NOURIANZ (istradefylline 40 mg) Dailymed
Generic: istradefylline is used for the treatment of Parkinson Disease
Go PRO for all pill images
1indications And Usage
NOURIANZ is indicated as adjunctive treatment to levodopa/carbidopa in adult patients with Parkinson's disease (PD) experiencing "off" episodes.
NOURIANZ is an adenosine receptor antagonist indicated as adjunctive treatment to levodopa/carbidopa in adult patients with Parkinson's disease (PD) experiencing "off" episodes (1 ).
2dosage And Administration
- The recommended dosage is 20 mg orally once daily. The dosage may be increased to a maximum of 40 mg once daily (
2.1 ).- May be taken with or without food (
2.1 ).- Patients with hepatic impairment: Maximum recommended dosage with moderate hepatic impairment is 20 mg once daily; use of NOURIANZ in patients with severe hepatic impairment should be avoided (
2.4 ,8.7 ).- Patients who smoke 20 or more cigarettes per day (or the equivalent of another tobacco product): Recommended dosage is 40 mg once daily (
2.5 ,8.8 ).2.1Dosing Information
The recommended dosage of NOURIANZ is 20 mg administered orally once daily. The dosage may be increased to a maximum of 40 mg once daily, based on individual need and tolerability. Initial dose titration is not required.
NOURIANZ can be taken with or without food [see Clinical Pharmacology (12.3)].
2.2Dosage Adjustment with Strong CYP3A4 Inhibitors
The maximum recommended dosage of NOURIANZ with concomitant use of strong CYP3A4 inhibitors is 20 mg once daily [see Drug Interactions (7.1)].
2.3Dosing with Strong CYP3A4 Inducers
Avoid use of NOURIANZ with strong CYP3A4 inducers [see Drug Interactions (7.1)].
2.4Dosage Adjustment in Patients with Hepatic Impairment
The maximum recommended dosage of NOURIANZ in patients with moderate hepatic impairment (Child-Pugh Class B) is 20 mg once daily. Closely monitor patients with moderate hepatic impairment for adverse reactions when on NOURIANZ treatment [see Adverse Reactions (6.1)]. Avoid use of NOURIANZ in patients with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.7)].
2.5Dosage Adjustment for Tobacco Smokers
The recommended dosage of NOURIANZ in patients who use tobacco in amounts of 20 or more cigarettes per day (or the equivalent of another tobacco product) is 40 mg once daily [see Use in Specific Populations (8.8) and Clinical Pharmacology (12.3)].
3dosage Forms And Strengths
- 20 mg tablets: Peach-colored, pillow-shaped, film-coated tablets with "20" debossed on one side.
- 40 mg tablets: Peach-colored, almond-shaped, film-coated tablets with "40" debossed on one side.
Tablets: 20 mg and 40 mg (3 ).
4contraindications
None.
None (4 ).
5warnings And Precautions
- Dyskinesia: Monitor patients for dyskinesia or exacerbation of existing dyskinesia (
5.1 ).- Hallucinations / Psychotic Behavior: Consider dosage reduction or stopping NOURIANZ if occurs (
5.2 ).- Impulse Control / Compulsive Behaviors: Consider dosage reduction or stopping NOURIANZ if occurs (
5.3 ).5.1Dyskinesia
NOURIANZ in combination with levodopa may cause dyskinesia or exacerbate pre-existing dyskinesia.
In controlled clinical trials (Studies 1, 2, 3, and 4) [see Clinical Studies (14)], the incidence of dyskinesia was 15% for NOURIANZ 20 mg, 17% for NOURIANZ 40 mg, and 8% for placebo, in combination with levodopa. One percent of patients treated with either NOURIANZ 20 mg or 40 mg discontinued treatment because of dyskinesia, compared to 0% for placebo.
5.2Hallucinations / Psychotic Behavior
Because of the potential risk of exacerbating psychosis, patients with a major psychotic disorder should not be treated with NOURIANZ. Consider dosage reduction or discontinuation if a patient develops hallucinations or psychotic behaviors while taking NOURIANZ.
