Oraverse Dailymed
Generic: phentolamine mesylate is used for the treatment of Intracranial Arteriosclerosis Coronary Artery Disease Extravasation of Diagnostic and Therapeutic Materials Hypersensitivity Hypertension, Malignant Kidney Diseases Necrosis Pheochromocytoma
Go PRO for all pill images
1. Indicatons And Usage
OraVerse an alpha adrenergic blocker, is indicated for adult and pediatric patients ages 3 years and older for the reversal of soft-tissue anesthesia, i.e., anesthesia of the lip and tongue, and the associated functional deficits resulting from an intraoral submucosal injection of a local anesthetic containing a vasoconstrictor.
OraVerse, an alpha adrenergic blocker, is indicated for adult and pediatric patients ages 3 years and older for the reversal of soft-tissue anesthesia, i.e., anesthesia of the lip and tongue, and the associated functional deficits resulting from an intraoral submucosal injection of a local anesthetic containing a vasoconstrictor. (1 )
2. Dosage And Administration
Amount of Local Anesthetic Administered Dose of OraVerse
1/4 Cartridge
1/4 Cartridge (0.1 mg)
½ Cartridge
½ Cartridge (0.2 mg) 1 Cartridge 1 Cartridge (0.4 mg) 2 Cartridges 2 Cartridges (0.8 mg)
OraVerse is administered using the same location(s) and same technique(s) (infiltration or block injection) used for the administration of local anesthetic. (2.1 )
2.1 General Dosing information
The recommended dose of OraVerse is based on the number of cartridges of local anesthetic with vasoconstrictor administered:
Amount of Local Anesthetic Administered Dose of OraVerse [mg] Dose of OraVerse [Cartridge(s)] 1/4 Cartridge 0.1 1/4 ½ Cartridge 0.2 ½ 1 Cartridge 0.4 1 2 Cartridges 0.8 2
OraVerse should be administered following the dental procedure using the same location(s) and technique(s) (infiltration or block injection) employed for the administration of the local anesthetic.
Chemically disinfect the carpule cap by wiping with either isopropyl alcohol (91%) or ethyl alcohol (70%). Many commercially available brands of isopropyl (rubbing) alcohol, as well as solutions of ethyl alcohol not of U.S.P. grade, contain denaturants that are injurious to rubber and therefore are not to be used. Inspect carpules visually prior to administration and do not use if particulate matter, discoloration, cracks in the glass, protruding plungers or other defects are observed.
Note: Do not administer OraVerse if particulate matter, discoloration, cracks in the glass, protruding plungers or other defects are observed.
2.2 Dosing in Special Populations
In pediatric patients weighing between ≥15 kg and <30 kg, the maximum dose of OraVerse recommend is ½ cartridge (0.2 mg).(Note: Use in pediatric patients under 3years of age or weighing less than15 kg (33 lbs) is not recommended. A dose of more than 1 cartridge [0.4 mg] of OraVerse has not been studied in children less than 4 years of age.)
3. Dosage Forms And Strengths
0.4 mg/1.7 mL solution per cartridge
0.4 mg/1.7 mL solution per cartridge (3 )
4. Contraindications
OraVerse is contraindicated in patients with:Hypersensitivity to the active substance or to any ingredients in the formulation
OraVerse is contraindicated in patients with:Hypersensitivity to the active substance or to any ingredients in the formulation. (4 )
5. Warnings And Precautions
Myocardial infarction, cerebrovascular spasm, and cerebrovascular occlusion have been reported to occur following the intravenous or intramuscular administration of phentolamine, usually in association with marked hypotensive episodes or shock-like states which occasionally follow parenteral administration.
Tachycardia and cardiac arrhythmiasmay occur with the use of phentolamine or other alpha-adrenergic blocking agents. (5.1 )
5.1 Cardiovascular Events
Myocardial infarction, cerebrovascular spasm, and cerebrovascular occlusion have been reported to occur following the parenteral administration of phentolamine. These events usually occurred in association with marked hypotensive episodes producing shock-like states.
