolonged in the ARF group (4 versus 136 mL/min), and the half-life was prolonged (12 hours versus >25 hours). Plasma levels accumulated in all ARF patients to about ten times that of the parent drug. The relationship between accumulating metabolite levels and prolonged sedation is unclear. In a study of chronic renal failure patients (n=15) receiving a single IV dose of midazolam, there was a 2-fold increase in the clearance and volume of distribution, but the half-life remained unchanged. Metabolite levels were not studied.
Patients with Hepatic Impairment
Midazolam pharmacokinetics were studied after a single IV dose (0.075 mg/kg) was administered to 7 patients with biopsy-proven alcoholic cirrhosis and 8 control patients. The mean half-life of midazolam increased 2.5-fold in the patients with cirrhosis. Clearance was reduced by 50% and Vd increased by 20%. In another study in 21 male patients with cirrhosis, without ascites and with normal kidney function as determined by creatinine clearance, no changes in the pharmacokinetics of midazolam or 1-hydroxy-midazolam were observed when compared to healthy individuals. The clinical significance of these findings is unknown.
Drug Interaction Studies
CYP3A4 Inhibitors
Drugs that inhibit the activity of CYP3A4 may inhibit midazolam clearance and elevate midazolam concentrations [see Drug Interactions (7.3)].
- The effect of single oral doses of 800 mg cimetidine and 300 mg ranitidine on steady-state concentrations of oral midazolam was examined in a randomized crossover study (n=8). Cimetidine increased the mean midazolam steady-state concentration from 57 to 71 ng/mL. Ranitidine increased the mean steady-state concentration to 62 ng/mL. No change in choice reaction time or sedation index was detected after dosing with the H2 receptor antagonists.
- In a placebo-controlled study, erythromycin administered as a 500 mg dose, three times a day, for 1 week (n=6), reduced the clearance of midazolam following a single 0.5 mg/kg IV dose. The half-life was approximately doubled.
- The effects of diltiazem (60 mg three times a day) and verapamil (80 mg three times a day) on the pharmacokinetics and pharmacodynamics of midazolam were investigated in a three-way crossover study (n=9). The half-life of midazolam increased from 5 to 7 hours when midazolam was taken in conjunction with verapamil or diltiazem. No interaction was observed in healthy subjects between midazolam and nifedipine.
- In a placebo-controlled study, where saquinavir or placebo was administered orally as a 1200 mg dose three times a day for 5 days (n=12), a 56% reduction in the clearance of midazolam following a single 0.05 mg/kg IV dose was observed. The half-life was approximately doubled.
CYP3A4 Inducers
Drugs that induce the activity of CYP3A4 may increase midazolam clearance and decrease midazolam concentrations.
Patient Counseling Information
Midazolam Injection is intended for administration by trained personnel. See the illustrated Instructions for Use. Patients having seizures will likely be unresponsive or may have difficulty in comprehending counseling information.
Risks from Concomitant Use with Opioids Inform patients and caregivers that potentially fatal additive effects may occur if Midazolam Injection is used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider [see Warnings and Precautions (5.1), Drug Interactions (7.1)]. Abuse, Misuse, and Addiction Inform patients that the use of Midazolam Injection more frequently than recommended, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug [see Warnings and Precautions (5.2) and Drug Abuse and Dependence (9.2)].
Withdrawal Reactions Inform patients that use of Midazolam Injection more frequently than recommended may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of Midazolam Injection may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months [see Warnings and Precautions (5.3) and Drug Abuse and Dependence (9.3)]. Concomitant Medications Advise patients to inform their healthcare provider about any alcohol consumption and medicine they are now taking, especially blood pressure medication and antibiotics, including drugs they buy without a prescription. Alcohol has an increased effect when consumed with benzodiazepines; therefore, caution should be exercised regarding simultaneous ingestion of alcohol during benzodiazepine treatment [see Warnings and Precautions (5.6), Drug Interactions (7)].
Impaired Cognitive Function Advise patients not to operate hazardous machinery or a motor vehicle until the effects of the drug, such as drowsiness, have subsided [see Warnings and Precautions (5.7)].
Pregnancy Advise pregnant females that the use of Midazolam Injection late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns [see Warnings and Precautions (5.9) and Use in Specific Populations (8.1)]. Instruct patients to inform their healthcare provider if they are pregnant Encourage patients to enroll in the North American Antiepileptic Drug Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy [see Use in Specific Populations (8.1)]. Lactation Counsel patients that midazolam, the active ingredient in Midazolam Injection, is excreted in breast milk. Instruct patients to inform their healthcare provider if they are breastfeeding or intend to breastfeed. Instruct breastfeeding patients who have been administered Midazolam Injection to monitor infants for excessive sedation, poor feeding and poor weight gain, and to seek medical attention if they notice these signs [see Use in Specific Populations (8.2)].
Manufactured and Distributed by:
Rafa Laboratories, Ltd.
3 Zeev Lev, Har Hotzvim,
Jerusalem, 9777515
Israel
Instructions For Use Section
Package Label.principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site