In controlled clinical trials (Studies 1, 2, 3, and 4) [see Clinical Studies (14)], the incidence of hallucinations was 2% for NOURIANZ 20 mg, 6% for NOURIANZ 40 mg, and 3% for placebo. In patients treated with NOURIANZ 40 mg, 1% discontinued because of hallucinations, compared to 0% for placebo and 0% for patients treated with NOURIANZ 20 mg. The incidence of "abnormal thinking and behavior" (paranoid ideation, delusions, confusion, mania, disorientation, aggressive behavior, agitation, or delirium) reported as an adverse reaction was 1% for NOURIANZ 20 mg, 2% for NOURIANZ 40 mg, and 1% for placebo.
5.3Impulse Control / Compulsive Behaviors
Patients treated with NOURIANZ and one or more medication(s) for the treatment of Parkinson's disease (including levodopa) may experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge or compulsive eating, and/or other intense urges, and the inability to control these urges. In controlled clinical trials (Studies 1, 2, 3 and 4) [see Clinical Studies (14)], one patient treated with NOURIANZ 40 mg was reported to have impulse control disorder, compared to no patient on placebo or NOURIANZ 20 mg.
In some postmarketing cases, these urges were reported to have stopped when the dose was reduced, or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending, binge or compulsive eating, or other urges while being treated with NOURIANZ. Consider dose reduction or discontinuation if a patient develops such urges while taking NOURIANZ [see Adverse Reactions (6.2)].
6adverse Reactions
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Dyskinesia [see Warnings and Precautions (5.1)]
- Hallucinations / Psychotic Behavior [see Warnings and Precautions (5.2)]
- Impulse Control / Compulsive Behaviors [see Warnings and Precautions (5.3)]
The most common adverse reactions (at least 5% and more frequent than placebo) were dyskinesia, dizziness, constipation, nausea, hallucination, and insomnia (6.1 ).
To report SUSPECTED ADVERSE REACTIONS, contact Kyowa Kirin Inc. at 1-844-768-3544 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of NOURIANZ was evaluated in 734 patients with Parkinson's disease (PD) taking a stable dose of levodopa and a DOPA decarboxylase inhibitor, with or without other PD medications, in four randomized, multicenter, double-blind, placebo-controlled trials 12 weeks in duration (Studies 1, 2, 3 and 4) [see Clinical Studies (14)]. Of the patient population exposed to NOURIANZ, 50% were male, 32% White, 67% Asian, and the mean age was 65 years (range: 33 to 84 years). Of these patients, 356 received NOURIANZ 20 mg and 378 received NOURIANZ 40 mg.
Adverse Reactions Leading to Discontinuation of Treatment
The incidence of patients discontinuing for any adverse reaction was 5% for NOURIANZ 20 mg, 6% for NOURIANZ 40 mg, and 5% for placebo. The most frequently reported adverse reaction causing study discontinuation was dyskinesia [see Warnings and Precautions (5.1)].
Common Adverse Reactions in Pooled Placebo-Controlled Trials
Table 1 shows adverse reactions with a frequency of at least 2% in patients treated with NOURIANZ 20 mg or 40 mg once daily. The most common adverse reactions in which the frequency for NOURIANZ was at least 5%, and greater than the incidence on placebo, were dyskinesia, dizziness, constipation, nausea, hallucination, and insomnia.
Table 1: Adverse Reactions with an Incidence of at Least 2% in Patients Treated with NOURIANZ, and Greater than on Placebo, in Pooled Studies 1, 2, 3, and 4 Adverse Reactions NOURIANZ 20 mg/day (N=356) % NOURIANZ 40 mg/day (N=378) % Placebo N=426 (%) Nervous system disorders   Dyskinesia 15 17 8   Dizziness 3 6 4 Gastrointestinal disorders   Constipation 5 6 3   Nausea 4 6 5   Diarrhea 1 2 1 Psychiatric disorders   Hallucination Includes hallucinations, hallucinations visual, hallucinations olfactory, hallucinations somatic, hallucinations auditory. 2 6 3   Insomnia 1 6 4 Metabolism and nutrition disorders   Decreased appetite 1 3 1 Investigations   Blood alkaline phosphatase increased 1 2 1   Blood glucose increased 1 2 0   Blood urea increased 1 2 0 Respiratory, thoracic and mediastinal disorders   Upper Respiratory Tract Inflammation 1 2 0 Skin and subcutaneous tissue disorders   Rash 1 2 1 6.2Postmarketing Experience
The following adverse reaction has been identified during post approval use of istradefylline outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: increased libido.