Tachycardia and cardiac arrhythmias may occur with the use of phentolamine or other alpha-adrenergic blocking agents. Although such effects are uncommon after administration of OraVerse, clinicians should be alert to the signs and symptoms of these events, particularly in patients with a prior history of cardiovascular disease.
6. Adverse Reactions
In clinical trials, the most common adverse reaction with OraVerse that was greater than the control group was injection site pain.
The most common adverse reaction with OraVerse (incidence ≥5% and > control) is injection-site pain. (6 )
To report SUSPECTED ADVERSE REACTIONS, contact Septodont at 1-888-888-1441 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Dental patients were administered a dose of either 0.2, 0.4 or 0.8mg of OraVerse. The majority of adverse reactions were mild and resolved within 48 hours. There were no serious adverse reactions and no discontinuations due to adverse reactions.
Table 1 uls adverse reactions where the frequency was greater than or equal to 3% in any OraVerse dose group and was equal to or exceeded that of the control group.
Table 1: Adverse Reactions with Frequency Greater Than or Equal to 3% and Equal to or Exceeding Control Adverse Event OraVerse Control 0.2 mg(N = 83) 0.4 mg(N = 284) 0.8 mg(N = 51) Total(N = 418) Total(N = 359) N (%) N (%) N (%) N (%) N (%) Patients with AEs 15 (18) 82 (29) 20 (39) 117 (28) 96 (27) Tachycardia 0 (0) 17 (6) 2 (4) 19 (5) 20 (6) Bradycardia 0 (0) 5 (2) 2 (4) 7 (2) 1 (0.3) Injection site pain 5 (6) 15 (5) 2 (4) 22 (5) 14 (4) Post procedural pain 3 (4) 17 (6) 5 (10) 25 (6) 23 (6) Headache 0 (0) 10 (4) 3 (6) 13 (3) 14 (4)
An examination of population subgroups did not reveal a differential adverse reaction incidence on the basis of age, gender, or race.
Results from the pain assessments in Study 1 and Study 2, involving mandibular and maxillary procedures, respectively, indicated that the majority of dental patients in both OraVerse and control groups experienced no or mild oral pain, with less than 10% of patients in each group reporting moderate oral pain with a similar distribution between the OraVerse and control groups. No patient experienced severe pain in these studies.
Study 4 included 150 pediatric patients between 2-5 years of age who received a dose of either ¼ cartridge (0.1 mg), ½ cartridge (0.2 mg) or 1 cartridge (0.4 mg) of OraVerse or sham injection (placebo). Safety in patients in Study 4 was similar to safety in older patients described above. Post-procedural revealed that oral pain was reported in the OraVerse group with a higher frequency (10.1%) than the placebo group (3.9%). The proportion of patients in the OraVerse and placebo groups was comparable with respect to the highest severity of pain experienced: 30.4% of OraVerse patients and 30% of placebo patients reported no pain; 43.1% of OraVerse patients and 45.0% of placebo patients reported mild pain; 19.0% of OraVerse subjects and 17.5% of placebo patients reported moderate pain; and 15.2% of OraVerse patients and 15.0% of placebo patients reported severe pain.
6.2 Adverse Reactions in Clinical Trials
Adverse reactions reported by less than 3% but at least 2 dental patients receiving OraVerse and occurring at a greater incidence than those receiving control, included diarrhea, facial swelling, increased blood pressure/hypertension, injection site reactions, jaw pain, oral pain, paresthesia, pruritus, tenderness, upper abdominal pain and vomiting. The majority of these adverse reactions were mild and resolved within 48 hours. The few reports of paresthesia were mild and transient and resolved during the same time period.
6.3 Post Marketing Adverse Reactions Reports from Literature and Other Sources
The following adverse reactions have been identified during postapproval parenteral use of phentolamine mesylate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Acute and prolonged hypotensive episodes and cardiac arrhythmias have been reported with the use of phentolamine. In addition, weakness, dizziness, flushing, orthostatic hypotension, and nasal stuffiness have occurred.
7. Drug Interactions
There are no known drug interactions with OraVerse.
