Corvita (.beta.-carotene 750 [iu] .alpha.-tocopherol succinate, d- 125 [iu] cholecalciferol 315 [iu] ascorbic acid 375 mg thiamine mononitrate 25 mg riboflavin 3.4 mg niacinamide 35 mg folic acid 1.25 mg pyridoxine hydrochloride 35 mg biotin 75 ug pantothenic acid 5 mg cyanocobalamin 70 ug magnesium oxide 35 mg zinc oxide 35 mg selenomethionine 125 ug chromium picolinate 150 ug copper gluconate 1 mg .alpha.-lipoic acid 10 mg lutein 7 mg lycopene 2.5 mg) Dailymed
Go PRO for all pill images
Supplement Facts
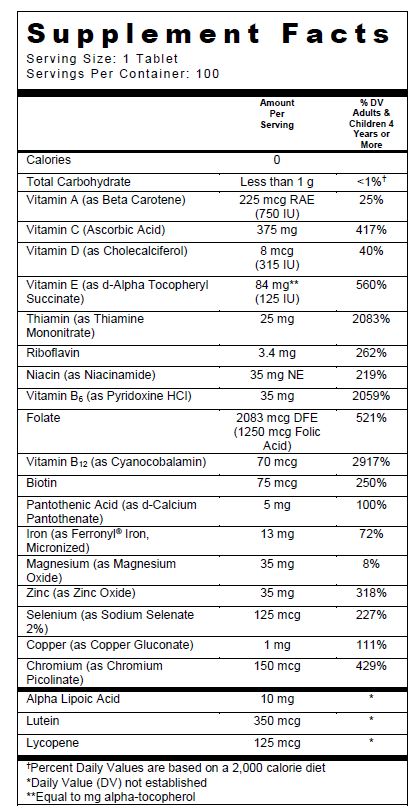
Other Ingredients: Silicified Microcrystalline Cellulose, Microcrystalline Cellulose, Green Color Coating (Hydroxypropylmethyl Cellulose, Titanium Dioxide, Polyvinyl Alcohol, Triacetin, Talc, FD&C Yellow #5 Lake, FD&C Blue #2 Lake, FD&C Yellow #6 Lake), Croscarmellose Sodium, Fumed Silica, Tri- Potassium Citrate, Magnesium Stearate, Stearic Acid, and Citric Acid.
Sugar, Lactose, and Gluten Free
CORVITA‚ĄĘ is a prescribed Multivitamin/Mineral Supplement with Iron that is specially formulated and indicated for the distinct nutritional requirements of individuals with vitamin deficiencies.
Indications And Usage
CORVITA‚ĄĘ Multivitamins/Minerals with Iron tablets are indicated for the distinctive nutritional requirements of persons being treated for vitamin deficiencies by a physician.
Contraindications
CORVITA‚ĄĘ Multivitamin/Mineral Supplement with Iron tablets should not be used by patients with known history of hypersensitivity to any of the uled ingredients or as directed by your physician.
Warning
 WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
Precautions
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily, may obscure pernicious anemia assessment, such that hematologic remission can occur while neurological manifestations remain progressive.
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Biotin levels higher than the recommended daily allowance may cause interference with some laboratory tests, including cardiovascular diagnostic test (e.g. troponin) and hormone tests, and may lead to incorrect test results. Tell your healthcare provider about all prescription and over-the-counter medicines, vitamins, and dietary supplements that you take, including biotin.
Drug Interactions
CORVITA‚ĄĘ Multivitamin & Mineral Supplement with Iron tablets are not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine. There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
Adverse Reactions
Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained in CORVITA‚ĄĘ. However, allergic and idiosyncratic reactions are possible at lower levels. Iron, even at the usual recommended levels has been associated with gastrointestinal intolerance in some patients.
Description
CORVITA‚ĄĘ is a green coated, oval shaped tablet debossed with ‚ÄúVP 011‚ÄĚon one side, bisect on the other side.
Directions For Use
Usual adult dosage is one bisected tablet daily, or as prescribed by a physician.
How Supplied
Corvita‚ĄĘ tablets are supplied in bottles of 100 tablets.
Product Code: 13811-028-10
Storage
Store at 20¬į-25¬įC (68¬į-77¬įF), excursion permitted to 15¬į-30¬įC (59¬į-86¬įF) [See USP Controlled Room Temperature]. Protect from light and moisture and avoid excessive heat. Dispense in a tight, light resistant container as defined by the USP with a child-resistant closure.
Health Claim Section
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-770-509-4500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.    
PLR-CORVITA-00001-02 Rev. 12/2021
Manufactured for:                                                                                             Trigen Laboratories, LLCAlpharetta, GA 30005
1-770-509-4500
Principal Display Panel - 100 Tablet Bottle Label
13811-028-10
CORVITA‚ĄĘ TABLETS
MULTIVITAMIN / MINERAL SUPPLEMENT WITH IRON
Sugar, Lactose and Gluten Free
100 TABLETS

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site
![Corvita tablet, coated - (.beta.-carotene 750 [iu] .alpha.-tocopherol succinate, d- 125 [iu] cholecalciferol 315 [iu] ascorbic acid 375 mg thiamine mononitrate 25 mg riboflavin 3.4 mg niacinamide 35 mg folic acid 1.25 mg pyridoxine hydrochloride 35 mg biotin 75 ug pantothenic acid 5 mg cyanocobalamin 70 ug magnesium oxide 35 mg zinc oxide 35 mg selenomethionine 125 ug chromium picolinate 150 ug copper gluconate 1 mg .alpha.-lipoic acid 10 mg lutein 7 mg lycopene 2.5 mg) image](https://pillboxwebp.pillsync.com/capsule.webp)