7drug Interactions
- Strong CYP3A4 inhibitors: Recommended maximum dosage with concomitant use is 20 mg once daily (
2.2 ,7.1 ).- Strong CYP3A4 inducers: Avoid use (
2.3 ,7.1 ).7.1Effect of Other Drugs on NOURIANZ
Strong CYP3A4 Inhibitors
Coadministration of NOURIANZ with a strong CYP3A4 inhibitor (ketoconazole) increased istradefylline AUCinf by 2.5-fold [see Clinical Pharmacology (12.3)]. Therefore, the recommended maximum dosage of NOURIANZ in patients concomitantly using strong CYP3A4 inhibitors (e.g., itraconazole, ketoconazole, clarithromycin) is 20 mg once daily [see Dosage and Administration (2.2)].
Strong CYP3A4 Inducers
Coadministration of NOURIANZ with a strong CYP3A4 inducer (rifampin) decreased istradefylline Cmax and AUCinf by 45% and 81%, respectively [see Clinical Pharmacology (12.3)]. Therefore, it is recommended to avoid use of NOURIANZ with strong CYP3A4 inducers (e.g., carbamazepine, rifampin, phenytoin, St. John's wort) [see Dosage and Administration (2.3)].
7.2Effect of NOURIANZ on Other Drugs
CYP3A4 Substrates
Coadministration of NOURIANZ 20 mg with a CYP3A4 substrate (midazolam) did not affect the CYP3A4 substrate exposure, while concomitant administration of NOURIANZ 40 mg increased the CYP3A4 substrate (atorvastatin) Cmax and AUCinf by 1.5-fold [see Clinical Pharmacology (12.3)]. Monitor for an increase in adverse reactions of concomitant drugs that are CYP3A4 substrates when coadministering with NOURIANZ 40 mg.
P-glycoprotein (P-gp) Substrates
Coadministration of NOURIANZ with a P-gp substrate (digoxin) increased the P-gp substrate Cmax and AUCinf by 33% and 21%, respectively [see Clinical Pharmacology (12.3)]. Monitor for an increase in adverse reactions of concomitant drugs that are P-gp substrates when coadministering with NOURIANZ.
8use In Specific Populations
- Pregnancy: Based on animal data, may cause fetal harm (
8.1 ).8.1Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of NOURIANZ in pregnant women. In animal studies (see Data ), oral administration of istradefylline during pregnancy resulted in teratogenicity (increased incidences of fetal structural abnormalities, embryofetal and offspring mortality and growth deficits) at clinically relevant exposures and in the absence of maternal toxicity. The teratogenic effects of istradefylline in pregnant rabbits were substantially greater when administered in combination with levodopa/carbidopa than when administered alone.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
Data
Animal Data
Oral administration of istradefylline (0, 40, 200, or 1000 mg/kg/day) to pregnant rats throughout organogenesis resulted in decreased fetal body weight and increased fetal skeletal and visceral variations at the highest dose tested. Plasma exposure (AUC) at the no-effect dose for adverse effects on embryofetal development in rats (200 mg/kg/day) is approximately 4 times that in humans at the maximum recommended human dose (MRHD) of 40 mg.
Oral administration of istradefylline (0, 50, 200, or 800 mg/kg/day) to pregnant rabbits throughout organogenesis resulted in increased embryofetal mortality at the mid and high doses, increased fetal malformations (external, visceral, skeletal) at all doses, and reduced fetal body weight at the highest dose tested. A no-effect dose for adverse effects on embryofetal development in rabbits was not identified. Plasma exposure (AUC) at the lowest dose tested (50 mg/kg/day) is less than that in humans at the MRHD.
In pregnant rabbits, oral administration of istradefylline (0, 50, 200, or 400 mg/kg/day) alone or in combination with oral levodopa/carbidopa (80/20 mg/kg/day) throughout the period of organogenesis resulted in an increase in embryofetal mortality and an increase (marked at the high dose) in malformations (including limb reduction, craniofacial, and cardiovascular) in fetuses from rats administered istradefylline at all doses in combination with levodopa/carbidopa. Istradefylline alone resulted in an increase in embryofetal mortality and visceral malformations; no increase in fetal malformations was observed with levodopa/carbidopa alone. Fetal body weight was reduced by istradefylline alone (400 mg/kg/day) and in combination (200 and 400 mg/kg/day) with levodopa/carbidopa. A no-effect dose for adverse effects on embryofetal development in rabbits when istradefylline was administered in combination with levodopa/carbidopa was not identified. Plasma exposure (AUC) at the lowest dose of istradefylline tested (50 mg/kg/day) in combination with levodopa/carbidopa is less than that in humans at the MRHD.