7.1 Lidocaine and Epinephrine
When OraVerse was administered as an intraoral submucosal injection 30 minutes after injection of a local anesthetic, 2% lidocaine HCl with 1:100,000 epinephrine, the lidocaine concentration increased immediately after OraVerse intraoral injection. Lidocaine AUC and Cmax values were not affected by administration of OraVerse. OraVerse administration did not affect the PK of epinephrine.
8. Use In Specific Populations
- Use in pediatric patients less than 3 years of age or <15 kg (33 lbs) has not been established. (
8.4 )- In pediatric patients weighing at least 10 kg (22 lbs), the maximum dose of OraVerse recommended is 1/4cartridge (0.1 mg). (
8.4 )8.1 Pregnancy
PREGNANCY SECTION
Pregnancy Category C
Risk summary
There are no available data with OraVerse in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. In animal toxicology studies, phentolamine administered orally to pregnant mice and rats during the period of organogenesis resulted in skeletal immaturity and decreased growth in the offspring at doses at least 24-times the recommended dose. Additionally, a lower rate of implantation was seen in pregnant rats treated with phentolamine at least 60-times the recommended dose. No malformations or embryofetal deaths were observed in the offspring of pregnant mice, rats, and rabbits administered phentolamine during the period of organogenesis at doses 24-, 60-, and 20-times, respectively, the recommended dose [see Data].The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Oral administration of phentolamine to pregnant rats and mice at doses at least 24-times the recommended dose (based on a mg/m2 comparison with a 60 kg human) resulted in slightly decreased growth and slight skeletal immaturity of the fetuses. Immaturity was manifested by increased incidence of incomplete or unossified calcanei and phalangeal nuclei of the hind limb and of incompletely ossified sternebrae. At oral phentolamine doses at least 60-times the recommended dose (based on a mg/m2 comparison with a 60 kg human),a slightly lower rate of implantation was found in the rat. Phentolamine did not affect embryonic or fetal development in the rabbit at oral doses at least 20-times the recommended dose (based on a mg/m2 comparison with a 60 kg human). No malformations or embryofetal deaths were observed in the rat, mouse or rabbit studies.
8.2 Lactation
Risk SummaryThere is no information regarding the presence of phentolamine in human milk, the effects on the breastfed infant or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OraVerse and any potential adverse effects on the breastfed infant from OraVerse, or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of OraVerse has not been established in patients younger than 3 years.The safety and effectiveness of OraVerse in pediatric patients ages 3 years and older is supported by evidence from adequate and well-controlled studies of OraVerse in adults, with additional adequate and well-controlled studies of OraVerse in pediatric patients ages 12-17 years old [Studies 1 (mandibular procedures) and 2 (maxillary procedures)], ages 6-11 years old [Study 3 (mandibular and maxillary procedures)], and another study in patients ages 2-5 years [Study 4]. Study 4 assessed safety and effectiveness in patients 4 to 5 years, but was not designed to demonstrate efficacy. Use in patients 3 to <4 years is supported by similar pharmacokinetics and safety in these patients compared with older pediatric patients (see Clinical Pharmacology (12.3)]. Use of OraVerse in this age group (3 to < 4 years) is also supported by the similarity in the exposure response of OraVerse for pediatric and adult patients, and the adequacy of the safety database for patients age ≥ 3. The safety database for patients age < 3 is limited, and therefore, use in patients age < 3 is not recommended. Dosages in pediatric patients may need to be limited based on body weight. [seeDosage and Administration (2)]
8.5 Geriatric Use
Of the total number of patients in clinical studies of OraVerse, 55 were 65 and over, while 21 were 75 and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
10. Overdosage
No deaths due to acute poisoning with phentolamine have been reported.
Overdosage with parenterally administered phentolamine is characterized chiefly by cardiovascular disturbances, such as arrhythmias, tachycardia, hypotension, and possibly shock. In addition, the following might occur: excitation, headache, sweating, pupillary contraction, visual disturbances, nausea, vomiting, diarrhea, or hypoglycemia.
