norelgestromin and ethinyl estradiol Dailymed
Generic: norelgestromin and ethinyl estradiol is used for the treatment of Amenorrhea Breast Neoplasms Cerebrovascular Disorders Coronary Disease Diabetes Mellitus Heart Valve Diseases Hypertension Liver Diseases Liver Neoplasms Neoplasms, Hormone-Dependent Pregnancy Pulmonary Embolism Thromboembolism Thrombophlebitis Tobacco Use Disorder Uterine Hemorrhage Thrombophilia Venous Thrombosis Headache Disorders Cerebral Arterial Diseases Coronary Artery Disease Hypogonadism Menopause, Premature Menorrhagia Prostatic Neoplasms Osteoporosis, Postmenopausal Primary Ovarian Insufficiency Endometrial Neoplasms Hot Flashes Tobacco Use
Go PRO for all pill images
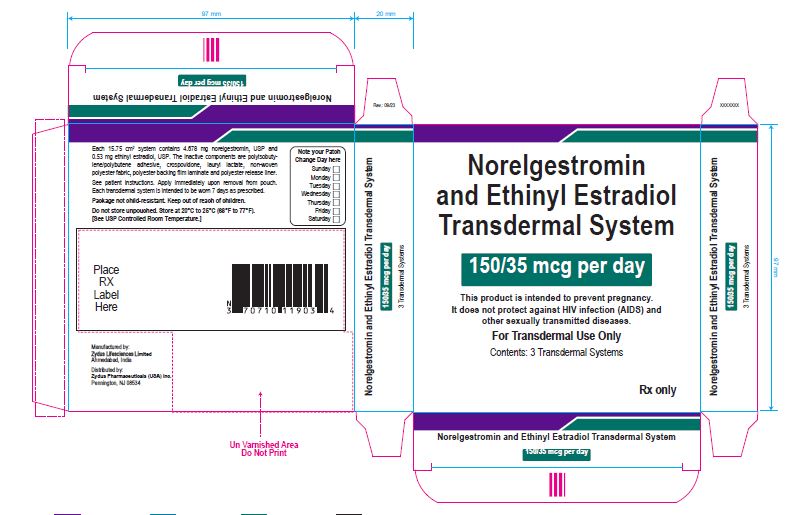
Package Label.principal Display Panel
NDC 70771-1777-3
Norelgestromin and Ethinyl Estradiol Transdermal System 150/35 mcg per day
This product is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
For Transdermal Use Only
Contents: 3 Transdermal Systems
Rx only
Each 15.75 cm2 system contains 4.678 mg norelgestromin, USP and 0.53 mg ethinyl estradiol, USP. The inactive components are polyisobutylene/polybutene adhesive, crospovidone, lauryl lactate, non-woven polyester fabric, polyester backing film laminate and polyester release liner.
See patient instructions. Apply immediately upon removal from pouch. Each transdermal system is intended to be worn 7 days as prescribed.
Package not child-resistant. Keep out of reach of children.
Do not store unpouched. Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Manufactured by:
Zydus Lifesciences Ltd.
Ahmedabad, India
Distributed by:
Zydus Pharmaceuticals (USA) Inc.
Pennington, NJ 08534
Rev.: 10/22
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site