Oral administration of istradefylline (0, 6, 25, 100, or 400 mg/kg/day) to female rats throughout gestation and lactation resulted in decreased pup survival and reduced pup body weight (which persisted into adulthood) at all but the lowest dose tested. Exposure to drug in the milk may have contributed to these effects, as demonstrated in pups of untreated (control) dams reared by dams receiving istradefylline (400 mg/kg/day). No adverse effects were observed on physical or neurobehavioral development, or reproductive function. Plasma exposure at the no-effect dose for adverse effects on pre- and postnatal development in rats (6 mg/kg/day) is less than that in humans at the MRHD.
8.2Lactation
Risk Summary
There are no data on the presence of istradefylline in human milk, the effects of istradefylline on the breastfed infant, or the effects of istradefylline on milk production. Istradefylline was present in the milk of lactating rats at concentrations up to 10 times that in maternal plasma.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for NOURIANZ, and any potential adverse effects on the breastfed infant from NOURIANZ or from the underlying maternal condition.
8.3Females and Males of Reproductive Potential
Contraception
Use of NOURIANZ during pregnancy is not recommended. Women of childbearing potential should be advised to use contraception during treatment with NOURIANZ [see Use in Specific Populations (8.1)].
8.4Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
No adjustment of NOURIANZ dosage is recommended on the basis of age. Of the total number of PD patients who received NOURIANZ in clinical trials, 53% were ≥65 years and 13% were ≥75 years of age. No overall differences in effectiveness were observed between these patients and younger patients.
8.6Renal Impairment
No adjustment of NOURIANZ dosage is needed in patients with mild renal impairment (estimated creatinine clearance (CrCL) by Cockcroft-Gault equation: 60-89 mL/min), moderate renal impairment (CrCL 30-59 mL/min), or severe renal impairment (CrCL 15-29 mL/min). NOURIANZ has not been evaluated in patients with end-stage renal disease (ESRD) (CrCL <15 mL/min) or ESRD requiring hemodialysis [see Clinical Pharmacology (12.3)].
8.7Hepatic Impairment
No adjustment of NOURIANZ dosage is needed in patients with mild hepatic impairment (Child-Pugh Class A).
In patients with moderate hepatic impairment (Child-Pugh Class B), the steady-state exposures (AUC0-24h) were predicted to be 3.3-fold higher than in healthy subjects, based on the estimated mean terminal half-life. Therefore, the maximum recommended dosage of NOURIANZ in patients with moderate hepatic impairment (Child-Pugh Class B) is 20 mg once daily [see Clinical Pharmacology (12.3)]. Closely monitor patients with moderate hepatic impairment for adverse events when on NOURIANZ treatment [see Adverse Reactions (6.1)].
NOURIANZ has not been studied in patients with severe hepatic impairment (Child-Pugh Class C). Avoid use of NOURIANZ in patients with severe hepatic impairment [see Clinical Pharmacology (12.3)].
8.8Tobacco Smokers
Tobacco smoking decreased NOURIANZ steady-state systemic exposures by 38% to 54% [see Clinical Pharmacology (12.3)], which may decrease efficacy. Therefore, the recommended NOURIANZ dosage in patients who smoke 20 or more cigarettes per day (or the equivalent amount of another tobacco product) is 40 mg once daily.
10overdosage
10.1 Human Experience
There is limited clinical experience regarding human overdosage with NOURIANZ. In clinical trials, one patient took 6 tablets (120 mg, 3 times the maximum recommended dosage) of istradefylline with alcoholic beverages and developed hallucinations, agitation, and worsening dyskinesia.
10.2 Management of Overdose
There are no known specific antidotes for NOURIANZ nor any specific treatment for istradefylline overdose. If an overdose occurs, NOURIANZ treatment should be discontinued and supportive treatment should be administered as clinically indicated. Consider the long terminal half-life of istradefylline (about 83 hours) and the possibility of multiple drug involvement.
Consult a Certified Poison Control Center for up-to-date guidance and advice.