There is no specific antidote; treatment consists of appropriate monitoring and supportive care. Substantial decreases in blood pressure or other evidence of shock-like conditions should be treated vigorously and promptly.
11. Description
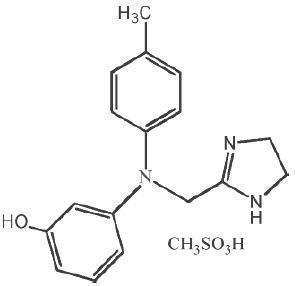
Phentolamine mesylate is phenol,3-[[(4,5-dihydro-1H-imidazol-2-yl)methyl](4-methyl-phenyl)amino]-,methanesulfonate (salt), a non-specific alpha adrenergic blocker.
Phentolamine mesylate USP is a white to off-white, odorless crystalline powder with a molecular weight of 377.46. It is sparingly soluble in water, soluble in alcohol, and slightly soluble in chloroform. The empirical formulation is C17H19N3O•CH4O3S, and the chemical structure is:
OraVerse (phentolamine mesylate) Injection is a clear, colorless, sterile, non pyrogenic, isotonic, preservative free solution. Each 1.7 mL cartridge contains 0.4 mg phentolamine mesylate, D-mannitol, edetate disodium, and sodium acetate. Either acetic acid or sodium hydroxide is used as necessary to adjust the pH.
12. Clinical Pharmacology
12.1 Mechanism of Action
The mechanism by which OraVerse accelerates reversal of soft-tissue anesthesia and the associated functional deficits is not fully understood. Phentolamine mesylate, the active ingredient in OraVerse, produces an alpha-adrenergic block of relatively short duration resulting in vasodilatation when applied to vascular smooth muscle. In an animal model, OraVerse increased local blood flow in submucosal tissue of the dog when given after an intraoral injection of lidocaine 2% with 1:100,000 epinephrine.
12.3 Pharmacokinetics
Following OraVerse administration, phentolamine is 100% available from the submucosal injection site and peak concentrations are achieved 10-20 minutes after injection. Phentolamine systemic exposure increased linearly after 0.8 mg compared to 0.4 mg OraVerse intraoral submucosal injection. The terminal elimination half-life of phentolamine in the blood was approximately 2-3 hours.
Pediatrics
Following OraVerse administration, the phentolamine Cmax was higher (approximately 3.5-fold) in children who weighed between 15 and 30 kg (33 and 66 lbs) than in children who weighed more than 30 kg. However, phentolamine AUC was similar between the two groups. It is recommended that in children weighing 15-30 kg, the maximum dose of OraVerse should be limited to ½ cartridge (0.2 mg) (seeDosage and Administrationsection).
The pharmacokinetics of OraVerse in adults and in children who weighed more than 30 kg (66 lbs) are similar after intraoral submucosal injection.
OraVerse has not been studied in children under 3 years of age or weighing less than 15 kg (33 lbs). The pharmacokinetics of OraVerse after administration of more than 1 cartridge (0.4mg) has not been studied in children.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis Carcinogenic studies with OraVerse have not been conducted.
Mutagenesis Phentolamine was not mutagenic in the in-vitro bacterial reverse mutation (Ames) assay. In the in-vitro chromosomal aberration study in Chinese hamster ovary cells, numerical aberrations were slightly increased after a 4-hour exposure to phentolamine without metabolic activation and structural aberrations were slightly increased after a 4-hour exposure to phentolamine with metabolic activation only at the highest concentrations tested, but neither numerical nor structural aberrations were increased after a 20-hour exposure without metabolic activation. Phentolamine was not clastogenic in two in-vivo mouse micronucleus assays.
Impairment of Fertility The effect of phentolamine on female fertility has not been studied. Male rats treated with oral phentolamine for nine weeks (four weeks prior to mating, 3 weeks during the mating period and 2 weeks after mating) were mated with untreated females. At doses up to 143-times human therapeutic exposure levels at the Cmax, no adverse effects on male fertility parameters or on reproductive parameters in the untreated females mated with the treated males were observed.