11description
NOURIANZ contains istradefylline, an adenosine receptor antagonist, which has a xanthine derivative structure. The chemical name is (E)-8-(3,4-dimethoxystyryl)-1,3-diethyl-7-methyl-3,7-dihydro-1H-purine-2,6-dione. Its molecular formula is C20H24N4O4. The molecular weight is 384.43. Istradefylline has the following structural formula:
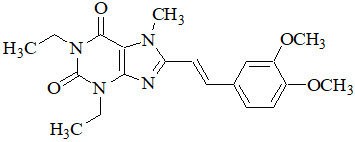
Istradefylline is a light yellow-green crystalline powder. Istradefylline has a dissociation constant (pK a) of 0.78. The aqueous solubility of istradefylline is ~0.5 µg/mL across the physiological pH range and 0.6 µg/mL in water.
NOURIANZ tablets are intended for oral administration only. Each tablet contains 20 mg or 40 mg of istradefylline and the following inactive ingredients: crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and polyvinyl alcohol. The film coating contains hypromellose, lactose monohydrate, polyethylene glycol 3350, titanium dioxide, triacetin, and the following dyes: iron oxide red and iron oxide yellow. Carnauba wax is used for polishing.
12clinical Pharmacology
12.1Mechanism of Action
The precise mechanism by which istradefylline exerts its therapeutic effect in Parkinson’s disease is unknown. In in vitro studies and in in vivo animal studies, istradefylline was demonstrated to be an adenosine A2A receptor antagonist.
12.2Pharmacodynamics
Cardiac Electrophysiology
The effect of NOURIANZ (40 mg or 160 mg [4 times the maximum recommended dosage] once daily for 14 days) on the QTc interval was evaluated in a randomized, placebo and moxifloxacin-controlled, multiple-dose, blinded, parallel group study. There was no clinically significant prolongation of QTc interval or relationship between changes in QTc and concentrations of istradefylline.
12.3Pharmacokinetics
Istradefylline exhibits dose-proportional pharmacokinetics after multiple oral doses from 20 mg to 80 mg (2 times the maximum recommended dosage). Steady-state was reached within 2 weeks of once-daily dosing. The pharmacokinetics of istradefylline were similar in PD patients and healthy subjects.
Absorption
The median time to reach the maximum concentration (Tmax) for istradefylline was about 4 hours under fasted dosing conditions.
Effect of Food
Istradefylline exposure, represented by the area under the curve over time to infinity (AUCinf), increased 1.25-fold when NOURIANZ was coadministered with a standard high-fat meal, compared with administration in a fasted state. Istradefylline maximum plasma concentrations (Cmax) increased by 1.64-fold and Tmax was shortened by 1 hour when NOURIANZ was administered with a high-fat meal. These differences in pharmacokinetic parameters are not expected to be clinically significant [see Dosage and Administration (2.1)].
Distribution
The plasma protein binding of istradefylline was approximately 98%. The apparent volume of distribution (Vd/F) of istradefylline is approximately 557 liters.
Elimination
The total clearance of istradefylline is approximately 4.6 L/hour. The mean terminal half-life (t1/2) for istradefylline at steady-state is approximately 83 hours.
Metabolism
In humans, istradefylline is exclusively eliminated via metabolism. In vitro studies indicate that istradefylline is primarily metabolized via CYP1A1 and CYP3A4, with minor contribution from CYP1A2, 2B6, 2C8, CYP2C9, CYP2C18, and 2D6. Six metabolites have been identified in human plasma. These metabolites each account for less than 10% of the exposure of the parent drug.
Excretion
Approximately 48% of a 40-mg oral dose of 14C-istradefylline was eliminated in feces, and 39% in urine. Unchanged istradefylline was not detected in urine.
Specific Populations
In patients with moderate hepatic impairment (Child-Pugh Class B), the steady-state exposure (AUC0-24) of istradefylline is predicted to be 3.3-fold higher relative to healthy subjects, based on the estimated mean terminal half-life [see Use in Specific Populations (8.7)]. Based on population pharmacokinetic analyses, no clinically relevant changes in the pharmacokinetics of istradefylline were observed based on age, sex, weight, or race. No clinically relevant changes in istradefylline exposure were observed in patients with severe renal impairment (CrCL 15-29 mL/min) or mild hepatic impairment. NOURIANZ has not been studied in patients with ESRD (CrCL < 15 mL/min), ESRD patients requiring hemodialysis, or severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.6, 8.7)].
Steady-state systemic exposure to istradefylline (40 mg) is 38% to 54% lower in tobacco smokers (who smoke 20 or more cigarettes per day) when compared with non-smokers matched for age, gender, and body weight [see Specific Populations (8.8)].
Drug Interaction Studies
In Vitro Assessment of Drug Interactions
In Vivo Assessment of Drug Interactions
13nonclinical Toxicology
13.1Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
In lifetime oral carcinogenicity studies, there was no evidence of carcinogenicity in mouse (0, 25, 125, or 250 mg/kg) or rat (0, 30, 100, or 320 mg/kg). Plasma exposures (AUC) at the highest doses tested were approximately 20 (mouse) and 10 (rat) times that in humans at the maximum recommended human dose (MRHD) of 40 mg/day.
Mutagenesis
Istradefylline was negative in in vitro (bacterial reverse mutation assay, chromosomal aberration in mammalian cells) and in vivo (mouse bone marrow micronucleus) assays.
Impairment of Fertility
Oral administration of istradefylline (0, 160, 360, or 800 mg/kg/day) to male and female rats prior to and during mating and continuing in females to gestation day 7 resulted in a decrease in fertility at the highest dose tested and an increase in preimplantation loss at the mid and high doses. Sperm motility was reduced at the highest dose tested. Plasma exposure (AUC) at the no-effect dose (160 mg/kg) for adverse effects on reproductive function is approximately 3 times that in humans at the MRHD.
13.2Animal Toxicology and/or Pharmacology
Oral administration of istradefylline (0, 30, 100, or 320 mg/kg/day) to rats for two years resulted in an increase in the incidence and severity of vascular mineralization in the brain (including in the caudate/putamen, globus pallidus, thalamus, and nucleus accumbens) at all doses tested. The vascular mineralization was composed of calcium and phosphorus and, at higher doses, were reported to partially or completely occlude the blood vessels. There was no evidence of neuronal degeneration, inflammation, or glial response associated with the foci of mineralization.
Brain mineralization was not detected in mice administered istradefylline (0, 25, 125, or 250 mg/kg/day) orally for two years or in dogs administered istradefylline (0, 10, 30, or 100 mg/kg/day) orally for 52 weeks.
14clinical Studies
The efficacy of NOURIANZ for the adjunctive treatment to levodopa/carbidopa in patients with Parkinson's disease experiencing "off" episodes was shown in four randomized, multicenter, double-blind, 12-week, placebo-controlled studies (Study 1, NCT00456586; Study 2, NCT00199407; Study 3, NCT00455507; and Study 4, NCT00955526). The studies enrolled patients with a mean duration of Parkinson's disease of 9 years (range: 1 month to 37 years) that were Hoehn and Yahr Stage II to IV, experiencing at least 2 hours (mean approximately 6 hours) of "off" time per day, and were treated with levodopa for at least one year, with stable dosage for at least 4 weeks before screening (mean total daily dosage range: 416 to 785 mg). Patients continued levodopa treatment with or without concomitant PD medications, including dopamine agonists (85%), COMT inhibitors (38%), MAO-B inhibitors (40%), anticholinergics (13%), and/or amantadine (33%), provided the medications were stable for at least 4 weeks before screening and throughout the study period. The studies excluded patients who had received a neurosurgical treatment for PD (e.g., pallidotomy, thalamotomy, deep brain stimulation).
The primary efficacy endpoint was the change from baseline in the daily awake percentage of "off" time, or the change from baseline in total daily "off" time, based on 24-hour diaries completed by patients. A change from baseline in "on" time without troublesome dyskinesia (i.e., "on" time without dyskinesia plus "on" time with non-troublesome dyskinesia) was a secondary efficacy endpoint.
Study 1 was conducted in the U.S. and Canada, and Study 2 was conducted in the U.S. In these studies, patients were randomized to once-daily treatment with NOURIANZ 20 mg, 40 mg, or placebo. Patients treated with NOURIANZ 20 mg or NOURIANZ 40 mg once daily experienced a statistically significant decrease from baseline in percentage of daily awake "off" time, compared with patients on placebo, as summarized in Table 2.