14. Clinical Studies
The safety and efficacy of OraVerse when used for reversal of soft-tissue anesthesia (STA), i.e., anesthesia of the lips and tongue following a dental procedure that required local anesthesia containing a vasoconstrictor, were evaluated in the following clinical studies. OraVerse-induced reversal of local anesthetic effects on the teeth, mandible and maxilla has not been assessed.
Two Phase 3, double-blinded, randomized, multi-center, controlled studies were conducted in dental patients who had mandibular (Study 1) or maxillary (Study 2) restorative or periodontal maintenance procedures and who had received a local anesthetic that contained a vasoconstrictor. The primary endpoint was time to normal lip sensation as measured by patient reported responses to lip palpation. The secondary endpoints included patients' perception of altered function, sensation and appearance, and their actual functional deficits in smiling, speaking, drinking and drooling, as assessed by both the patient and an observer blinded to the treatment. In the mandibular study, the time to recovery of tongue sensation was also a secondary endpoint. Patients were stratified by type and amount of anesthetic administered. OraVerse was administered at a cartridge ratio of 1:1 to local anesthetic. The control was a sham injection.
OraVerse reduced the median time to recovery of normal sensation in the lower lip by 85 minutes (55%) compared to control. The median time to recovery of normal sensation in the upper lip was reduced by 83 minutes (62%). The differences between these times for both studies are depicted in Kaplan-Meier plots for time to normal lip sensation in
Figures 1 and 2. Within 1 hour after administration of OraVerse, 41% of patients reported normal lower lip sensation as compared to 7% in the control group, and 59% of patients in the OraVerse group reported normal upper lip sensation as compared to 12% in the control group.
Figure 1: Kaplan-Meier Plot of Time to Recovery of Normal Sensation in the Lower Lip (ITT Analysis Data Set) 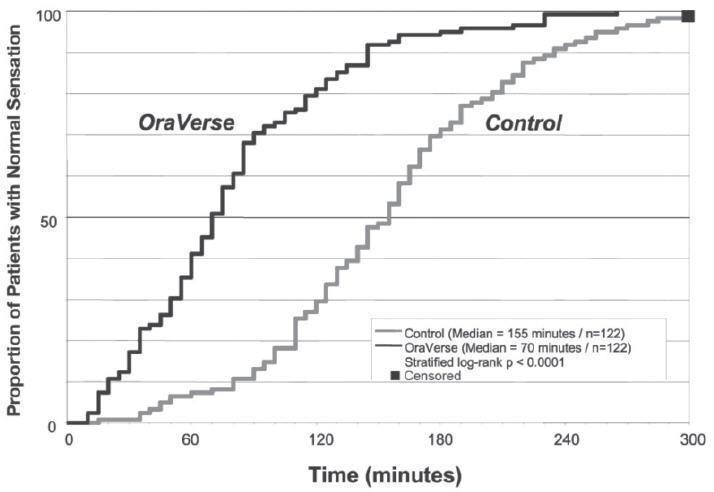
Figure 2: Kaplan-Meier Plot of Time to Recovery of Normal Sensation in the Upper Lip (ITT Analysis Data Set) 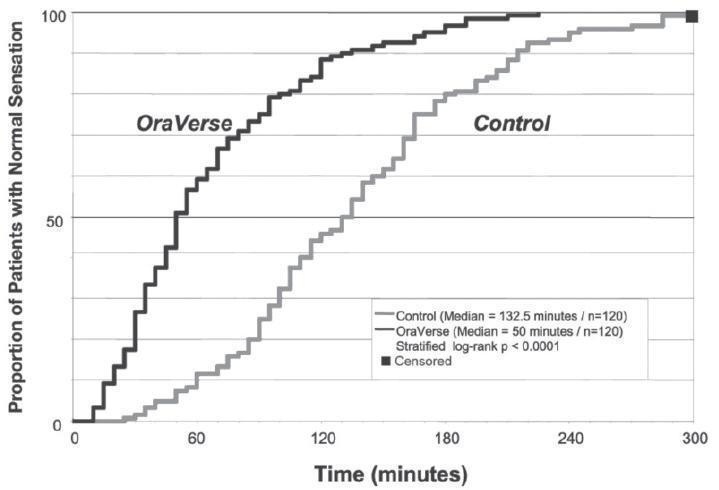
In Study 1 (mandibular), OraVerse accelerated: a) the recovery of the perception of normal appearance and function by 60 minutes (40%), b) the recovery of normal function by 60 minutes (50%), and c) the recovery of normal sensation in the tongue by 65 minutes (52%). In Study 2 (maxillary), the recovery of the perception of normal appearance and function was reduced by 60 minutes (50%) and the recovery of normal function was reduced by 45 minutes (43%).