Table 2: Studies 1 and 2: Change From Baseline in Daily Awake OFF Time Baseline Change from Baseline to Endpoint N (mean ± SD) % of awake "off" hours N (LSMD LSMD: Least squares mean difference; a negative value indicates a greater reduction from baseline in Percentage Daily Awake "off" time for NOURIANZ, relative to placebo. vs. placebo), % awake "off" hours, (p-value)SD: Standard Deviation Study 1 Placebo 66 37.2 ± 13.8 65 -- NOURIANZ 40 mg 129 38.4 ± 16.2 126 - 6.78 (p=0.007) Study 2 Placebo 113 38.7 ± 11.6 113 -- NOURIANZ 20 mg 112 39.8 ± 14.0 112 - 4.57 (p=0.025)
Compared with patients on placebo, patients treated with NOURIANZ experienced an additional increase from baseline in "on" time without troublesome dyskinesia of 0.96 hours (nominal p=0.026) in Study 1, and of 0.55 hours (nominal p=0.135) in Study 2.
Study 3 and Study 4 were conducted in Japan. In these studies, patients were randomized equally to treatment with NOURIANZ 20 mg, 40 mg, or placebo. Patients treated with NOURIANZ 20 mg or NOURIANZ 40 mg once daily experienced a statistically significant decrease from baseline in "off" time compared with patients on placebo, as summarized in Table 3.
Table 3: Studies 3 and 4: Change From Baseline in Daily OFF Time Baseline Change from Baseline to Endpoint N (mean ± SD) hours N (LSMD LSMD: Least squares mean difference; a negative value indicates a greater reduction from baseline in "off" time for NOURIANZ, relative to placebo. vs. placebo) hours ( p-value)SD: Standard Deviation Study 3 Placebo 118 6.4 ± 2.7 118 -- NOURIANZ 20 mg 115 6.8 ± 2.9 115 -0.65 (p=0.028) NOURIANZ 40 mg 124 6.6 ± 2.5 124 -0.92 (p=0.002) Study 4 Placebo 123 6.3 ± 2.5 123 -- NOURIANZ 20 mg 120 6.6 ± 2.7 120 -0.76 (p=0.006) NOURIANZ 40 mg 123 6.0 ± 2.5 123 -0.74 (p=0.008)
In Study 3, compared with placebo, an additional increase from baseline in "on" time without troublesome dyskinesia of 0.57 hours (nominal p=0.085) and of 0.65 hours (nominal p=0.048), respectively, were observed in patients treated with NOURIANZ 20 mg or NOURIANZ 40 mg. In Study 4, the corresponding increases in "on" time without troublesome dyskinesia were 0.83 hours (nominal p=0.008) for NOURIANZ 20 mg and 0.81 hours (nominal p=0.008) for NOURIANZ 40 mg.
16how Supplied/storage And Handling
16.1How Supplied
NOURIANZ (istradefylline) tablets are available as:
20 mg Tablets:
Peach-colored, pillow-shaped, film-coated tablets with "20" debossed on one side.
Bottle of 90: NDC 42747-602-90
40 mg Tablets:
Peach-colored, almond-shaped, film-coated tablets with "40" debossed on one side.
Bottle of 90: NDC 42747-604-90
16.2Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
17patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Dyskinesia
Advise patients that NOURIANZ may cause dyskinesia or exacerbate pre-existing dyskinesia [see Warnings and Precautions (5.1)].
Hallucinations / Psychotic Behavior
Advise patients that NOURIANZ may cause hallucinations or psychotic behavior and they should report any of these adverse reactions to their healthcare provider [see Warnings and Precautions (5.2)].
Impulse Control / Compulsive Behaviors
Inform patients that they may experience intense urges to gamble, increased sexual urges, and other intense urges and the inability to control these urges while taking NOURIANZ and one or more medication(s) for the treatment of Parkinson's disease (including levodopa). Advise patients that they should report any of these adverse reactions to their healthcare provider [see Warnings and Precautions (5.3)].
Concomitant Medications
Certain medications can cause an interaction with NOURIANZ. Advise patients to inform their healthcare provider about their smoking status and about all of the medicines they are taking or plan to take, including over-the-counter medicines, dietary supplements, and herbal products [see Drug Interactions (7.1 , 7.2) and Use in Specific Populations (8.8)].
NOURIANZ® (istradefylline)Manufactured by:Kyowa Kirin, Inc.Princeton, NJ 08540
Spl Patient Package Insert Section
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 3/2023 Patient InformationNOURIANZ®  (nue'–ree–anz) (istradefylline)tablets, for oral use What is NOURIANZ? NOURIANZ is a prescription medicine used with levodopa and carbidopa to treat adults with Parkinson's disease (PD) who are having "off" episodes.It is not known if NOURIANZ is safe and effective in children. Before you take NOURIANZ, tell your healthcare provider about all your medical conditions, including if you: Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.NOURIANZ and other medicines may affect each other causing side effects. NOURIANZ may affect the way other medicines work, and other medicines may affect how NOURIANZ works.Know the medicines you take. Keep a ul of them to show your healthcare provider and pharmacist when you get a new medicine.
- have a history of abnormal movement (dyskinesia).
- have reduced liver function.
- smoke cigarettes.
- are pregnant or plan to become pregnant. NOURIANZ may harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if NOURIANZ passes into breast milk. You and your healthcare provider should decide if you will take NOURIANZ or breastfeed.
How should I take NOURIANZ?
- Take NOURIANZ exactly as your healthcare provider tells you to.
- Take NOURIANZ one time each day.
- You can take NOURIANZ with or without food.
- If you take too much NOURIANZ, call your health care provider or go to the nearest hospital emergency room right away.
What are the possible side effects of NOURIANZ?NOURIANZ may cause serious side effects, including:
- uncontrolled sudden movements (dyskinesia). Uncontrolled sudden movements is one of the most common side effects. NOURIANZ may cause uncontrolled sudden movements or make such movements you already have worse or more frequent. Tell your healthcare provider if this happens.
- hallucinations and other symptoms of psychosis. NOURIANZ can cause abnormal thinking and behavior including:
- being overly suspicious or feeling people want to harm you (paranoid ideation)
- believing things that are not real (delusions)
- seeing or hearing things that are not real (hallucinations)
- confusion
- increase activity or talking (mania)
- disorientation
- aggressive behavior
- agitation
- delirium (decreased awareness of things around you)
If you have hallucinations or any other abnormal thinking or behavior, talk with your healthcare provider. The most common side effects of NOURIANZ include uncontrolled movements (dyskinesia), dizziness, constipation, nausea, hallucinations, and problems sleeping (insomnia).These are not all the possible side effects of NOURIANZ.Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
- unusual urges (impulse control or compulsive behaviors). Some people taking NOURIANZ get urges to behave in a way unusual for them. Examples of this are unusual urges to gamble, increased sexual urges, strong urges to spend money, binge eating, and the inability to control these urges. If you notice or your family notices that you are developing any unusual behaviors, talk to your healthcare provider.
How should I store NOURIANZ?
- Store NOURIANZ at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep NOURIANZ and all medicines out of the reach of children.
General information about the safe and effective use of NOURIANZ. Medicines are sometimes prescribed for purposes other than those uled in a Patient Information leaflet. Do not use NOURIANZ for a condition for which it was not prescribed. Do not give NOURIANZ to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about NOURIANZ that is written for health professionals. What are the ingredients in NOURIANZ? Active ingredient: istradefyllineInactive ingredients: crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyvinyl alcohol, hypromellose, polyethylene glycol 3350, titanium dioxide, triacetin, iron oxide red, iron oxide yellow, and carnauba wax.Manufactured by: Kyowa Kirin, Inc., Princeton, NJ 08540 U.S. NOURIANZ is a registered trademark of Kyowa Kirin, Inc.For more information, call 1-844-768-3544 or go to www.NOURIANZ.com.
Principal Display Panel - 20 Mg Tablet Label
NDC 42747-602-90
NOURIANZ® (istradefylline) tablets
20 mg
90 tablets Rx Only KYOWA KIRIN

Principal Display Panel - 20 Mg Tablet Carton Label
NDC 42747-602-07 Rx Only
NOURIANZ® (istradefylline) tablets
20 mg per tablet
7 tablets(7-count buler card) KYOWA KIRIN
Principal Display Panel - 40 Mg Tablet Label
NDC 42747-604-90
NOURIANZ® (istradefylline) tablets
40 mg
90 tablets Rx Only KYOWA KIRIN

Principal Display Panel - 40 Mg Tablet Carton Label
NDC 42747-604-07 Rx Only
NOURIANZ® (istradefylline) tablets
40 mg per tablet
7 tablets(7-count buler card) KYOWA KIRIN
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