Study 3, a pediatric, Phase 2, double-blinded, randomized, multi-center, controlled study was conducted in dental patients who had received 2% lidocaine with 1:100,000 epinephrine. Dental patients (n=152, ages 4-11 years) received 1/2 cartridge of OraVerse if they weighed ≥15 kg but <30 kg, and one-half or one full cartridge if they weighed ≥30 kg at a cartridge ratio of 1:1 to local anesthetic.The median time to normal lip sensation in patients 6 to 11years of age who were trainable in the lip-palpation procedures, for mandibular and maxillary procedures combined, was reduced by 75 minutes (56%). Within 1 hour after administration of OraVerse, 44 patients (61%) reported normal lip sensation, while 9 patients (21%) randomized to the control group reported normal lip sensation. In this study, neither the patients' perception of their appearance or ability to function nor their actual ability to function was evaluated.
Study 4, a pediatric, Phase 4, double-blinded, randomized, multi-center, controlled study was conducted in dental patients undergoing mandibular and maxillary procedures after receiving 2% lidocaine with 1:100,000 epinephrine. Patients 2-5 years of age received sham injection (n=51) or 1/4 cartridge of OraVerse if they weighed ≥10 kg but <15kg (n=5), 1/2 cartridge if they weighed ≥15 kg but <30kg (n=91), and a full cartridge if they weighed >30kg (n=3). This study was not designed to demonstrate efficacy.
The median time to normal lip sensation in patients 4 and 5 years of age who were trainable in the lip-palpation procedure, for mandibular and maxillary procedures combined, was reduced by 48 minutes (44%). Within 2 hours after administration of OraVerse, 57 patients (80%) reported normal lip sensation, while 19 patients (51%) randomized to the sham injection group reported normal lip sensation. There were no significant differences between OraVerse and sham injection for time to return of normal function in pediatric functional assessment battery and time to recovery of normal tongue sensation (for mandibular procedures only).
16. How Supplied/storage And Handling
OraVerse (phentolamine mesylate) Injection 0.4 mg/1.7 mL is supplied in a dental cartridge, in cartons of 10 and 50 cartridges. Each cartridge is individually packaged in a separate compartment of a 10 cartridge buler pack.
NDC 0362-0101-50NDC 0362-0101-10
STORAGE AND HANDLING SECTION
Store at controlled room temperature, 20-25°C (68-77°F) with brief excursions permitted between 15-30°C (59-86°F) (see USP Controlled Room Temperature)
Protect from direct heat and light. Do not permit to freeze.
Manufactured by
Novocol Pharmaceutical of Canada, Inc. Cambridge, Ontario, Canada
for
Septodont, Inc Louisville, CO 80027
US Patent Nos.: 6,764,678; 6,872,390; 7,229,630Trademark of Septodont Holdings SAS
17. Patient Counseling Information
Patients should be instructed not to eat or drink until normal sensation returns.
septodont
Rev. 03/16 (2604-5)
Principal Display Panel - 10 Pack Of 0.4mg/1.7 Ml Carton
NDC 0362-0101-10
OraVerse™
(Phentolamine Mesylate) Injection 0.4 mg/1.7 mL
For Intraoral Submucosal Injection Only 10 x 0.4 mg/1.7 mL
septodont
Distributed by Septodont, Inc.Louisville, CO, 80027
Made in Canada byNovocol Pharmaceutical of Canada, Inc.
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site



